Abstract
Purpose
Current clinical guidelines recommended the routine use of adjuvant chemotherapy for locally advanced rectal cancer (LARC) patients. However, the effects of adjuvant chemotherapy in patients with pathological complete response (pCR) after neoadjuvant chemoradiotherapy and radical surgery showed discrepancies in different investigations.
Methods
A systematic review and meta-analysis were conducted using PubMed, Embase and Web of Science databases. All original comparative studies published in English that were related to adjuvant versus non-adjuvant chemotherapy for LARC patients with pCR were included.
Results
A total of 6 studies based on 18 centres or databases involving 2948 rectal cancer patients with pCR (adjuvant group = 1324, non-adjuvant group = 1624) were included in our overall analysis. Based on our meta-analysis, LARC patients with pCR who received adjuvant chemotherapy showed a significantly improved overall survival (OS) when compared to patients with observation (HR = 0.65, 95% CI = 0.46–0.90, P = 0.01). In addition, investigations focused on this issue based on the National Cancer Database (NCDB) were systematically reviewed in our current study. Evidence from all three analyses demonstrated that LARC patients with clinical nodal positive disease that achieved pCR might benefit the most from additional adjuvant chemotherapy.
Conclusion
Our meta-analysis indicated that adjuvant chemotherapy is associated with improved OS in LARC patients with pCR after neoadjuvant chemoradiotherapy and radical surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The standard treatment for locally advanced rectal cancer (LARC) has been established, including neoadjuvant chemoradiotherapy, radical surgery and adjuvant chemotherapy based on the latest National Comprehensive Cancer Network (NCCN) guidelines [1]. Several phase III randomised controlled trials (RCTs) were also conducted to explore the effects of adjuvant chemotherapy for LARC patients after neoadjuvant chemoradiotherapy and radical surgery [2,3,4,5]. Based on current evidence, a systematic review and meta-analysis demonstrated that rectal cancer patients who received adjuvant chemotherapy had improved survival outcomes in comparison with those without postoperative chemotherapy [6]. However, with the development of precision therapy, whether all the LARC patients should receive adjuvant chemotherapy has remained controversial since limited data has been provided based on populations of different subgroups. Issues of whether adjuvant chemotherapy should be routinely delivered have been proposed, especially for potential subgroups with a good prognosis [7,8,9].
LARC patients who achieved a pathological complete response (pCR) represented the subgroup to benefit the most from neoadjuvant chemoradiotherapy. Evidence based on a meta-analysis indicated that LARC patients with pCR following neoadjuvant chemoradiotherapy are associated with excellent long-term survival, with low rates of local recurrence and distant failure [10]. However, whether LARC patients with pCR might still further benefit from adjuvant chemotherapy remains unknown. Thus, in subgroups with a good prognosis, such as those patients achieving pCR, it is defensible to argue against the routine inclusion of adjuvant chemotherapy, given the association with toxicity, expense and impact on the quality of life. A pooled analysis of 3313 patients by Maas et al. [11] indicated that patients with pCR after neoadjuvant chemoradiotherapy might not benefit from adjuvant chemotherapy, whereas patients with residual tumour had superior outcomes when adjuvant chemotherapy was administered. Notably, two recent published analyses based on the National Cancer Database (NCDB) demonstrated that adjuvant chemotherapy was associated with improved overall survival (OS) in patients with pCR after neoadjuvant chemoradiotherapy for resected LARC [12, 13].
Based on the aforementioned findings, the issue of whether adjuvant chemotherapy is necessary for LARC patients with pCR remained controversial. In addition, to the best of our knowledge, the evidence for this issue was mainly based on different single centres. Therefore, we aimed to provide a comprehensive evaluation and updated evidence for this issue by using meta-analysis.
Methods
Search strategy
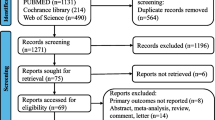
Our meta-analysis was conducted in accordance with Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines (http://www.prisma-statement.org/). The checklist in accordance with PRISMA was shown in Supplementary Table 1. A comprehensive search of published studies was performed using PubMed, Embase and the Cochrane Database (up to August 2018). Language was restricted to English, and the following MeSH/main keywords were employed: “rectal cancer”, “pathological complete response”, “PCR”, “yPCR”, “ypT0N0”, “neoadjuvant”, “adjuvant chemotherapy” and “postoperative chemotherapy”. Based on our search strategy (Supplementary Table 2), we identified relevant studies from Pubmed and other databases and removed the duplicates for the further screening (Identification). Based on the titles and abstracts, we identified studies which in accordance with our issue for full-text reviewing (Screening). Based on the inclusion and exclusion criteria (Eligibility), we selected the studies for our meta-analysis (Included). In addition, for multiple studies that were published using the same patient population based on the same outcomes, we only included the most informative study. If multiple studies reported different outcomes based on the same patient population, the results were combined for a more comprehensive analysis. The lists of references in the relevant studies were also screened for additional studies.
Inclusion and exclusion criteria
According to the PICOS criteria (population, intervention, comparison, outcomes and study design), studies were selected in our present meta-analysis according to the following eligibility criteria: (1) population: patients with primary LARC (T3–4 N0 or TanyN+; TNM stage of II and III), (2) intervention: LARC patients with pCR (ypT0N0) after neoadjuvant chemoradiotherapy and total mesorectal excision (mainly anterior resection, abdminonal peritoneal resection, Hartmann or intersphincteric resection), (3) comparison: patients received postoperative adjuvant chemotherapy (adjuvant group: adjuvant chemotherapy was mainly based on infusion of 5-fluorouracil/oral capecitabine with or without oxaliplatin generally within 6–8 weeks after neoadjuvant therapy) versus observation (non-adjuvant group), (4) outcomes: OS compared between two groups and OS was defined as time to death from any cause or time to the most current follow up and (5) study design: comparative studies (observational studies or pooled analysis based on comparative studies).
The exclusion criteria were as follows: (1) population: patients without pCR, (2) intervention: patients who received preoperative radiotherapy but not chemoradiotherapy, (3) comparison: studies compared different adjuvant chemotherapy regimens but without observational group (non-adjuvant group), (4) outcomes: studies with insufficiently detailed data (reference abstracts) or that lacked the outcomes of interest and (5) study design: single-arm study without a control group or reviews.
Data extraction and quality assessment of included studies
Two reviewers (Bin Ma and Yupeng Ren) reviewed and assessed each of the included studies. Data extraction was performed independently and the following information was collected: first author, year of publication, duration, study type, patient source, number of patients with pCR enrolled, rectal cancer stage, follow-up, regimens of adjuvant chemotherapy and survival data. In addition, the Newcastle-Ottawa Scale (NOS) criterion was used to evaluate the quality of the studies included [14]. All disagreements were resolved by discussion between the two reviewers (Bin Ma and Yupeng Ren).
Statistical analysis
In our meta-analysis, the most appropriate statistic to use for evaluating primary endpoints (time-to-event outcomes) was the hazard ratio (HR). If studies did not provide the HR directly, we obtained an estimated HR by methods designed by Tierney [15], mainly using Kaplan-Meier curves or P values from log-rank tests and the number of observed events in each group. All analyses were performed using Stata software, version 12.0 (2011; Stata Corp., College Station, TX, USA). All the analyses in this study used a random-effect model because it provided more conservative estimates and was tailored to multicentre studies in which heterogeneity was typically present [16]. All statistical values are reported with 95% confidence intervals (CI) and a two-tailed P value of less than 0.05 was defined as statistically significant. Finally, publication bias was assessed using Begg’s and Egger’s tests [17, 18].
Results
Selected studies
Based on our search strategy, a total of 1113 published studies were identified. After the removal of duplicates, title and abstract screening and further evaluation, 10 comparative studies were reviewed by full-text reading [8, 11,12,13, 19,20,21,22,23,24]. Amongst these 10 studies, Shahab et al. [21], Polanco et al. [12] and Dossa et al. [13] reported their outcomes from the same patient population of the National Cancer Database (NCDB) and Gamaleldin et al. [24] and Kiran et al. [8] also presented their outcomes from the same patient population of the Cleveland Clinic Foundation. Hence, we only included the most informative and qualitative study in our overall analysis [12, 24]. In addition, Zhou et al. [19] did not provide the detailed primary outcome of OS between adjuvant and non-adjuvant chemotherapy groups of pCR patients and was excluded from our overall analysis. Finally, six comparative studies [11, 12, 20, 22,23,24] were included in our study and the reasons for study exclusion are illustrated in Fig. 1.
Amongst our included studies, five [12, 20, 22,23,24] were retrospective comparative studies, and the study by Maas et al. [11] was a pooled analysis (collect individual data (not published) from original authors and reanalyse based on another relevant issue) from 17 papers, and finally, 13 studies had gave consents to share their individual data (3 studies with insufficient information and one author confused). Hence, 6 studies based on 18 centres or databases involving 2948 rectal cancer patients with pCR (adjuvant group = 1324, non-adjuvant group = 1624) were included in our meta-analysis. All the patients enrolled were LARC (T3–4 N0 or TanyN+) after neoadjuvant chemoradiotherapy and achieved pCR. The detailed information is shown in Table 1 (five respective studies and one pooled analysis). In addition, the detailed information of 13 studies from pooled analysis of Maas et al. [11] was summarised in Supplementary Table 3.
Adjuvant versus non-adjuvant for pCR patients
Of the original data from included studies, all the HR was derived based on OS. Five studies [11, 20, 22,23,24] indicated that adjuvant chemotherapy could not improve the OS for rectal cancer patients with pCR in comparison with patients without adjuvant chemotherapy. However, four studies presented the advantage of adjuvant chemotherapy for pCR patients, though with no statistical significance [11, 22,23,24]. Based on our overall analysis of included studies, rectal cancer patients with pCR receiving adjuvant chemotherapy showed significantly improved OS when compared with the non-adjuvant chemotherapy group (HR = 0.65, 95% CI = 0.46–0.90, P = 0.01) (Fig. 2). In addition, we did not observe significant heterogeneity amongst the studies (I2 = 2.90%, P = 0.40), and there was no publication bias based on the results of Begg’s (P = 1.00) and Egger’s (P = 0.69) tests.
Which pCR patients would benefit the most from adjuvant chemotherapy?
The NCDB is a clinical oncology-specific database established in 1989 as a joint programme of the American College of Surgeons Commission on Cancer and the American Cancer Society. The NCDB estimates that approximately 70% of newly diagnosed malignant neoplasms in the USA are captured by this database annually. Shahab et al. [21], Polanco et al. [12] and Dossa et al. [13] reported their results of this issue based on the NCDB. All three studies demonstrated that patients with pCR who received adjuvant chemotherapy had improved OS when compared to patients without adjuvant chemotherapy. For the subgroup analysis of these three studies, Shahab et al. [21] indicated that adjuvant chemotherapy were more likely to be given for clinical ≥ T3 disease and clinical N+ rectal cancer patients with pCR. In addition, Polanco et al. [12] showed that pCR patients with clinical stage T3/T4 and node-positive disease benefited the most from adjuvant chemotherapy (HR = 0.47, 95% CI = 0.25–0.91). Meanwhile, Dossa et al. [13] demonstrated that only patients with node-positive disease exhibited improved OS with the administration of adjuvant chemotherapy (HR = 0.24, 95% CI = 0.10–0.58), but not the patients without adjuvant chemotherapy. We have summarised the subgroup analysis results from these three studies in Fig. 3.
Discussion
Over the past decade, the rationale for the routine use of adjuvant chemotherapy has been mainly based on an extrapolation of the survival benefits amongst colon cancer patients [25,26,27]. In fact, the efficacy of adjuvant chemotherapy in rectal cancer patients is less clear and challenges remain [28]. Numerous randomised trials have been conducted to investigate the benefit of adjuvant chemotherapy in rectal cancer patients after neoadjuvant therapy and resection. Regarding to overall analyses, a pooled analyses based on individual data from five RCTs [4] had demonstrated that significant benefits of adjuvant chemotherapy in local control (P < 0.01), distant control (P = 0.03) and OS (P = 0.02). However, the results from several randomised trials (EORTC 22921 [3], I-CNR-RT [29] and the PROCTOR arm of the Dutch PROCTOR-SCRIPT [30] trials) failed to demonstrate a benefit to its use in overall analyses. In terms of subgroup analyses, an exploratory subgroup analyses in terms of tumour response of EORTC 22921 trial with a follow-up of 5 years indicated that only ypT0–2 patients but not ypT3–4 patients benefit from adjuvant chemotherapy irrespective OS or disease-free survival [7]. Meanwhile, a previous meta-analysis based on five comparative studies indicated that patients with ypT0–2N0M0 showed improved 5-year OS in adjuvant group significantly [6]. However, I-CNR-RT trial also conducted subgroup analyses based on ypT0–2, ypT3–4 and ypN+ patients, but there was no significant difference between two groups in the subgroup population. Based on aforementioned, poor adherence to adjuvant chemotherapy, a limited number of patients and a mixed patient population might be some of the reasons preventing a further exploration into the advantages of adjuvant therapy. Hence, which subgroup of resected rectal cancer would benefit from adjuvant therapy after neoadjuvant chemoradiotherapy has become a specific issue. It is clear that patients who achieve tumour downstaging (especially pCR) have better oncologic outcomes [10] and, in practice, patients who achieve pCR are less likely to undergo adjuvant chemotherapy than those with residual disease after neoadjuvant chemoradiotherapy [31]. Whether patients with pCR would further benefit from adjuvant therapy remained controversial.
Investigations focused on which subgroups (pCR or non-pCR) of patients could benefit from adjuvant chemotherapy after neoadjuvant chemoradiotherapy showed discrepancies in their results. A previous propensity score-adjusted analysis evaluated adherence with adjuvant chemotherapy amongst patients with pCR and subgroup analysis indicated that adjuvant chemotherapy appears to be independently associated with improved OS regardless of ypStage [32]. An early subgroup analysis of the EORTC trial suggested a beneficial effect of adjuvant chemotherapy for patients with ypT0–2 tumours but not in ypT3–4 tumours [7]. Notably, two recent original investigations based on the NCDB further indicated that adjuvant chemotherapy was associated with improved OS in LARC patients with pCR after neoadjuvant chemoradiotherapy and radical resection [12, 13]. However, Tay et al. demonstrated that a significant OS benefit favouring adjuvant chemotherapy was seen in the non-pCR subset of patients, but not pCR patients [20]. Meanwhile, Maas et al. also indicated that patients with pCR may not benefit from adjuvant chemotherapy, whereas patients with residual tumour had superior outcomes when adjuvant chemotherapy was administered [11]. Hence, it was time to specifically evaluate the effect of adjuvant chemotherapy in the pCR population with the primary aim of providing comprehensive evidence on this issue. In our overall analysis from 18 different centres or databases involving 2948 rectal cancer patients with pCR (adjuvant group = 1324, non-adjuvant group = 1624), we indicated that locally advanced rectal cancer patients with pCR after neoadjuvant chemoradiotherapy and radical resection showed an improved OS in the adjuvant chemotherapy group.
In contrast with the results from previous small studies [20, 22,23,24], our overall analysis indicated a significant advantage of adjuvant chemotherapy in rectal cancer patients with pCR since we provided larger sample sizes (pCR patients = 2948) from 18 centres or databases to detect a statistically significant difference. One theory for our finding is that tumour downstaging after neoadjuvant treatment may indicate favourable tumour biology and can be correlated with further responsivity for patients with a proven responsivity to additional chemotherapy [13]. Adjuvant chemotherapy for patients with a proven responsivity to treatment may be beneficial by potentially eradicating residual micrometastatic disease [6, 7]. Notably, based on the NCDB analysis from three studies [12, 13, 21] on this issue, the evidence potentially demonstrated that patients with clinical nodal positive disease that achieved pCR after neoadjuvant chemoradiotherapy might benefit the most from additional adjuvant chemotherapy. This seems intuitive given that nodal disease is a major marker for systemic recurrence in colorectal cancer [4, 12, 33, 34]. However, the subgroup results were only summarised qualitatively and required further investigation for confirmation. In addition, the NCDB did not contain the information of disease-free survival or local recurrence survival; hence, in our present analysis, we only provide the primary outcome of OS. In fact, the potential advantage of adjuvant chemotherapy in improving disease-free survival for pCR patients could be observed in some small comparative studies [19, 24], though with no statistical significance.
For LARC patients who received neoadjuvant treatment, the pCR rate ranged from 9 to 22% from 13 different centres [11]. In addition, Xu et al. indicated that LARC patients who have achieved pCR after neoadjuvant chemoradiotherapy showed a poor compliance with adjuvant chemotherapy based on the NCDB. This analysis showed that only 28% of the patients (484/1727) with pCR received adjuvant chemotherapy [32]. Based on the aforementioned evidence, only approximately 5% of the overall LARC patients may benefit from the adjuvant chemotherapy. Xu et al. [32] further indicated that adjuvant therapy appears to be independently associated with improved OS regardless of stage of disease and the greatest survival benefit was observed in patients who had achieved pCR. Hence, how to improve the compliance with adjuvant chemotherapy of patients with pCR remains an urgent issue for current practice. Age and co-morbidities were found to be significantly associated with nonreceipt of adjuvant therapy, and improved rehabilitation and physical conditioning may improve the odds of patients receiving adjuvant therapy [32].
The choice of adjuvant chemotherapy agents used may also affect the outcomes for LARC. A review summarised by Carvalho et al. [28] indicated that the addition of oxaliplatin to 5-fluorouracil-based adjuvant chemotherapy showed a significant improved 3-year disease-free survival in trials of CAO/ARO/AIO-4 [35] and ADORE [36]. However, significant advantages of adjuvant chemotherapy in RCTs comparing 5-fluorouracil versus observation were not observed for EORTC 22921 [3], I-CNR-RT and the PROCTOR [29] arm of the Dutch PROCTOR-SCRIPT [30]. In our overall analysis of the included studies, Tay et al. indicated that the regimen of adjuvant chemotherapy included folinic acid, fluorouracil and oxaliplatin [20]. Meanwhile, the pooled analysis conducted by Maas et al. [11] showed that10 centres used 5-fluorouracil-based agents in adjuvant chemotherapy and 3 centres delivered oxaliplatin and 5-fluorouracil-based agents in adjuvant chemotherapy. However, the other included studies did not present the detailed regimens of adjuvant therapy; hence, whether the addition of oxaliplatin to 5-fluorouracil-based chemotherapy can further improve the outcomes for LARC patients with pCR is unknown based on current evidence.
To the best of our knowledge, we performed the first meta-analysis to explore whether adjuvant chemotherapy is necessary for LARC patients with pCR after neoadjuvant chemoradiotherapy. Our study provided updated evidence based on 18 different centres or databases in support of the administration of adjuvant chemotherapy for rectal cancer patients with pCR. However, there are some limitations in our present study. First, all the included studies are observational studies with a retrospective nature. Although current guidelines recommended the routine use of adjuvant chemotherapy for patients with pCR, the evidence is still not verified by prospective or randomised trials. The individual data of patients with pCR from previous RCTs are needed to update our findings. In addition, study by Polanco et al. [12] had provided the highest influence to the overall analysis (48.4%) due to NCDB enrolled the largest patient population; therefore, we expected that future studies based on multicentres and larger-scale population to further update or findings. Meanwhile, although OS is the primary aim we focused on, the issue of whether improved OS would be balanced by the increased toxicities and impaired quality of life needs to be studied further. Toxicities and quality of life outcomes are absent and insufficient from the current published evidence. Furthermore, our study only provided the data of OS; in fact, four included studies also provided the data on recurrence [11, 20, 23, 24]. However, our overall results showed that adjuvant group did not show a significant improved of recurrence-free survival in comparison with non-adjuvant group (HR = 1.33, 95% CI = 0.80–2.20, P = 0.27). There are several biases and limitations for this result: (1) not all the included studies had provided the data of recurrence, the analysis based on the dataset of the NCDB had no data on recurrence, and (2) included studies based on recurrence had no detailed information to distinguish local or distant recurrence. The basis of giving adjuvant chemotherapy is to rid of any micrometastasis; therefore, we might need large-scale and prospective studies to further explore the effect of adjuvant chemotherapy on recurrence between two groups. Finally, the detailed regimens of adjuvant chemotherapy were not presented in some of our included studies [12, 22,23,24]. Therefore, we could still not determine what the most appropriate regimens and cycles of adjuvant chemotherapy are for rectal cancer patients with pCR.
Conclusion
Our meta-analysis indicated that adjuvant chemotherapy is associated with improved OS in LARC patients with pCR after neoadjuvant chemoradiotherapy and radical surgery.
References
Benson AB 3rd, Venook AP, Al-Hawary MM, Cederquist L, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D, Engstrom PF, Grem JL, Grothey A, Hochster HS, Hoffe S, Hunt S, Kamel A, Kirilcuk N, Krishnamurthi S, Messersmith WA, Meyerhardt J, Mulcahy MF, Murphy JD, Nurkin S, Saltz L, Sharma S, Shibata D, Skibber JM, Sofocleous CT, Stoffel EM, Stotsky-Himelfarb E, Willett CG, Wuthrick E, Gregory KM, Gurski L, Freedman-Cass DA (2018) Rectal Cancer, version 2.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw 16(7):874–901. https://doi.org/10.6004/jnccn.2018.0061
Glynne-Jones R, Counsell N, Quirke P, Mortensen N, Maraveyas A, Meadows HM, Ledermann J, Sebag-Montefiore D (2014) Chronicle: results of a randomised phase III trial in locally advanced rectal cancer after neoadjuvant chemoradiation randomising postoperative adjuvant capecitabine plus oxaliplatin (XELOX) versus control. Ann Oncol 25(7):1356–1362. https://doi.org/10.1093/annonc/mdu147
Bosset JF, Calais G, Mineur L, Maingon P, Stojanovic-Rundic S, Bensadoun RJ, Bardet E, Beny A, Ollier JC, Bolla M, Marchal D, Van Laethem JL, Klein V, Giralt J, Clavere P, Glanzmann C, Cellier P, Collette L, Group ERO (2014) Fluorouracil-based adjuvant chemotherapy after preoperative chemoradiotherapy in rectal cancer: long-term results of the EORTC 22921 randomised study. Lancet Oncol 15(2):184–190. https://doi.org/10.1016/S1470-2045(13)70599-0
Valentini V, van Stiphout RG, Lammering G, Gambacorta MA, Barba MC, Bebenek M, Bonnetain F, Bosset JF, Bujko K, Cionini L, Gerard JP, Rodel C, Sainato A, Sauer R, Minsky BD, Collette L, Lambin P (2011) Nomograms for predicting local recurrence, distant metastases, and overall survival for patients with locally advanced rectal cancer on the basis of European randomized clinical trials. J Clin Oncol 29(23):3163–3172. https://doi.org/10.1200/JCO.2010.33.1595
Bujko K, Glynne-Jones R, Bujko M (2010) Does adjuvant fluoropyrimidine-based chemotherapy provide a benefit for patients with resected rectal cancer who have already received neoadjuvant radiochemotherapy? A systematic review of randomised trials. Ann Oncol 21(9):1743–1750. https://doi.org/10.1093/annonc/mdq054
Petrelli F, Coinu A, Lonati V, Barni S (2015) A systematic review and meta-analysis of adjuvant chemotherapy after neoadjuvant treatment and surgery for rectal cancer. Int J Color Dis 30(4):447–457. https://doi.org/10.1007/s00384-014-2082-9
Collette L, Bosset JF, den Dulk M, Nguyen F, Mineur L, Maingon P, Radosevic-Jelic L, Pierart M, Calais G, European Organisation for R, Treatment of Cancer Radiation Oncology G (2007) Patients with curative resection of cT3-4 rectal cancer after preoperative radiotherapy or radiochemotherapy: does anybody benefit from adjuvant fluorouracil-based chemotherapy? A trial of the European Organisation for Research and Treatment of Cancer radiation oncology group. J Clin Oncol 25(28):4379–4386. https://doi.org/10.1200/JCO.2007.11.9685
Kiran RP, Kirat HT, Burgess AN, Nisar PJ, Kalady MF, Lavery IC (2012) Is adjuvant chemotherapy really needed after curative surgery for rectal cancer patients who are node-negative after neoadjuvant chemoradiotherapy? Ann Surg Oncol 19(4):1206–1212. https://doi.org/10.1245/s10434-011-2044-1
Hu X, Li YQ, Li QG, Ma YL, Peng JJ, Cai SJ (2018) Adjuvant chemotherapy seemed not to have survival benefit in rectal Cancer patients with ypTis-2N0 after preoperative radiotherapy and surgery from a population-based propensity score analysis. Oncologist. https://doi.org/10.1634/theoncologist.2017-0600
Martin ST, Heneghan HM, Winter DC (2012) Systematic review and meta-analysis of outcomes following pathological complete response to neoadjuvant chemoradiotherapy for rectal cancer. Br J Surg 99(7):918–928. https://doi.org/10.1002/bjs.8702
Maas M, Nelemans PJ, Valentini V, Crane CH, Capirci C, Rodel C, Nash GM, Kuo LJ, Glynne-Jones R, Garcia-Aguilar J, Suarez J, Calvo FA, Pucciarelli S, Biondo S, Theodoropoulos G, Lambregts DM, Beets-Tan RG, Beets GL (2015) Adjuvant chemotherapy in rectal cancer: defining subgroups who may benefit after neoadjuvant chemoradiation and resection: a pooled analysis of 3,313 patients. Int J Cancer 137(1):212–220. https://doi.org/10.1002/ijc.29355
Polanco PM, Mokdad AA, Zhu H, Choti MA, Huerta S (2018) Association of Adjuvant Chemotherapy with Overall Survival in patients with rectal Cancer and pathologic complete response following neoadjuvant chemotherapy and resection. JAMA Oncol 4(7):938–943. https://doi.org/10.1001/jamaoncol.2018.0231
Dossa F, Acuna SA, Rickles AS, Berho M, Wexner SD, Quereshy FA, Baxter NN, Chadi SA (2018) Association between adjuvant chemotherapy and overall survival in patients with rectal Cancer and pathological complete response after neoadjuvant chemotherapy and resection. JAMA Oncol 4(7):930–937. https://doi.org/10.1001/jamaoncol.2017.5597
Stang A (2010) Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25(9):603–605. https://doi.org/10.1007/s10654-010-9491-z
Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR (2007) Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 8:16. https://doi.org/10.1186/1745-6215-8-16
Schmidt FL, Oh IS, Hayes TL (2009) Fixed- versus random-effects models in meta-analysis: model properties and an empirical comparison of differences in results. Br J Math Stat Psychol 62(Pt 1):97–128. https://doi.org/10.1348/000711007X255327
Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50(4):1088–1101
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. Bmj 315(7109):629–634
Zhou J, Qiu H, Lin G, Xiao Y, Wu B, Wu W, Sun X, Lu J, Zhang G, Xu L, Liu Y (2016) Is adjuvant chemotherapy necessary for patients with pathological complete response after neoadjuvant chemoradiotherapy and radical surgery in locally advanced rectal cancer? Long-term analysis of 40 ypCR patients at a single center. Int J Color Dis 31(6):1163–1168. https://doi.org/10.1007/s00384-016-2579-5
Tay RY, Jamnagerwalla M, Steel M, Wong HL, McKendrick JJ, Faragher I, Kosmider S, Hastie I, Desai J, Tacey M, Gibbs P, Wong R (2017) Survival impact of adjuvant chemotherapy for resected locally advanced rectal adenocarcinoma. Clin Colorectal Cancer 16(2):e45–e54. https://doi.org/10.1016/j.clcc.2016.09.011
Shahab D, Gabriel E, Attwood K, Ma WW, Francescutti V, Nurkin S, Boland PM (2017) Adjuvant chemotherapy is associated with improved overall survival in locally advanced rectal Cancer after achievement of a pathologic complete response to Chemoradiation. Clin Colorectal Cancer 16(4):300–307. https://doi.org/10.1016/j.clcc.2017.03.005
Kuan FC, Lai CH, Ku HY, Wu CF, Hsieh MC, Liu TW, Yeh CY, Lee KD (2017) The survival impact of delayed surgery and adjuvant chemotherapy on stage II/III rectal cancer with pathological complete response after neoadjuvant chemoradiation. Int J Cancer 140(7):1662–1669. https://doi.org/10.1002/ijc.30562
Geva R, Itzkovich E, Shamai S, Shacham-Shmueli E, Soyfer V, Klausner JM, Tulchinsky H (2014) Is there a role for adjuvant chemotherapy in pathological complete response rectal cancer tumors following neoadjuvant chemoradiotherapy? J Cancer Res Clin Oncol 140(9):1489–1494. https://doi.org/10.1007/s00432-014-1712-5
Gamaleldin M, Church JM, Stocchi L, Kalady M, Liska D, Gorgun E (2017) Is routine use of adjuvant chemotherapy for rectal cancer with complete pathological response justified? Am J Surg 213(3):478–483. https://doi.org/10.1016/j.amjsurg.2016.11.028
Andre T, Boni C, Navarro M, Tabernero J, Hickish T, Topham C, Bonetti A, Clingan P, Bridgewater J, Rivera F, de Gramont A (2009) Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol 27(19):3109–3116. https://doi.org/10.1200/JCO.2008.20.6771
Twelves C, Wong A, Nowacki MP, Abt M, Burris H 3rd, Carrato A, Cassidy J, Cervantes A, Fagerberg J, Georgoulias V, Husseini F, Jodrell D, Koralewski P, Kroning H, Maroun J, Marschner N, McKendrick J, Pawlicki M, Rosso R, Schuller J, Seitz JF, Stabuc B, Tujakowski J, Van Hazel G, Zaluski J, Scheithauer W (2005) Capecitabine as adjuvant treatment for stage III colon cancer. N Engl J Med 352(26):2696–2704. https://doi.org/10.1056/NEJMoa043116
O'Connell MJ, Mailliard JA, Kahn MJ, Macdonald JS, Haller DG, Mayer RJ, Wieand HS (1997) Controlled trial of fluorouracil and low-dose leucovorin given for 6 months as postoperative adjuvant therapy for colon cancer. J Clin Oncol 15(1):246–250. https://doi.org/10.1200/JCO.1997.15.1.246
Carvalho C, Glynne-Jones R (2017) Challenges behind proving efficacy of adjuvant chemotherapy after preoperative chemoradiation for rectal cancer. Lancet Oncol 18(6):e354–e363. https://doi.org/10.1016/S1470-2045(17)30346-7
Sainato A, Cernusco Luna Nunzia V, Valentini V, De Paoli A, Maurizi ER, Lupattelli M, Aristei C, Vidali C, Conti M, Galardi A, Ponticelli P, Friso ML, Iannone T, Osti FM, Manfredi B, Coppola M, Orlandini C, Cionini L (2014) No benefit of adjuvant fluorouracil Leucovorin chemotherapy after neoadjuvant chemoradiotherapy in locally advanced cancer of the rectum (LARC): long term results of a randomized trial (I-CNR-RT). Radiother Oncol 113(2):223–229. https://doi.org/10.1016/j.radonc.2014.10.006
Breugom AJ, van Gijn W, Muller EW, Berglund A, van den Broek CB, Fokstuen T, Gelderblom H, Kapiteijn E, Leer JW, Marijnen CA, Martijn H, Meershoek-Klein Kranenbarg E, Nagtegaal ID, Pahlman L, Punt CJ, Putter H, Roodvoets AG, Rutten HJ, Steup WH, Glimelius B, van de Velde CJ, Cooperative Investigators of Dutch Colorectal Cancer G, Nordic Gastrointestinal Tumour Adjuvant Therapy G (2015) Adjuvant chemotherapy for rectal cancer patients treated with preoperative (chemo)radiotherapy and total mesorectal excision: a Dutch colorectal Cancer group (DCCG) randomized phase III trial. Ann Oncol 26(4):696–701. https://doi.org/10.1093/annonc/mdu560
Khrizman P, Niland JC, ter Veer A, Milne D, Bullard Dunn K, Carson WE 3rd, Engstrom PF, Shibata S, Skibber JM, Weiser MR, Schrag D, Benson AB 3rd (2013) Postoperative adjuvant chemotherapy use in patients with stage II/III rectal cancer treated with neoadjuvant therapy: a national comprehensive cancer network analysis. J Clin Oncol 31(1):30–38. https://doi.org/10.1200/JCO.2011.40.3188
Xu Z, Mohile SG, Tejani MA, Becerra AZ, Probst CP, Aquina CT, Hensley BJ, Arsalanizadeh R, Noyes K, Monson JR, Fleming FJ (2017) Poor compliance with adjuvant chemotherapy use associated with poorer survival in patients with rectal cancer: an NCDB analysis. Cancer 123(1):52–61. https://doi.org/10.1002/cncr.30261
Ryuk JP, Choi GS, Park JS, Kim HJ, Park SY, Yoon GS, Jun SH, Kwon YC (2014) Predictive factors and the prognosis of recurrence of colorectal cancer within 2 years after curative resection. Ann Surg Treat Res 86(3):143–151. https://doi.org/10.4174/astr.2014.86.3.143
Augestad KM, Bakaki PM, Rose J, Crawshaw BP, Lindsetmo RO, Dorum LM, Koroukian SM, Delaney CP (2015) Metastatic spread pattern after curative colorectal cancer surgery. A retrospective, longitudinal analysis. Cancer Epidemiol 39(5):734–744. https://doi.org/10.1016/j.canep.2015.07.009
Rodel C, Graeven U, Fietkau R, Hohenberger W, Hothorn T, Arnold D, Hofheinz RD, Ghadimi M, Wolff HA, Lang-Welzenbach M, Raab HR, Wittekind C, Strobel P, Staib L, Wilhelm M, Grabenbauer GG, Hoffmanns H, Lindemann F, Schlenska-Lange A, Folprecht G, Sauer R, Liersch T, German Rectal Cancer Study G (2015) Oxaliplatin added to fluorouracil-based preoperative chemoradiotherapy and postoperative chemotherapy of locally advanced rectal cancer (the German CAO/ARO/AIO-04 study): final results of the multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 16(8):979–989. https://doi.org/10.1016/S1470-2045(15)00159-X
Hong YS, Nam BH, Kim KP, Kim JE, Park SJ, Park YS, Park JO, Kim SY, Kim TY, Kim JH, Ahn JB, Lim SB, Yu CS, Kim JC, Yun SH, Kim JH, Park JH, Park HC, Jung KH, Kim TW (2014) Oxaliplatin, fluorouracil, and leucovorin versus fluorouracil and leucovorin as adjuvant chemotherapy for locally advanced rectal cancer after preoperative chemoradiotherapy (ADORE): an open-label, multicentre, phase 2, randomised controlled trial. Lancet Oncol 15(11):1245–1253. https://doi.org/10.1016/S1470-2045(14)70377-8
Acknowledgements
We thank Pengliang Wang from the department of Surgical Oncology of First Hospital of China Medical University for the statistical assistance and guidance.
Funding
This work was funded by Natural Science Foundation of Liaoning Province (No. 20180550485).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
No informed consent.
Disclosure statement
The authors have nothing to disclose.
Rights and permissions
About this article
Cite this article
Ma, B., Ren, Y., Chen, Y. et al. Is adjuvant chemotherapy necessary for locally advanced rectal cancer patients with pathological complete response after neoadjuvant chemoradiotherapy and radical surgery? A systematic review and meta-analysis. Int J Colorectal Dis 34, 113–121 (2019). https://doi.org/10.1007/s00384-018-3181-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-018-3181-9