Abstract
Background
There is increasing evidence to support the use of neoadjuvant chemotherapy (NAC) in locally advanced colon cancer (LACC). However, its safety, efficacy and side effect profile is yet to be completely elucidated. This review aims to assess NAC regimens, duration, compare completion rates, intra-operative and post-operative complication profiles and oncological outcomes, in order to provide guidance for clinical practice and further research.
Methods
PubMed, EMBASE and MEDLINE were searched for a systematic review of the literature from 2000 to 2020. Eight eligible studies were included, with a total of 1213 patients, 752 (62%) of whom received NAC. Of the eight studies analysed, two were randomised controlled trials comparing neoadjuvant chemotherapy followed by oncological resection to upfront surgery and adjuvant chemotherapy, three were prospective single-arm phase II trials analysing neoadjuvant chemotherapy followed by surgery only, one was a retrospective study comparing neoadjuvant chemotherapy followed by surgery versus surgery first followed by adjuvant chemotherapy and the remaining two were single-arm retrospective studies of neoadjuvant chemotherapy followed by surgery.
Results
All cases of LACC were determined and staged by computed tomography; majority of the studies defined LACC as T3 with extramural depth of 5 mm or more, T4 and/or nodal positivity. NAC administered was either folinic acid, fluorouracil and oxaliplatin (FOLFOX) or capecitabine and oxaliplatin (XELOX) with the exception of one study which utilised 5-fluorouracil and mitomycin. Most studies had NAC completion rates of above 83% with two notable exceptions being Zhou et al. and The Colorectal Cancer Chemotherapy Study Group of Japan who both recorded a completion rate of 52%. Time to surgery from completion of NAC ranged on average from 16 to 31 days. The anastomotic leak rate in the NAC group ranged from 0 to 4.5%, with no cases of postoperative mortality. The R0 resection rate in the NAC group was 96.1%. Meta-analysis of both RCTs included in this study showed that neoadjuvant chemotherapy increased the likelihood of a negative resection margin T3/4 advanced colon cancer (pooled relative risk of 0.47 with a 95% confidence interval) with no increase in adverse consequence of anastomotic leak, wound infection or return to theatre.
Conclusions
Our systematic review and meta-analysis show that NAC is safe with an acceptable side effect profile in the management of LACC. The current data supports an oncological benefit for tumour downstaging and increased in R0 resection rate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer is the third most commonly diagnosed cancer and the fourth most common cause of cancer death worldwide [1]. One of the challenges is mitigating the risk of local and distant recurrences, which is estimated to be 20–30% in patients with locally advanced colon cancer (LACC) [2]. The definition of LACC includes T3 tumours with ≥ 5 mm invasion beyond the muscularis propria, T4 (direct invasion into adjacent structures) or extensive regional lymph node involvement, without distant metastases [3,4,5].
Several trials have validated neoadjuvant chemotherapy (NAC) as a strategy for other tumour types, such as locally advanced gastroesophageal, breast and rectal cancers [6,7,8,9]. Hence, there has been an interest in applying this strategy for LACC as well. The theoretical advantages for this include the early treatment of potential lymph node and/or distant micrometastases, increased likelihood of a clear resection (R0) margin and the ability to evaluate chemosensitivity and assess tumour biology by the degree of downstaging that may occur after treatment.
However, use of neoadjuvant treatment is not without risk: it has been associated with chemotoxicity resulting in delay to surgery, disease progression, risk of bowel obstruction or perforation requiring emergency surgery [6, 7, 10]. Several recent trials have assessed the role of neoadjuvant chemotherapy in LACC [10,11,12,13,14,15,16,17]. Many of these studies have been small, observational studies with only two multicentre randomised controlled trials (RCT) that have been published thus far [11, 12]. As a result, most studies have inconsistent results with no international consensus on the value of neoadjuvant chemotherapy compared with adjuvant chemotherapy.
Therefore, this study aims to assess NAC regimens and duration, compare completion rates, intra-operative and post-operative complication profiles and oncological outcomes, in order to provide guidance for clinical practice and further research.
Methods
A systematic literature search was performed of all published articles in English from January 2000 to January 2020. This systematic review was performed in accordance with guidelines from the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) group [18]. Randomised control trials, non-randomised prospective trials, retrospective studies and conference abstracts were included given paucity of available literature. Studies were identified by searching MEDLINE (via PubMed) and EMBASE (via OvidSP) databases. Ongoing trails were identified via the clinical trials registry: https://clinicaltrials.gov/.
Studies included in data analysis were reporting the use of preoperative chemotherapy in non-metastatic locally advanced colon cancer and included the terms “neoadjuvant chemotherapy”, “preoperative chemotherapy” and “perioperative chemotherapy”. Articles were excluded if (i) it was not possible to differentiate between colon and rectal cancers, (ii) treatment for rectal cancer only and (iii) studies that investigate the role of neoadjuvant therapy in the setting of colon cancers with distant metastasis. Data was collated using a well-defined data extraction sheet.
Data collection and endpoints
Data regarding trial design, patient selection protocols, number of patients, patient demographics, radiological stage, pre- and post-operative chemotherapy protocols, completion rate of chemotherapy, type of surgery, rate of surgery, histopathological outcomes, perioperative morbidity and mortality, length of follow-up and oncological outcomes were recorded. The primary end point of the study assessed was the tumour regression grade (TRG) to NAC. Secondary end points included completion rates of neoadjuvant and adjuvant chemotherapy, R0 resection rates and chemotherapy toxicity. Adverse events included anastomotic leak, wound infection and overall rate of return to theatre.
TRG was graded in 3 categories by Ryan et al., from 1 (complete or near-complete response) to 3 (no response) based on the presence of residual tumour cells in resection specimen [19]. R0 resection was defined as a microscopically margin-negative resection. Completion rate was defined as the number of patients in whom the full course of chemotherapy was completed, as a percentage of all patients that started chemotherapy. Toxicity was graded in accordance with the Cancer Therapy Evaluation Program Common Toxicity Criteria. Grade 3 toxicity was defined as a severe and undesirable adverse event [20]. Time to surgery was defined as the time in days from the date of completion of neoadjuvant chemotherapy to the date surgery was performed.
Data were entered into an Excel spreadsheet and verified for accuracy.
Statistical analysis
All categorical data was pooled using the random effect model to yield a relative risk and associated 95% confidence interval (CI). I2 statistic was formed to assess for inter-study heterogeneity. The Newcastle-Ottawa scale is used to assess the quality of each non-randomised study where Jadad score was used to assess the quality of RCTs. A p value of < 0.05 was considered significant. All data analysis was performed in R Studio Team (2015). RStudio: Integrated Development for R Studio, Inc., Boston, MA, and using the metaphor package for meta-analysis [21].
Results
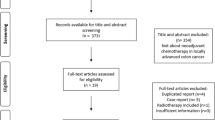
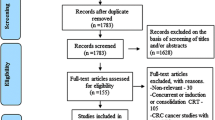
The preliminary search identified 17 studies pertinent to neoadjuvant treatment of LACC. Nine studies were excluded from analysis: three involved neoadjuvant radiotherapy either as a single modality or in combination as chemotherapy [22,23,24], three studies had outcome measures not relevant to this meta-analysis [25,26,27] and one study was a literature review with no original findings [28]. Of the eight studies included, two were randomised controlled trials comparing neoadjuvant chemotherapy followed by oncological resection to upfront surgery and adjuvant chemotherapy [11, 29], three were prospective single-arm phase II trials analysing neoadjuvant chemotherapy followed by surgery only [10, 15, 30], one was a retrospective study comparing neoadjuvant chemotherapy followed by surgery versus surgery first followed by adjuvant chemotherapy [31] and the remaining two were single-arm retrospective studies of neoadjuvant chemotherapy followed by surgery [32, 33].
Design features of all eligible studies are shown in Tables 1 and 2. All cases of LACC were determined and staged by computed tomography; majority of the studies defined LACC as T3 with extramural depth of 5 mm or more, T4 and/or nodal positivity. Neoadjuvant chemotherapy administered was either folinic acid, fluorouracil and oxaliplatin (FOLFOX) or capecitabine and oxaliplatin (XELOX) with the exception of one study which utilised 5-fluorouracil (5-FU) and mitomycin (MMC). Most studies had NAC completion rates of above 83% with two notable exceptions being Zhou et al. and The Colorectal Cancer Chemotherapy Study Group of Japan who both recorded a completion rate of 52%. Time to surgery from completion of NAC ranged on average from 16 to 31 days.
Completion of adjuvant chemotherapy ranged from 67 to 100%, with Zhou et al. reporting 47.1% adjuvant chemotherapy completion. On specific analysis of the two RCTs included, Karoui et al. documented an adjuvant chemotherapy completion rate of 74.5% in their control arm while the FOxTROT Collaborative Group recorded 72.5%. Completion rates in their intervention arms were 98% and 81.7%, respectively. The median days to adjuvant chemotherapy was consistently 5–6 weeks in both intervention and control groups.
Both RCTs included in this study showed that neoadjuvant chemotherapy increased the likelihood of a negative resection margin T3/4 advanced colon cancer (pooled relative risk of 0.47 with a 95% confidence interval) (Fig. 1) with no increase in adverse consequence of anastomotic leak, wound infection or return to theatre (Figs. 2, 3 and 4). Further assessment on the complication profile associated with neoadjuvant chemotherapy is outlined in Table 3. This includes risk of neutropenia (13.1%), neurotoxicity (7.4%) with an overall grade 3 toxicity rate of 9.8%.
Discussion
To the best of our knowledge, this is the first published meta-analysis reviewing the use of NAC in LACC. Our study shows that NAC can be delivered safely with acceptable toxicity and is associated with higher R0 resection rates. Neoadjuvant chemotherapy is already an accepted gold standard treatment for rectal, gastric, pancreatic and breast cancer [8, 34,35,36]. Our study results support the hypothesis that NAC in selected colon cancer can encourage tumour regression with the aim of downstaging tumours, thus resulting in better rates of local control. These results are novel.
The specific patient cohort for analysis in this systematic review and meta-analysis was patients that had high-risk features such as T4 or high-risk T3 (> 5-mm tumour invasion beyond the muscularis propria, lymphovascular involvement or poorly differentiated tumours) without distant metastases (T3/4, N0, M0). Identifying this patient population relies heavily on accurate CT staging as it guides the need for neoadjuvant therapy. In a recent meta-analysis, Nerad et al. [37] found CT staging to be accurate with an overall sensitivity of 90% in detecting tumour invasion beyond the bowel wall and nodal involvement.
Another potential modality is the magnetic resonance imaging (MRI), which is the current gold standard for staging rectal cancer [38]. The comparative diagnostic performance of MRI and CT for LACC has been investigated in multiple previous studies [39,40,41,42], in which all unanimously concluded that MRI is superior in defining T3 tumours with serosal involvement and T4 tumours compared to CT. Combined with this known precision for detecting liver metastases, MRI may rapidly become the most optimal staging modality for patients with locally advanced colon cancer.
The Colorectal Cancer Chemotherapy Study Group of Japan [17] employed the use of neoadjuvant MMC and 5-FU; this regime is largely usurped by agents such as capecitabine, oxaliplatin and irinotecan. MMC is an old drug that has been tested in multiple phase III trials for its use in colorectal cancer, and although it has a favourable toxicity profile when administered synergistically with capecitabine [43, 44], it has failed to show a survival benefit when compared with 5-FU alone [45] and hence has no role as the first or second line of therapy in colorectal cancer.
Other concerns raised about NAC were related to unnecessary patient morbidity from chemotherapeutic toxicities and delays to operative intervention. In addition, the response of tumours to neoadjuvant therapies remains variable, a subgroup of patients may not achieve any down-staging of the tumour and some of them even show disease progression as observed in locally advanced rectal cancer [46, 47]. This subgroup tended to have worse tumour biology and poorer overall prognosis; hence, administering NAC would allow potential stratification and select patients out that will benefit the most from curative surgery as many may progress to distant metastasis. The results of this study clearly show that NAC is well tolerated with an acceptable side effect profile with on average less than 30 days to surgery. Clinically significant neoadjuvant therapy toxicity (grade 3) was only observed in 9.8% of all patients included.
The FOxTROT trial demonstrated that there was no increased risk of tumour progression leading to emergency surgery. Karoui et al. [11] and the FOxTROT Collaborative Group [12] both reported higher rates of completion of therapy in NAC group as compared to the adjuvant therapy alone group, 88% vs 76% and 68% vs 57%, respectively. Importantly, pooled relative risk analysis of both RCTs showed no significant difference in post-operative anastomotic leaks, wound infections or return to theatre between the neoadjuvant and control arms. In a recent presentation at the European Society for Medical Oncology (ESMO) Congress in 2019, the FOxTROT Collaborative Group presented an interim analysis of a further 1053 patients across 98 hospitals in the UK, Denmark and Sweden. The group reported that compared to the NAC arm patients in the control arm had double the number of incomplete resections (10% vs %; p = 0.001) requiring an additional operation (7.1% vs 4.3%; p = 0.05) and suffered higher rates of anastomotic leaks or intra-abdominal abscesses (7.4% vs 4.7%; p = n.s.); however, this data is yet to be published and hence could not be used for statistical analysis [48].
Complete oncological resection followed by adjuvant chemotherapy is the current standard of treatment for patients with LACC. This approach may necessitate an extensive en bloc multivisceral resection for T4b to maintain a high-quality surgical resection, with the aim of a negative resection margin to decrease the risk of recurrence. However, this is often associated with an increased postoperative morbidity and known to have a lower rate of R0 resection, varying between 40 and 90% [49, 50]. As such, the current NCCN guidelines have added NAC as a treatment option for patients with clinical T4b disease [51]. Pooled outcome of all the patients analysed in this study has revealed that 59.2% demonstrated at least a partial histopathological response with 5% demonstrating a complete response. Additionally, the aforementioned interim analysis by the FOxTROT group also reported that of the 699 patients allocated to the NAC arm, 88% completed the three cycles of neoadjuvant FOLFOX and had marked histological downstaging with a lower pT and pN stage (p < 0.0001 for both). A subset of patients in the NAC arm displayed a complete (3.8%) and near-complete (4.6%) tumour regression [48]. However, larger studies are required to further elucidate the true effect of tumour regression after NAC.
This review was limited primarily by a paucity of data available for comparative analysis. There were only two randomised control trials eligible for pooled meta-analysis. There are currently, however, many ongoing trials assessing both pathological response and survival of LACC after NAC.
Conclusion
Neoadjuvant chemotherapy is safe for the management of LACC as highlighted by this review. The current data supports an oncological benefit for tumour downstaging and increased in R0 resection rate. Hence, NAC can be considered as alternate strategy before surgery for clinically staged advanced colon cancers (T4b), particularly where a clear resection margin is questionable.
References
Favoriti P, Carbone G, Greco M, Pirozzi F, Pirozzi REM, Corcione F (2016) Worldwide burden of colorectal cancer: a review. Updat Surg 68(1):7–11
Xue L, Williamson A, Gaines S, Andolfi C, Paul-Olson T, Neerukonda A, Steinhagen E, Smith R, Cannon LM, Polite B, Umanskiy K, Hyman N (2018) An update on colorectal cancer. Curr Probl Surg 55(3):76–116
Edge SB, Edge SB (2017). AJCC Cancer Staging Manual 8th Ed: Springer
Vieira RA, Lopes A, Almeida PA, Rossi BM, Nakagawa WT, Ferreira FO et al (2004) Prognostic factors in locally advanced colon cancer treated by extended resection. Revista do Hospital das Clínicas 59(6):361–368
Eisenberg S, Kraybill WG, Lopez M (1990) Long-term results of surgical resection of locally advanced colorectal carcinoma. Surgery. 108(4):779
Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF, Karstens JH, Liersch T, Schmidberger H, Raab R (2004) Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 351(17):1731–1740
Wang X, Zhao L, Liu H, Zhong D, Liu W, Shan G, Dong F, Gao W, Bai C, Li X (2016) A phase II study of a modified FOLFOX6 regimen as neoadjuvant chemotherapy for locally advanced gastric cancer. Br J Cancer 114(12):1326–1333
Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M et al (2006) Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 355(1):11–20
Medical Research Council Oesophageal Cancer Working Group (2002) Surgical resection with or without preoperative chemotherapy in oesophageal cancer: a randomised controlled trial. Lancet 359(9319):1727–1733
Liu F, Yang L, Wu Y, Li C, Zhao J, Keranmu A, Zheng H, Huang D, Wang L, Tong T, Xu J, Zhu J, Cai S, Xu Y, Department of Colorectal Surgery, Fudan University Shanghai Cancer Center, Shanghai 200032, China, Department of Oncology, Shanghai Medical College, Fudan University, Shanghai 200032, China, Department of Pathology, Fudan University Shanghai Cancer Center, Shanghai 200032, China, Department of Radiology, Fudan University Shanghai Cancer Center, Shanghai 200032, China, Department of Nuclear Medicine, Fudan University Shanghai Cancer Center, Shanghai 200032, China, Department of Radiation Oncology, Fudan University Shanghai Cancer Center, Shanghai 200032, China, Clinical Statistics Center, Fudan University Shanghai Cancer Center, Shanghai 200032, China (2016) CapOX as neoadjuvant chemotherapy for locally advanced operable colon cancer patients: a prospective single-arm phase II trial. Chin J Cancer Res 28(6):589–597
Karoui M, Rullier A, Piessen G, Legoux JL, Barbier E, De Chaisemartin C et al (2020) Perioperative FOLFOX 4 versus FOLFOX 4 plus cetuximab versus immediate surgery for high-risk stage II and III colon cancers: a phase II multicenter randomized controlled trial (PRODIGE 22). Ann Surg 271(4):637–645
Foxtrot CG (2012) Feasibility of preoperative chemotherapy for locally advanced, operable colon cancer: the pilot phase of a randomised controlled trial. Lancet Oncol 13(11):1152–1160
Arredondo J, Baixauli J, Pastor C, Chopitea A, Sola JJ, Gonzalez I et al (2017) Mid-term oncologic outcome of a novel approach for locally advanced colon cancer with neoadjuvant chemotherapy and surgery. Clin Transl Oncol 19(3):379–385
Arredondo J, Gonzalez I, Baixauli J, Martinez P, Rodriguez J, Pastor C et al (2014) Tumor response assessment in locally advanced colon cancer after neoadjuvant chemotherapy. J Gastrointest Oncol 5(2):104–111
Zhou H, Song Y, Jiang J, Niu H, Zhao H, Liang J, Su H, Wang Z, Zhou Z, Huang J, Department of Colorectal Surgery, Department of Medical Oncology, Department of Imageology, Department of Abdominal Surgery, National Cancer Center/Cancer Hospital, Chinese Academy of Medical Science & Peking Union Medical College, Beijing 100021, China (2016) A pilot phase II study of neoadjuvant triplet chemotherapy regimen in patients with locally advanced resectable colon cancer. Chin J Cancer Res 28(6):598–605
Jakobsen A, Andersen F, Fischer A, Jensen LH, Jorgensen JC, Larsen O et al (2015) Neoadjuvant chemotherapy in locally advanced colon cancer. A phase II trial. Acta Oncol 54(10):1747–1753
Colorectal Cancer Chemotherapy Study Group of Japan (2003) The 2nd T. Results of a randomized trial with or without 5-FU-based preoperative chemotherapy followed by postoperative chemotherapy in resected colon and rectal carcinoma. Jpn J Clin Oncol 33(6):288–296
Salameh JP, Bossuyt PM, McGrath TA, Thombs BD, Hyde CJ, Macaskill P et al (2020) Preferred reporting items for systematic review and meta-analysis of diagnostic test accuracy studies (PRISMA-DTA): explanation, elaboration, and checklist. BMJ. 370:m2632
Ryan R, Gibbons D, Hyland J, Treanor D, White A, Mulcahy H et al (2005) Pathological response following long-course neoadjuvant chemoradiotherapy for locally advanced rectal cancer. Histopathology. 47(2):141–146
DCTD N, NIH D (1999) Cancer Therapy Evaluation Program Common Toxicity Criteria. Version. 2:1–32
RStudio (2020) RStudio: Integrated Development for R. RStudio Team, Boston
Hawkins AT, Ford MM, Geiger TM, Hopkins MB, Kachnic LA, Muldoon RL, Glasgow SC (2019) Neoadjuvant radiation for clinical T4 colon cancer: a potential improvement to overall survival. Surgery. 165(2):469–475
Huang C-M, Huang M-Y, Ma C-J, Yeh YS, Tsai H-L, Huang C-W et al (2017) Neoadjuvant FOLFOX chemotherapy combined with radiotherapy followed by radical resection in patients with locally advanced colon cancer. Radiat Oncol 12(1):48
Zhou J, Guo Z, Yu W, Li S, Qiao W (2019) Clinical evaluation of preoperative radiotherapy combined with FOLFOX chemotherapy on patients with locally advanced colon cancer. Am Surg 85(4):313–320
Baretti M, Rimassa L, Personeni N, Giordano L, Tronconi MC, Pressiani T, Bozzarelli S, Santoro A (2018) Effect of comorbidities in stage II/III colorectal cancer patients treated with surgery and neoadjuvant/adjuvant chemotherapy: a single-center, observational study. Clin Colorectal Cancer 17(3):e489–ee98
Nørgaard A, Dam C, Jakobsen A, Pløen J, Lindebjerg J, Rafaelsen SR (2014) Selection of colon cancer patients for neoadjuvant chemotherapy by preoperative CT scan. Scand J Gastroenterol 49(2):202–208
Mullen MG, Shah PM, Michaels AD, Hassinger TE, Turrentine FE, Hedrick TL, Friel CM (2018) Neoadjuvant chemotherapy is associated with lower lymph node counts in colon cancer. Am Surg 84(6):996–1002
Zhou Z, Nimeiri HS, Benson AB III (2013) Preoperative chemotherapy for locally advanced resectable colon cancer-a new treatment paradigm in colon cancer? AnnTransl Med 1(2):11
FOxTROT Collaborative Group (2012) Feasibility of preoperative chemotherapy for locally advanced, operable colon cancer: the pilot phase of a randomised controlled trial. Lancet Oncol 13(11):1152–1160
Jakobsen A, Andersen F, Fischer A, Jensen LH, Jørgensen JC, Larsen O et al (2015) Neoadjuvant chemotherapy in locally advanced colon cancer. A phase II trial. Acta Oncol 54(10):1747–1753
Colorectal Cancer Chemotherapy Study Group of Japan, The 2nd Trial. Results of a randomized trial with or without 5-FU-based preoperative chemotherapy followed by postoperative chemotherapy in resected colon and rectal carcinoma (2003) Jpn J Clin Oncol 33(6):288–296
Arredondo J, Baixauli J, Pastor C, Chopitea A, Sola JJ, González I, A-Cienfuegos J, Martínez P, Rodriguez J, Hernández-Lizoain JL (2017) Mid-term oncologic outcome of a novel approach for locally advanced colon cancer with neoadjuvant chemotherapy and surgery. Clin Transl Oncol 19(3):379–385
Arredondo J, González I, Baixauli J, Martínez P, Rodríguez J, Pastor C, Ribelles MJ, Sola JJ, Hernández-Lizoain JL (2014) Tumor response assessment in locally advanced colon cancer after neoadjuvant chemotherapy. J gastrointest Oncol 5(2):104–111
Allum WH, Stenning SP, Bancewicz J, Clark PI, Langley RE (2009) Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J Clin Oncol 27(30):5062–5067
Maas M, Nelemans PJ, Valentini V, Das P, Rodel C, Kuo LJ et al (2010) Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol 11(9):835–844
Eltahir A, Heys SD, Hutcheon AW, Sarkar TK, Smith I, Walker LG et al (1998) Treatment of large and locally advanced breast cancers using neoadjuvant chemotherapy. Am J Surg 175(2):127–132
Nerad E, Lahaye MJ, Maas M, Nelemans P, Bakers FC, Beets GL et al (2016) Diagnostic accuracy of CT for local staging of colon cancer: a systematic review and meta-analysis. AJR Am J Roentgenol 207(5):984–995
Balyasnikova S, Brown G (2016) Optimal imaging strategies for rectal cancer staging and ongoing management. Curr Treat Options in Oncol 17(6):32
Dam C, Lindebjerg J, Jakobsen A, Jensen LH, Rahr H, Rafaelsen SR (2017) Local staging of sigmoid colon cancer using MRI. Acta Radiol Open 6(7):2058460117720957
Hunter C, Siddiqui M, Georgiou Delisle T, Blake H, Jeyadevan N, Abulafi M, Swift I, Toomey P, Brown G (2017) CT and 3-T MRI accurately identify T3c disease in colon cancer, which strongly predicts disease-free survival. Clin Radiol 72(4):307–315
Liu LH, Lv H, Wang ZC, Rao SX, Zeng MS (2019) Performance comparison between MRI and CT for local staging of sigmoid and descending colon cancer. Eur J Radiol 121:108741
Nerad E, Lambregts DM, Kersten EL, Maas M, Bakers FC, van den Bosch HC et al (2017) MRI for local staging of colon cancer: can MRI become the optimal staging modality for patients with colon cancer? Dis Colon Rectum 60(4):385–392
Maisano R, Caristi N, Mare M, Raffaele M, Iorfida M, Mafodda A, Zavettieri M, Nardi M (2007) Mitomycin C plus capecitabine (mixe) in anthracycline- and taxane-pretreated metastatic breast cancer. A multicenter phase II study. Anticancer Res 27(4C):2871–2875
Massacesi C, La Cesa A, Marcucci F, Pilone A, Rocchi MB, Zepponi L et al (2006) Capecitabine and mitomycin C is an effective combination for anthracycline- and taxane-resistant metastatic breast cancer. Oncology. 70(4):294–300
Dimou A, Syrigos KN, Saif MW (2010) Is there a role for mitomycin C in metastatic colorectal cancer? Expert Opin Investig Drugs 19(6):723–735
Gollins S, Sebag-Montefiore D (2016) Neoadjuvant treatment strategies for locally advanced rectal cancer. Clin Oncol (R Coll Radiol) 28(2):146–151
Travaini LL, Zampino MG, Colandrea M, Ferrari ME, Gilardi L, Leonardi MC et al (2016) PET/CT with fluorodeoxyglucose during neoadjuvant chemoradiotherapy in locally advanced rectal cancer. E Cancer Med Sci 10:629
Morton D. (2019) FOxTROT: An international randomised controlled trial in 1053 patients evaluating neoadjuvant chemotherapy (NAC) for colon cancer. On behalf of the FOxTROT Collaborative Group. In: Committee ECS, editor. 44th ESMO Congress; Barcelona: Annals of Oncology, p. 198-252.
Lehnert T, Methner M, Pollok A, Schaible A, Hinz U, Herfarth C (2002) Multivisceral resection for locally advanced primary colon and rectal cancer: an analysis of prognostic factors in 201 patients. Ann Surg 235(2):217–225
Croner RS, Merkel S, Papadopoulos T, Schellerer V, Hohenberger W, Goehl J (2009) Multivisceral resection for colon carcinoma. Dis Colon Rectum 52(8):1381–1386
Benson AB, Venook AP, Al-Hawary MM, Cederquist L, Chen YJ, Ciombor KK et al (2018) NCCN Guidelines Insights: Colon Cancer, Version 2.2018. J Natl Compr Cancer Netw 16(4):359–369
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gosavi, R., Chia, C., Michael, M. et al. Neoadjuvant chemotherapy in locally advanced colon cancer: a systematic review and meta-analysis. Int J Colorectal Dis 36, 2063–2070 (2021). https://doi.org/10.1007/s00384-021-03945-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-021-03945-3