Abstract
Objectives
According to practice guidelines, adjuvant chemotherapy (ACT) is required for all patients with locally advanced rectal cancer who have received neoadjuvant chemoradiotherapy (NCRT) and total mesorectal excision (TME). The objective of this study was to determine whether ACT is necessary for patients achieving pathological complete response (pCR) after NCRT followed by surgery.
Methods
By retrospectively reviewing a prospectively collected database in our single tertiary care center, 210 patients with locally advanced rectal cancer who underwent NCRT followed by TME were identified between February 2005 and August 2013. All patients achieving ypCR were enrolled in this study, in which who underwent ACT (chemo group) and who did not (non-chemo group) were compared in terms of local recurrence (LR) rate, 5-year disease-free survival (DFS) rate and overall survival (OS) rate.
Results
Forty consecutive patients with ypCR were enrolled, 19 (47.5 %) in chemo group and 21 (52.5 %) in non-chemo group. After a median follow-up of 57 months, five patients developed systemic recurrences, with the 5y-DFS rate of 83.5 %. No LR occurred in the two groups. The 5y-DFS rates for patients in chemo group and non-chemo group was 90.9 and 76.0 %, respectively, showing no statistically significant difference (p = 0.142). Multivariate analysis showed that tumor grade was the only independent prognostic factor for 5y-DFS and 5y-OS.
Conclusions
Results of this study suggested that it may not be necessary for all rectal cancer patients with ypCR after NCRT and radical surgery to receive ACT. Prospective randomized trials are warranted to further determine the value of ACT for ypCR patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neoadjuvant chemoradiotherapy (NCRT) followed by total mesorectal excision (TME) has become the recommended treatment strategy for mid to low locally advanced rectal cancers. NCRT has been proved to improve the local disease control with decreased medicine-related toxicity [1, 2]. Patients with rectal cancer exhibit different responses to NCRT. Those who achieve a pathologic complete response (pCR) after NCRT are considered a more favorable subpopulation with very low local recurrence (LR) rate and improved long-term survival [3–5]. According to the present guidelines, adjuvant chemotherapy (ACT) is offered as a standard treatment for all patients having completed NCRT and the radical surgery, regardless of the postoperative pathological results [2, 6]. Several authors have proposed that not all the patients benefit from the ACT after NCRT and the curative resection, however their studies showed inconsistent results [7–14]. The main aim of this study was to evaluate the necessity of undergoing ACT in patients achieving ypCR after NCRT.
Patients and methods
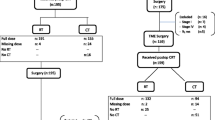
This study was a retrospective review of a prospectively collected database maintained since 1997. The database includes data for all patients with colorectal cancer undergoing surgery in the department of general surgery of our tertiary care center. Between February 2005 and August 2013, a total of 210 patients with mid to low locally advanced rectal cancer (clinically staged T3-4 or N+, M0) underwent NCRT followed by TME. Among which 40 consecutive patients (aged up to 80 years) with pathologically confirmed ypCR were enrolled in this study. Patients with a previous history of malignant disease were excluded.
For all the cases, the diagnosis was based on the pathological result of tumor biopsy, and the clinical staging both prior and after NCRT was based on imaging studies including the transanal rectal ultrasound (TRUS), and or rectal magnetic resonance imaging (MRI), and pulmonary and abdominopelvic contrast-enhanced computed tomography (CT) scan. The informed consent was obtained from all the patients before initiation of NCRT. NCRT regimen included a total of 50Gy radiation (2Gy per day for 25 fractions over 5 weeks) with concurrent chemotherapy of CapeOX (29 cases), FOLFOX 4 (seven cases), or single-agent Capecitabine (four cases) regimen.
The median time interval between the completion of radiotherapy and surgery was 7 weeks (range, 4–9 weeks). During the surgery, the total mesorectal excision principle was followed in all the 40 cases. After the surgery, pathological examination was performed by two pathologists independently.
After the surgery, nineteen patients (47.5 %) underwent ACT (chemo group), with the regimen of CapeOX (12 cases), FOLFOX 4 (two cases), or single-agent Capecitabine (five cases). Another 21 patients (52.5 %) did not accept ACT (non-chemo group), nine of them were influenced by unfavorable factors including severe neutropenia complicated by NCRT, postoperative complications such as small bowel obstruction or infection of surgical incision, or concomitant diseases (repeated biliary tract infection due to cholangiolithiasis in one case and lower limb atherosclerotic occlusive disease in another patient). Another 12 patients refused ACT owing to personal unwillingness, although having been fully informed about the risks of increased disease relapse rate and the impairment to their long-term outcome. Another four patients from the database accomplishing ypCR received the postoperative ACT, whereas not completing the entire regimens for various reasons. They were not included in this study.
Follow-up was conducted by interview in the outpatient clinic and by telephone. All patients were required to keep routine follow-up visits for 5 years. Examinations including the serum tumor markers CEA and CA 19–9, stool occult blood test, chest X-ray, abdominal ultrasonography, colonoscopy, and CT scan were performed regularly according to our follow-up criteria for colorectal cancer patients. All the follow-up information was recorded for the final analysis.
By the deadline for the evaluation on June 20, 2015, the median follow-up period was 57 (range, 20–130) months. Evaluation of the data as well as the survival analysis was calculated by using the SPSS 19.0, relative to the date of disease recurrence, the patient death or to the last follow-up time point. Survival was measured from the date of the surgery. Patients alive and free of tumor (distant metastases (DM) and/or LR) were censored for the disease-free survival (DFS) analysis. Differences between curves were assessed by the Mantel log-rank test for censored survival data. Multivariate analysis was performed according to the Cox proportional hazards model by backward elimination of factors found with p < 0.12 on univariate analyses. Results are presented as hazard ratios with the corresponding 95 % confidence intervals. All p values resulted from two-sided statistical tests, and p < 0.05 was considered to be significant.
Results
The demographic and tumor characteristics, treatment modalities (including the regimens of preoperative chemotherapy, the surgical approaches, and the postoperative complications) in the chemo and non-chemo groups are shown in Table 1. The non-chemo group had a higher male/female ratio, lower body mass index (BMI), a smaller proportion of patients undergoing the anterior resection, and had more postoperative complications than the chemo group (p < 0.05). Other characteristics including age, ASA score, tumor size, grade, clinical staging, as well as the treatment modalities and regimens all matched well between the two groups. There was no statistically significant difference in terms of intraoperative blood loss and number of lymph nodes retrieved between the two groups (p > 0.05) (Table 1). Postoperative pathological examination revealed complete tumor response in all the 40 patients with an average lymph node retrieval of 9.2 ± 4.4.
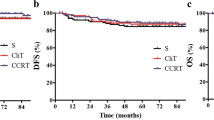
During a median follow-up of 57 (range, 20–130) months, five patients (two in the chemo group and three in the non-chemo group) developed systemic recurrences at 12 through 57 postoperative months and died during the period between 20 and 62 months after the surgery, with the total 5y-DFS being 83.5 % (Fig 1). No LR occurred in the two groups. The 5y-DFS rates for patients in the chemo group and the non-chemo group were 90.9 and 76.0 %, respectively, showing no significant difference (p = 0.142) (Fig 2). In the univariate analyses, only the age of patients significantly correlated with the 5y-DFS and 5y-OS. Patients aged 60 years and older had significantly poor long-term survival than younger patients, with the 5y-DFS of age group I (<60 years) and II (≥60 years) being 100.0 and 52.7 %, respectively (p = 0.001). Other parameters including the patient gender, BMI, ASA score, tumor size, tumor distance above the anal verge, pretreatment clinical stage, neoadjuvant chemotherapy regimen, surgical approaches, postoperative complications, number of the lymph nodes retrieved, and postoperative ACT regimen did not correlate significantly with 5y-DFS or 5y-OS. In the multivariate analysis using Cox’s regression model, only tumor grade was found to correlate with 5y-DFS and 5y-OS. The hazard ratio (HR) for death from rectal cancer was about 7.339 times greater for every increased tumor grade (95 % CI, 1.234–43.645, p = 0.028). Although age of patients was not significantly associated with the long-term survival in the multivariate analysis, there was a trend toward a worse outcome in terms of 5y-DFS in older patients (HR, 1.184; 95 % CI, 0.997–1.405, p = 0.054).
Discussion
According to the current guidelines, postoperative ACT is recommended as standard of care for patients with rectal cancers who have received NCRT. In the actual clinical practice, however, patients’ adherence to ACT is poor, with only about half to two thirds patients receive it [15, 16]. Better evidences are needed to define the role of ACT in patients with locally advanced rectal cancer after NCRT.
Some studies have investigated upon this issue, whereas showing inconsistent results. The 10-year follow-up results of EORTC trial 22921 showed that adjuvant fluorouracil-based chemotherapy after preoperative radiotherapy (with or without chemotherapy) does not affect DFS or OS [17]. A recently published meta-analysis drew similar conclusions [11]. On the contrary, Jung et al. [9] reported a retrospective study of 476 cases, which revealed that the 5y-DFS was significantly higher for patients who underwent ACT than those who did not, after NCRT and radical surgery (78.5 vs. 63.1 %, p = 0.016). A more recent study showed similar results [18].
Some authors performed subgroup analyses with the aim of defining the subpopulation who may or may not benefit from ACT after NCRT. Most of them focused on the patients who respond well to the neoadjuvant therapy (ypT0-2 N0, or ypN-patients). Some studies showed that patients with good response to NCRT were a subgroup of patients being expected to have improved outcomes, and ACT was unnecessary for them [10, 14], whereas others suggested that ACT is likely most beneficial for patients who respond to NCRT [7, 13]. In a retrospective cohort analysis, Chang et al.[19] further divided ypT1-2 N0 from ypCR patients, and found that patients with an intermediate response to NCRT (ypT1-2 N0) treated with ACT had a significantly longer recurrence-free time [19]. This implies that there may be a need for exploring patients of ypCR, ypT1-2 N0, and other stages separately.
Few studies have demonstrated the efficacy of ACT for ypCR patients after NCRT so far. In the present study, we evaluated the oncological outcomes of 40 patients with ypCR at our single center and carefully followed them for a prolonged period of time. In this study, a pCR rate of 19.0 % (40/210) was achieved, which was consistent with the reported pCR rate of 15–27 % from previous literatures [4]. Our results in terms of LR (0), rate of disease relapse (12.5 %), and 5y-DFS (83.5 %) of ypCR cases are comparable to those of the previous literatures [5, 14].
Patients in the two groups had comparable characteristics mostly, except for higher BMI, larger proportion of anterior resection, and lower postoperative complication rate in the chemo-group than its counterpart. Such differences may be explained by the fact that patients with better physical status after the surgery were easier to accept ACT and better adhere to the required regimens. In spite of having the adverse factors, patients in the non-chemo group achieved similar long-term oncological outcomes comparing with their counterparts (p = 0.142 for DFS and p = 0.140 for OS). Our findings questioned the routine use of ACT for rectal cancer patients with ypCR after NCRT and radical surgery.
Previous literatures have proved that patients achieving ypCR after NCRT are a favorable subpopulation with good prognosis [3–5, 7]. In the present study, we found some possible adverse factors for the long-term outcome of ypCR patients. Tumor grade was revealed an independent adverse prognostic factor. Higher tumor grade was associated with significantly decreased 5y-DFS and 5y-OS, regardless of whether the patient received ACT or not. In this study, all five patients who developed disease recurrence and finally died of disease aged 60 years or older. In the multivariate analysis, old age (≥60 years) was an borderline independent adverse prognostic factor for the 5y-DFS (p = 0.054). Similarly, a previous study of 566 ypCR patients demonstrated that patients older than 60 years had significantly lower 5y-DFS and 5y-OS than younger patients [3]. These results suggest that old age patients seem to have relatively worse prognosis, even after achieving ypCR.
We therefore hypothesized that in ypCR patients with the possible adverse prognostic factors including high tumor grade and old age, postoperative ACT may still be necessary to control the systemic disease recurrence and improve survival. Prospective randomized trials are warranted to determine this issue.
It is generally considered that about 80 % of disease recurrences after radical resection of colorectal cancer occur within the first 2 years after the surgery [20, 21]. However, it does not seem to be the case for patients with ypCR in this study. Some authors have inferred from their studies that LR was postponed in patients with ypCR, with 61 % of the LR cases being reported after 5 years post treatment (5y LR: 1.6 %, 10y LR: 4.1 %) [5]. In our study, with a median follow-up of 57 months, we did not observe any LR. Besides, among the five cases having developed systemic disease recurrence, two of them (40 %) occurred more than 4 years after the surgery. We therefore agreed with the perspective that patients with ypCR require prolonged observation [5, 22].
This retrospective cohort study has certain limitations. Here, we analyzed data from a single center, which guaranteed the consistency of therapeutic and follow-up methods among cases and minimized data variations. However, the limitation of relatively small sample size may hamper the elimination of confounding factors. Besides, because of the low disease relapse rate and mortality rate of ypCR patients, the percentage of censored data was high, even with a long median follow-up period. These factors limited the power of the statistical analysis. Further studies by accumulating more cases or by conducting a prospective randomized trial may solve these problems, which may also potentially define the subpopulation who will actually benefit from ACT.
Conclusions
Our results suggested that it may not be necessary for all rectal cancer patients with ypCR after NCRT and radical surgery to receive ACT. However, for patients with high tumor grade, and those old aged, ACT may still be beneficial and even more aggressive chemotherapy regimens are indicated. Prospective randomized trials or large multicenter cohort studies are warranted to further determine the value of ACT for ypCR patients.
References
Scott NA, Susnerwala S, Gollins S et al (2009) Preoperative neo-adjuvant therapy for curable rectal cancer--reaching a consensus 2008. Colorectal Dis 11:245–248
Glimelius B, Oliveira J (2009) Rectal cancer: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol 20(Suppl 4):54–56
Capirci C, Valentini V, Cionini L et al (2008) Prognostic value of pathologic complete response after neoadjuvant therapy in locally advanced rectal cancer: long-term analysis of 566 ypCR patients. Int J Radiat Oncol Biol Phys 72:99–107
Maas M, Nelemans PJ, Valentini V et al (2010) Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol 11:835–844
Valentini V, van Stiphout RG, Lammering G et al (2015) Selection of appropriate end-points (pCR vs 2yDFS) for tailoring treatments with prediction models in locally advanced rectal cancer. Radiother Oncol 114:302–309
Benson AB 3rd, Bekaii-Saab T, Chan E et al (2012) Rectal cancer. J Natl Compr Canc Netw 10:1528–1564
Collette L, Bosset JF, den Dulk M et al (2007) Patients with curative resection of cT3-4 rectal cancer after preoperative radiotherapy or radiochemotherapy: does anybody benefit from adjuvant fluorouracil-based chemotherapy? A trial of the European Organisation for Research and Treatment of Cancer Radiation Oncology Group. J Clin Oncol 25:4379–4386
Fietkau R, Barten M, Klautke G et al (2006) Postoperative chemotherapy may not be necessary for patients with ypN0-category after neoadjuvant chemoradiotherapy of rectal cancer. Dis Colon Rectum 49:1284–1292
Jung KU, Kim HC, Park JO et al (2015) Adjuvant chemotherapy after neoadjuvant chemoradiation and curative resection for rectal cancer: is it necessary for all patients? J Surg Oncol 111:439–444
Park IJ, Kim DY, Kim HC et al (2015) Role of adjuvant chemotherapy in ypT0-2N0 patients treated with preoperative chemoradiation therapy and radical resection for rectal cancer. Int J Radiat Oncol Biol Phys 92:540–547
Breugom AJ, Swets M, Bosset JF et al (2015) Adjuvant chemotherapy after preoperative (chemo)radiotherapy and surgery for patients with rectal cancer: a systematic review and meta-analysis of individual patient data. Lancet Oncol 16:200–207
Garcia-Albeniz X, Gallego R, Hofheinz RD et al (2014) Adjuvant therapy sparing in rectal cancer achieving complete response after chemoradiation. World J Gastroenterol 20:15820–15829
De Stefano A, Moretto R, Bucci L et al (2014) Adjuvant treatment for locally advanced rectal cancer patients after preoperative chemoradiotherapy: when, and for whom? Clin Colorectal Cancer 13:185–191
Kiran RP, Kirat HT, Burgess AN et al (2012) Is adjuvant chemotherapy really needed after curative surgery for rectal cancer patients who are node-negative after neoadjuvant chemoradiotherapy? Ann Surg Oncol 19:1206–1212
Haynes AB, You YN, Hu CY et al (2014) Postoperative chemotherapy use after neoadjuvant chemoradiotherapy for rectal cancer: analysis of surveillance, epidemiology, and end results-Medicare data, 1998–2007. Cancer 120:1162–1170
Bosset JF, Collette L, Calais G et al (2006) Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med 355:1114–1123
Bosset JF, Calais G, Mineur L et al (2014) Fluorouracil-based adjuvant chemotherapy after preoperative chemoradiotherapy in rectal cancer: long-term results of the EORTC 22921 randomised study. Lancet Oncol 15:184–190
Ahn DH, Wu C, Wei L et al (2015) The efficacy of adjuvant chemotherapy in patients with stage II/III resected rectal cancer treated with neoadjuvant chemoradiation therapy. Am J Clin Oncol. doi:10.1097/COC.0000000000000185
Chang GJ PI, Eng C (2012) Exploratory analysis of adjuvant chemotherapy benefits after preoperative chemoradiotherapy and radical resection for rectal cancer. J Clin Oncol 30:abstr 557
Scholefield JH, Steele RJ (2002) Guidelines for follow up after resection of colorectal cancer. Gut 51(Suppl 5):V3–V5
Umpleby HC, Fermor B, Symes MO, Williamson RC (1984) Viability of exfoliated colorectal carcinoma cells. Br J Surg 71:659–663
Habr-Gama A, Perez RO, Proscurshim I et al (2006) Patterns of failure and survival for nonoperative treatment of stage c0 distal rectal cancer following neoadjuvant chemoradiation therapy. J Gastrointest Surg 10:1319–1328, discussion 1328–1319
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Zhou, J., Qiu, H., Lin, G. et al. Is adjuvant chemotherapy necessary for patients with pathological complete response after neoadjuvant chemoradiotherapy and radical surgery in locally advanced rectal cancer? Long-term analysis of 40 ypCR patients at a single center. Int J Colorectal Dis 31, 1163–1168 (2016). https://doi.org/10.1007/s00384-016-2579-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-016-2579-5