Abstract
Purpose
Despite the increasing use of telemanipulators in colorectal surgery, an additional benefit in terms of improved perioperative results is not proven. The aim of the study was to compare clinical, oncological, and functional results of Da Vinci (Xi)–assisted versus conventional laparoscopic (low) anterior resection for rectal cancer.
Methods
Monocenter, prospective, controlled cohort study with a 12-month follow-up of bladder and sexual function using the validated questionnaires International Prostate Symptom Score, International Index of Erectile Function, and Female Sexual Function Index.
Results
Fifty-one patients were included (18, Da Vinci (Xi) assisted; 33, conventional laparoscopy). Conversion to an open approach was more common in the Da Vinci cohort (p = 0.012). In addition, surgery and resumption of a normal diet took longer in the robotic group (p = 0.005; p = 0.042). Surgical morbidity and oncological quality did not differ. There was no difference in most functional domains, except for worsened ability to orgasm (p = 0.047) and sexual satisfaction (p = 0.034) in women after conventional laparoscopy. Moreover, we found a higher rate of improved bladder function in the conventional laparoscopy group (p = 0.023) and less painful sexual intercourse among women in the robot-assisted group (p = 0.049).
Conclusion
In contrast to the ROLARR trial, a higher conversion rate was found in the robotic cohort, which may in part be explained by a learning curve effect. Nevertheless, the Da Vinci–assisted approach showed favorable results regarding sexual function.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The mainstay of treatment of rectal cancer is oncological resection of the tumor as either a (low) anterior resection, an intersphincteric resection, or an abdominoperineal resection. Because of the multiple advantages of laparoscopy over open surgery regarding the patient’s postoperative recovery and the proven oncological equivalence [1, 2], minimally invasive rectal resection should be regarded as the gold standard. As conventional laparoscopic rectal cancer surgery is technically demanding, especially for low rectal tumors and in obese, male patients with a narrow pelvis and extensive visceral fat, the robotic-assisted laparoscopic approach may considerably increase maneuverability and visibility during the pelvic dissection and therefore facilitate the identification and preservation of the hypogastric plexus and the sacral splanchnic nerve, which could have a positive impact on postoperative functional results.
Despite the growing popularity of telemanipulators in colorectal surgery, an additional benefit compared with conventional laparoscopy in terms of improved perioperative results, especially regarding oncological and functional aspects, is not proven. The aim of the current study was to compare the results of Da Vinci (Xi)–assisted versus conventional laparoscopic (low) anterior resection for rectal cancer. The main focus was on the comparison of sexual and bladder functions, since clinical and oncological outcomes have already been identified as equivalent according to current evidence [3].
Methods
This is a monocenter, prospective, controlled cohort study from the University Hospital Mannheim in Mannheim, Germany, comparing robot-assisted versus conventional laparoscopic surgery in patients with high- to low-lying (≤ 15 cm from the anal margin) histologically proven rectal adenocarcinoma. The same team of surgeons (Peter Kienle (PK), Georgi Vassilev (GV), Julia Hardt (JH)) performed all operations. The trial received institutional ethical approval by the ethics committee of the Medical Faculty Mannheim, Heidelberg University, Mannheim, Germany (2015-636N-MA). The study was performed according to the Declaration of Helsinki. All participants provided written informed consent. Patients > 18 years scheduled to undergo elective minimally invasive curative treatment for rectal cancer in the form of (low) anterior resection were eligible. All patients were placed on the same perioperative enhanced recovery pathway. The bowel preparation comprised 2–3 L of Oralav® (B. Braun Melsungen AG, Melsungen, Germany) given the day before surgery. At the induction of anesthesia, all patients received prophylactic intravenous antibiotics (metronidazole, cefazolin).
Patient self-reported bladder and sexual functions were measured at baseline (on the day before surgery) and 1 year after surgery using the standardized and validated questionnaires International Prostate Symptom Score (IPSS) [4], International Index of Erectile Function (IIEF15) [5], Female Sexual Function Index (FSFI) [6], and Sexual Activity Questionnaire (SAQ) [7]. The prespecified clinical and histopathological endpoints were 30-day surgical morbidity (classified according to the Clavien–Dindo classification [8]), the rate of conversion (defined as any kind of laparotomy to facilitate mesorectal excision; the small incision (~ 4 cm) for specimen extraction routinely performed at the left lower quadrant trocar site was not considered a conversion), rate of reoperation, anastomotic leakage, postoperative ileus, intraabdominal abscess, surgical site infection (SSI), readmission within 30 days after surgery, local recurrence, distant metastasis, TME (total mesorectal excision) quality (MERCURY grades 1–3), pathologic circumferential resection margin (pCRM, positive if the distance between the tumor and the mesorectal fascia was ≤ 1 mm), resection status, number of resected lymph nodes, operative time, postoperative length of stay, blood loss, time to first bowel movement, time to resumption of regular diet, and postoperative pain (visual analogue scale (VAS), 1–10).
Robot-assisted procedure
A single-docking robotic approach was performed using the Da Vinci Xi Surgical System (Intuitive Surgical, Sunnyvale, CA, USA) as taught by Amjad Parvaiz, the proctor who supervised the first robotic colorectal cases at our institution. All operations were performed in a hybrid technique which is composed of an exclusively robotic part (medial-to-lateral approach with ligation of the main vessels using clips, left colon and splenic flexure mobilization, and PME (partial mesorectal excision) or TME) followed by a conventional laparoscopic part after (resection, anastomosis, and loop ileostomy placement if a low anastomosis had been fashioned). Trocars were positioned as follows: the four 8-mm robotic trocars are placed 7–8 cm apart from each other in a straight line on the right side of the abdomen oblique to the midline. The distance between the most caudally placed trocar and the right anterior superior iliac spine had to be at least two fingers. Lateral to and between the most caudally located robotic trocars, a 12-mm hybrid trocar was placed as an assistant trocar for suction, retraction, vessel clip application, and insertion of the Endo GIA.
Conventional laparoscopic procedure
Conventional laparoscopic surgery was performed in the same manner as robot-assisted surgery using laparoscopic instruments. Trocars were positioned differently: a 10-mm trocar for the laparoscope was placed just below the umbilicus; one 12-mm trocar, through which the Endo GIA was inserted for stapling, and one 5-mm trocar in the right lower quadrant; and a 5-mm trocar in the left lower quadrant (this trocar incision was extended to a 4-cm incision to extract the specimen).
Statistical analysis
The mean and standard deviation were calculated for quantitative variables. The median, together with the range or interquartile range (IQR), is presented for skewed or ordinal-scaled parameters. Qualitative variables were quoted as absolute and relative frequencies. Student’s t test was used for comparing approximately normally distributed quantitative variables. The Mann–Whitney U test was used for variables that were not normally distributed. For qualitative variables, an Χ2 test or Fisher’s exact test was used, as appropriate. All statistical tests for the comparison of two groups were two-tailed. In general, a test result was considered statistically significant if p ≤ 0.05. All analyses were performed using the SAS statistical analysis software (release 9.4; Cary, NC, USA).
In order to investigate whether there was any improvement or worsening regarding sexual and bladder function, the difference between preoperative and postoperative scores was calculated for each domain if applicable. The differences between and within the two groups (robot-assisted and conventional laparoscopic surgery) were compared. For normally distributed differences, student’s t test for paired samples was used to test within the groups; for non-normalized data, the Wilcoxon test for paired samples was applied.
Results
Between February 2016 and December 2017, 51 patients were included. Eighteen were operated using the Da Vinci (Xi)–assisted approach; 33 underwent conventional laparoscopy. The baseline characteristics of the cohorts were comparable (Table 1).
Perioperative clinical and histopathological outcomes are presented in Table 2. Conversion to an open approach (either a Pfannenstiel incision or a median laparotomy) was significantly more common in the Da Vinci cohort (4/18 vs. 0/33, p = 0.012). Reasons for conversion to a Pfannenstiel incision were bulky tumors (n = 2) and insufficient exposure due to an elongated sigmoid colon (n = 1). In one case, the operating surgeon converted to a median laparotomy due to a T4 situation. In addition, surgery and resumption of a normal diet took longer in the robotic group (mean operative time ± SD, 394 min ± 78.5 vs. 324 ± 80.9, p = 0.005; median duration until resumption of a normal diet, 4.5 (range, 1–30) vs. 2 days (range, 1–6), p = 0.042). Postoperative length of stay, intraoperative blood loss, time to first bowel movement, and postoperative pain levels were comparable in both cohorts (Table 2).
Surgical morbidity was comparable in the two cohorts. Six of 18 (33.3%) in the Da Vinci group vs. 12 of 21 (36.4%) in the control group suffered from at least one surgical complication which were all classified as grade II or III according to the Clavien–Dindo classification. There were no > grade III complications. Four patients had to be re-operated: one patient each in the Da Vinci and the control cohort due to kinking and stenosis of the proximal loop of the diverting ileostomy with consecutive ileus in combination with anastomotic leakage, and two further patients in the control group due to the following reasons: (1) irreversible ischemia of a 10-cm segment of the descending colon with concomitant anastomotic leakage and development of a recto-vaginal fistula and (2) iatrogenic ureteral lesion during rectal resection requiring reimplantation of the ureter in psoas hitch technique. Anastomotic leakage was diagnosed in one patient in the Da Vinci cohort (1/18 (5.6%)) vs. seven patients in the control cohort (7/33 (21.2%); p = 0.233). The rates of postoperative ileus, intraabdominal abscess, surgical site infection (SSI), and readmission were similar between groups (Table 2).
Oncological quality of the specimen did also not differ between the two surgical approaches (Table 2): none of the patients was diagnosed with local recurrence during the follow-up; 4 of 18 (22.2%) in the robot-assisted and 3 of 33 (9.1%) in the conventional laparoscopic group developed distant metastasis. Except for one case in the control group, TME quality was classified as MERCURY grade 1 (good) concordantly by the operating surgeon and the pathologist. pCRM was negative (distance between the tumor and the mesorectal fascia > 1 mm) and resection status was R0 in all cases but one in the Da Vinci cohort with advanced tumor infiltrating the presacral mesorectal fascia. The number of resected lymph nodes was 14.3 ± 2.1 (mean ± SD) in the robot-assisted cohort vs. 16.0 ± 3.8 in the control group. Except for one specimen, which included only ten lymph nodes, all resections fulfilled the guideline recommendation to harvest at least twelve lymph nodes.
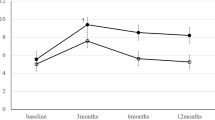
Table 3 presents the changes (delta 1 year postop–preop) in functional outcomes from baseline until 1 year after surgery. Eleven of 18 in the robotic cohort vs. 21 of 33 in the conventional laparoscopic cohort answered the questionnaires for the 1-year follow-up, whereas all except for one patient had filled out the IPSS, FSFI, and IIEF15 baseline questionnaire. Regarding the impact of surgery on bladder and sexual function, there was no difference between the two groups in most domains, except for impaired ability to orgasm (FSFI orgasm, 0.6 ± 2.1 (− 3.2–3.6) vs. − 2.6 ± 2.3 (− 5.2–0.4), p = 0.047) as well as worsened sexual satisfaction (FSFI satisfaction, 0.7 ± 1.8 (− 0.4–4.8) vs. − 1.6 ± 2.1 (− 4.0–(− 0.4)), p = 0.034) in female patients in the conventional laparoscopic group.
Comparing the functional changes (improvement vs. deterioration/no change) within groups (Table 4), we found a higher rate of improved bladder function in the conventional laparoscopy group (14/19 vs. 3/11, p = 0.023) and less painful sexual intercourse among women in the robot-assisted group (3/8 vs. 0/12, p = 0.049).
Unfortunately, there were only two complete datasets (pre- and postoperative scores) of the SAQ, which inhibits any valuable statistical analysis and conclusion.
Discussion
In the present monocenter, prospective, controlled cohort study of 51 consecutive cases, we compared clinical, oncological, and functional outcomes of Da Vinci (Xi)–assisted versus conventional laparoscopic resection for rectal cancer.
In contrast to the highest-quality evidence from the ROLARR trial [9] and a recently published phase II randomized trial from Korea [10], a significantly higher conversion rate was found in the robotic cohort (22.2% vs. 0%), which may at least in part be explained by a certain learning curve effect regarding the robot-assisted approach. Moreover, the operating colorectal specialist (PK) possessed great expertise in conventional laparoscopy and during the last decade the vast majority of cases (75%) were operated laparoscopically at our institution [11], which may explain the zero conversions in the conventional laparoscopic cohort. According to a recent systematic review, the learning curve for robotic rectal cancer surgery is relatively short if compared with the learning curve for the conventional laparoscopic approach [12]. Especially for experienced laparoscopic colorectal surgeons, the learning process may be short to perform as technically adept as in conventional laparoscopy [13]. However, the mean number of cases for a surgeon to be classed as an expert in robotic surgery was calculated to be 39 [12], which was not achieved at our institution within the time frame of the present study.
Surgical morbidity, graded according to the Clavien–Dindo classification, as well as the oncological quality of the resection, measured by surrogate parameters, such as TME quality, pCRM, and the number of resected lymph nodes, did not differ between the groups, which is consistent with the findings of the ROLARR trial.
In line with the current level 1 evidence [10], surgery took longer in the Da Vinci–assisted group. Moreover, transition to a normal diet was delayed in the robotic group, which could be the result of the prolonged operative time and the higher conversion rate in this group, as the risk of postoperative ileus is increased after long operations and open surgery.
Nevertheless, the Da Vinci–assisted approach showed—despite the assumed ongoing learning curve—favorable results regarding sexual function 12 months after rectal cancer resection, i.e., less painful sexual intercourse among women. Furthermore, we found lower scores (indicating worse function) 1 year after surgery regarding the ability to orgasm and sexual satisfaction in women who had undergone the conventional laparoscopic approach. This is not in line with the findings of the ROLARR trial, which could not detect significant differences in functional outcomes [9]. The other recently published phase II randomized trial comparing the results of robot-assisted versus laparoscopic rectal cancer surgery by Kim et al. [10] did investigate neither sexual nor urinary function. However, a prospective comparative study with 69 patients (39 underwent laparoscopic TME, 30 robotic-assisted TME) could demonstrate similar results: erectile function recovered earlier after surgery in patients in the robotic cohort. Moreover, sexual desire increased significantly faster after robotic-assisted surgery [14]. Unfortunately, female sexual function was not investigated in this study. Favorable results regarding urogenital function have been demonstrated by several previous studies [14,15,16]. A comparative study including 158 patients (89 laparoscopic, 69 robotic-assisted) conducted by Panteleimonitis et al. [17] provided further evidence on the potential benefits of robot-assisted rectal cancer resection in regard to postoperative sexual function: male patients in the robotic group deteriorated less across all components of the IIEF5-questionnaire. In females, there was no difference in postoperative sexual function between the two groups. However, only 13 female patients were sexually active and answered the questionnaire, which makes a statistical comparison problematic. In general, the evidence on female sexual function and how it is influenced by rectal cancer surgery is very limited and requires further well-designed, large-scale prospective studies to shed light on this topic. According to the present state of our knowledge, the current study is the first to detect an improved sexual function after robotic-assisted rectal cancer resection in female patients.
Except for a higher rate of improved bladder function in the conventional laparoscopy group, there were no differences between the groups regarding voiding function, which is consistent with the ROLARR trial, but in contradiction to the comparative studies by Kim et al. [14] and Panteleimonitis et al. [17] who found superior outcomes in the robotic group regarding the IPSS-Score and urinary flow.
Our study had some limitations. First, the non-randomized nature and the small sample size may limit the validity of our data. Second, the colorectal surgeons at our institution had considerable experience in conventional laparoscopic surgery, but only limited robotic experience. Thus, learning curve effects cannot be excluded. Third, statistical analysis of the SAQ was not possible because of the small number of respondents. Fourth, only two-thirds of the study cohort answered the questionnaires for the 1-year follow-up of functional outcomes which may further limit the significance of our results. Fifth, we did neither analyze costs nor assessed the cost-effectiveness of the robot-assisted procedure based on clinical, oncological, or functional outcomes. Given the high costs of robotic surgery mainly due to initial investments, maintenance costs, and longer operating times, future studies should properly evaluate the cost-effectiveness of the robot-assisted technique in comparison with conventional laparoscopy.
The potential of robot-assisted rectal cancer surgery to improve functional outcomes via facilitated identification and preservation of the autonomous pelvic nerves, especially in technically challenging cases, should be further investigated in future trials. Moreover, long-term oncological and functional results of the ROLARR trial and other randomized controlled trials must be awaited before final conclusions can be made regarding the value and significance of robotic-assisted rectal cancer surgery.
References
Green BL, Marshall HC, Collinson F, Quirke P, Guillou P, Jayne DG, Brown JM (2013) Long-term follow-up of the Medical Research Council CLASICC trial of conventional versus laparoscopically assisted resection in colorectal cancer. Br J Surg 100(1):75–82. https://doi.org/10.1002/bjs.8945
Bonjer HJ, Deijen CL, Haglind E, Group CIS (2015) A randomized trial of laparoscopic versus open surgery for rectal cancer. N Engl J Med 373(2):194. https://doi.org/10.1056/NEJMc1505367
Prete FP, Pezzolla A, Prete F, Testini M, Marzaioli R, Patriti A, Jimenez-Rodriguez RM, Gurrado A, Strippoli GFM (2018) Robotic versus laparoscopic minimally invasive surgery for rectal cancer: a systematic review and meta-analysis of randomized controlled trials. Ann Surg 267(6):1034–1046. https://doi.org/10.1097/SLA.0000000000002523
Barry MJ, Fowler FJ Jr, O’Leary MP, Bruskewitz RC, Holtgrewe HL, Mebust WK, Cockett AT (1992) The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol 148(5):1549–1557 discussion 1564
Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A (1997) The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology 49(6):822–830
Rosen R, Brown C, Heiman J, Leiblum S, Meston C, Shabsigh R, Ferguson D, D’Agostino R Jr (2000) The Female Sexual Function Index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther 26(2):191–208. https://doi.org/10.1080/009262300278597
Thirlaway K, Fallowfield L, Cuzick J (1996) The Sexual Activity Questionnaire: a measure of women’s sexual functioning. Qual Life Res 5(1):81–90
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240(2):205–213
Jayne D, Pigazzi A, Marshall H, Croft J, Corrigan N, Copeland J, Quirke P, West N, Rautio T, Thomassen N, Tilney H, Gudgeon M, Bianchi PP, Edlin R, Hulme C, Brown J (2017) Effect of robotic-assisted vs conventional laparoscopic surgery on risk of conversion to open laparotomy among patients undergoing resection for rectal cancer: the ROLARR randomized clinical trial. JAMA 318(16):1569–1580. https://doi.org/10.1001/jama.2017.7219
Kim MJ, Park SC, Park JW, Chang HJ, Kim DY, Nam BH, Sohn DK, Oh JH (2018) Robot-assisted versus laparoscopic surgery for rectal cancer: a phase II open label prospective randomized controlled trial. Ann Surg 267(2):243–251. https://doi.org/10.1097/SLA.0000000000002321
Hardt J, Pilz L, Magdeburg J, Kienle P, Post S, Magdeburg R (2017) Preoperative hypoalbuminemia is an independent risk factor for increased high-grade morbidity after elective rectal cancer resection. Int J Color Dis 32(10):1439–1446. https://doi.org/10.1007/s00384-017-2884-7
Jimenez-Rodriguez RM, Rubio-Dorado-Manzanares M, Diaz-Pavon JM, Reyes-Diaz ML, Vazquez-Monchul JM, Garcia-Cabrera AM, Padillo J, De la Portilla F (2016) Learning curve in robotic rectal cancer surgery: current state of affairs. Int J Color Dis 31(12):1807–1815. https://doi.org/10.1007/s00384-016-2660-0
Odermatt M, Ahmed J, Panteleimonitis S, Khan J, Parvaiz A (2017) Prior experience in laparoscopic rectal surgery can minimise the learning curve for robotic rectal resections: a cumulative sum analysis. Surg Endosc 31(10):4067–4076. https://doi.org/10.1007/s00464-017-5453-9
Kim JY, Kim NK, Lee KY, Hur H, Min BS, Kim JH (2012) A comparative study of voiding and sexual function after total mesorectal excision with autonomic nerve preservation for rectal cancer: laparoscopic versus robotic surgery. Ann Surg Oncol 19(8):2485–2493. https://doi.org/10.1245/s10434-012-2262-1
D’Annibale A, Pernazza G, Monsellato I, Pende V, Lucandri G, Mazzocchi P, Alfano G (2013) Total mesorectal excision: a comparison of oncological and functional outcomes between robotic and laparoscopic surgery for rectal cancer. Surg Endosc 27(6):1887–1895. https://doi.org/10.1007/s00464-012-2731-4
Morelli L, Ceccarelli C, Di Franco G, Guadagni S, Palmeri M, Caprili G, D’Isidoro C, Marciano E, Pollina L, Campani D, Massimetti G, Di Candio G, Mosca F (2016) Sexual and urinary functions after robot-assisted versus pure laparoscopic total mesorectal excision for rectal cancer. Int J Color Dis 31(4):913–915. https://doi.org/10.1007/s00384-015-2301-z
Panteleimonitis S, Ahmed J, Ramachandra M, Farooq M, Harper M, Parvaiz A (2017) Urogenital function in robotic vs laparoscopic rectal cancer surgery: a comparative study. Int J Color Dis 32(2):241–248. https://doi.org/10.1007/s00384-016-2682-7
Authorship and contributions
J. Hardt, C. Galata, G. Vassilev, P. Kienle, C. Reißfelder: conceived of the study
J. Hardt, F. Haas, S. Büttner: acquired and analyzed the data
All authors interpreted the data, revised the article for important intellectual content, and gave final approval of the version to be published.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The trial received institutional ethical approval by the ethics committee of the Medical Faculty Mannheim, Heidelberg University, Mannheim, Germany (2015-636 N-MA). The study was performed according to the Declaration of Helsinki. All participants provided written informed consent.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Galata, C., Vassilev, G., Haas, F. et al. Clinical, oncological, and functional outcomes of Da Vinci (Xi)–assisted versus conventional laparoscopic resection for rectal cancer: a prospective, controlled cohort study of 51 consecutive cases. Int J Colorectal Dis 34, 1907–1914 (2019). https://doi.org/10.1007/s00384-019-03397-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-019-03397-w