Abstract
Introduction
Crohn’s disease (CD) is a progressive inflammatory disease affecting the entire gastrointestinal tract. The need for a definitive stoma (DS) is considered as the ultimate phase of damage. It is often believed that the risk of further disease progression is small when a DS has been performed.
Aims
The goals of the study were to establish the rate of CD recurrence above the DS and to identify predictive factors of CD recurrence at the time of DS.
Methods
We retrospectively reviewed all medical records of consecutive CD patients having undergone DS between 1973 and 2010. We collected clinical data at diagnosis, CD phenotype, treatment, and surgery after DS and mortality. Stoma was considered as definitive when restoration of continuity was not possible due to proctectomy, rectitis, anoperineal lesions (APL), or fecal incontinence. Clinical recurrence (CR) was defined as the need for re-introduction or intensification of medical therapy, and surgical recurrence (SR) was defined as a need for a new intestinal resection.
Results
Eighty-three patients (20 males, 63 females) with a median age of 34 years at CD diagnosis were included. The median time between diagnosis and DS was 9 years. The median follow-up after DS was 10 years. Thirty-five patients (42%) presented a CR after a median time of 28 months (2–211) and 32 patients (38%) presented a SR after a median time of 29 months (4–212). In a multivariate analysis, APL (HR = 5.1 (1.2–21.1), p = 0.03) and colostomy at time of DS (HR = 3.8 (1.9–7.3), p = 0.0001) were associated factors with the CR.
Conclusion
After DS for CD, the risk of clinical recurrence was high and synonymous with surgical recurrence, especially for patients with APL and colostomy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Crohn’s disease (CD) is a chronic inflammatory disorder of the digestive tract that leads progressively to stricturing and penetrating complications [1, 2]. These complications concern the vast majority of CD patients and frequently require surgery with approximately 80% of patients who still undergo intestinal surgical resection at some point in their life [3, 4].
Definitive stoma (DS) can be the ultimate therapeutic option in some circumstances for CD patients. The cumulative probability of DS 20 years after diagnosis of CD has been estimated at 14% [3–6]. Although the risk of DS in patients undergoing surgery for Crohn’s colitis with anorectal involvement has been falling (notably thanks to the use of biologics), it is still present in 19% of these individuals [7]. For many patients, DS is a major surgical concern [1] because they fear a change in body image and the quality of their social, professional, and sex lives [8–10]. It is commonly thought that CD recurs less frequently in patients with stoma than in patients with intestinal anastomosis [11]. However, the risk of clinical recurrence of CD above the DS varies from study to study [12–14]. One study reported a clinical recurrence rate of 22% after 30 years in 182 patients with coloproctectomy and definitive ileostomy [14], whereas most series report a clinical recurrence rate of between 35 and 60% 10 years after DS [15–20]. It has been estimated that between 5 and 33% of patients with DS will require further surgery after a median follow-up period of 1 to 19 years [13, 14, 16–18, 20–24]. Despite these dramatic results, most clinicians appear to underestimate the risk of clinical recurrence after DS and consider that the post-DS treatment of CD is not necessarily required.
The goals of the present study were to (i) establish the rate of CD recurrence above the DS in consecutive patients attending a university medical center and to (ii) identify predictive factors of CD recurrence at the time of DS.
Methods
Selection of patients
We retrospectively reviewed the medical records of all consecutive CD patients having undergone DS in the Digestive Surgery and Transplantation Unit at the Lille University Hospital between 1973 and 2010. All medical records were reviewed by the study’s principal investigator (DK). DS was defined as an end or loop ileostomy or colostomy with no likelihood of restoring bowel continuity due to anatomic reasons (abdominoperineal resection) or functional reasons (anal incontinence, active perineal lesion, refractory proctitis, short bowel syndrome, etc.). In case of loop colostomy, fecal diversion was considered as a viable option for severe perirectal fistulizing CD and was the preferred option for patients with the intent of re-hook after perineal healing, or for patients who had refused rectal amputation. Finally, in patients with a high risk of perineal fistulizing evolution after sustained intestinal continuity, this diverting stoma was considered as DS. For all patients, intestinal resections bellow the DS was systematically performed for end ileostomy or colostomy. Furthermore, we checked that the surgeon had noted the absence of macroscopic lesions above the DS during the surgical procedure.
Data collection
The following socio demographic and clinical characteristics were recorded: age, gender, family history of inflammatory bowel disease (IBD), smoking status (smoking cessation at least 12 months previously), extraintestinal manifestations (EIMs such as arthritis and ocular, skin, hepatic, and associated immune-mediated inflammatory diseases), and the CD phenotype according to the Montreal classification [25] (Table 1). Anoperineal lesions (APLs) were defined as anal or rectovaginal fistulae, anal abscesses, anal ulcerations, and/or anal stenosis. The post-DS use of immunosuppressants (such as azathioprine, 6-mercaptopurine, methotrexate, and cyclosporine) and/or anti-tumor necrosis factor alpha (TNFα) antibodies (infliximab and adalimumab) was recorded. Surgical procedures after DS (including ileocolectomy, small bowel resection, proctectomy, J-pouch resection and partial, subtotal or total colectomy) were reported, as were mortality and causes of death.
Outcomes
CD clinical recurrence after DS was defined as the recurrence of digestive symptoms (pain, occlusion, fistula, or stoma stenosis) and evidence of macroscopic lesions (in an endoscopic examination and/or bowel follow-through) and/or microscopic CD lesions (in a histopathologic examination). Colonic or stoma stricture in the absence of microscopic CD lesions was not considered to be a CD recurrence. Surgical recurrence was defined as further intestinal resection above the DS. Surgery for APL was not regarded as surgical recurrence. A histopathologic examination was performed in all cases of surgical recurrence in order to confirm the recurrence of CD at the resection site. Given that one of the study’s objectives was to determine the rate of CD recurrence above the DS, the need for intestinal resection below the DS (i.e., secondary proctectomy) was not considered here.
Statistical analysis
Qualitative variables were reported as the frequency and its 95% confidence interval (CI). Quantitative variables were reported as the median (range). The cumulative probability of clinical recurrence and its 95% CI were calculated using the Kaplan Meier method. Associated factors for clinical CD recurrence were searched for by applying bivariate Cox proportional hazards models. The hazard ratio (HR) (95%CI) was used to quantify the risk of clinical recurrence. Parameters with a p value below 0.2 in a bivariate analysis were introduced into a Cox proportional hazards multivariable regression model with p < 0.1 for backward selection.
All statistical analyses were performed with SAS software (version 9.2, SAS Inc., Chicago, IL). The threshold for statistical significance was set to p ≤ 0.05.
Results
Socio demographic and clinical characteristics
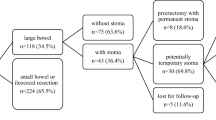
Eighty-three patients, including 63 females (76%), underwent DS during the study period; 63 patients had an ileostomy and 20 patients had a colostomy (Fig. 1). The clinical characteristics of the CD at the time of the DS and the indications for surgery are summarized in Table 2. Median (range) time between CD diagnosis and last follow-up was 22 years (1–39). Thirteen patients (16%) had a family history of IBD (eight for CD, four for ulcerative colitis, and one for both conditions). Twenty-two patients had extraintestinal manifestations of CD (27%). The median time between CD diagnosis and DS was 9 years (0–35). Almost half the patients had never undergone surgery for CD prior to the DS (46%; n = 38). Sixty-three patients (76%) underwent an ileostomy and 20 patients (24%) underwent a colostomy. Fifty patients (60%) had a diverting DS without rectal resection.
CD outcome after DS
Median duration of post-DS follow-up was 10 years (0–24). None of the patients were lost to follow-up. Among the 83 patients with DS, 48 (58%) had no clinical CD recurrence. Clinical recurrence of CD above the DS occurred in 35 patients (42%) (18 with ileostomy and 17 with colostomy) after a median post-DS time of 28 months (2–211). The rate of clinical recurrence above the DS was greater among patients with colostomies (17/20, 85%) than among those with ileostomies (18/63, 29%) (p < 0.001). This recurrence resulted in further intestinal resection in 32 of the 35 patients (91%) after a median post-DS time interval of 29 months (4–212) (Fig. 1). Histopathologic examination confirmed the recurrence of CD in all patients having undergone further intestinal resection.
The cumulative probabilities of clinical recurrence at 1, 5, and 12 years were respectively 14, 43, and 51% (Fig. 2). In a multivariate analysis, APLs at time of DS (HR = 5.1 (1.2–21.1), p = 0.03) and colostomy at time of DS (HR = 3.8 (1.9–7.3), p = 10−4) were associated factors with the clinical recurrence of CD above the DS (Table 3). In our study we did not find any influence on the risk of CD recurrence of the digestive location, behavior, smoking status, and presence of EIMs at the time of DS has been performed.
Seven patients died during follow-up. Among them, three had clinical CD recurrence with microscopic and/or macroscopic lesions above the DS. The causes of death were suicide (n = 1, 6 years after DS), septic shock after esophageal cancer resection (n = 1, 17 years after DS), and sepsis related to use of a central venous catheter for parenteral nutrition (n = 1, 24 years after DS). The other four patients had no clinical recurrence and died from septic shock (n = 1, 13 days after DS), an unknown etiology (n = 1, 4 years after DS), pulmonary embolism (n = 1, 1 year after DS), and geriatric cachexia (n = 1, 10 years after DS).
Discussion
Prevention of CD recurrence in patients with DS represents a major issue but regarding the debated risk of clinical recurrence in these patients, none post-operative treatment strategy is currently recommended. Our study’s major finding was that 2 years after DS, 42% of patients had a clinical recurrence of CD above the DS. This required further intestinal resection in almost all the affected patients (91%) a median of 2.4 years after DS (with the histopathologic confirmation of CD recurrence for all cases of further resection). The second important finding was the greater frequency of CD recurrence in patients with colostomy than in patients with ileostomy. On the basis of comprehensive data collection and long-term follow-up (10 years after DS and 22 years after CD diagnosis) of a cohort attending a single regional referral center, we determined the cumulative clinical probability of CD recurrence to be 43% at 5 years and 51% at 12 years. The risk of relapse was highest in the first 5 years post-DS and fell thereafter. Our findings are in agreement with a reported clinical recurrence rate of 39% 8 years after total coloproctectomy resulting in a 44% of surgical recurrence rate [22]. The high surgical recurrence rate (91%) in the present study may have been due to our long follow-up period (a median of 10 years vs. 5 years in the study by Amiot et al. [22]) and a high proportion of colostomies (identified as a risk factor for the clinical recurrence of CD in our present multivariate analysis). All the patients in Amiot’s study underwent ileostomy [22].
Given that our data were collected in patients with severe CD attending a regional referral center, our findings may not necessarily be applicable to the general population of patients with CD. The high surgical recurrence rate (reflected the disabling course of CD in our cohort) was confirmed by the fact that almost half of these individuals had undergone DS as their first surgery for the indication of CD.
The rate of clinical recurrence above the DS was greater among patients with colostomies than among those with ileostomies (85 and 29%, respectively). We suggest that with a view to decreasing the clinical recurrence rate, coloproctectomy with definitive ileostomy might be an alternative to definitive colostomy.
In our multivariate analysis, APLs and colostomy were independently associated with the risk of clinical recurrence of CD. The presence of APLs at the time of DS was associated with a five-fold increase in the risk for clinical recurrence in the digestive tract above the DS. Thus, patients with APLs at the time of DS and those having undergone colostomy should be monitored more intensively. Our observation is in agreement with previous reports in which APLs were associated with more severe CD. Indeed, the presence of APLs is a criterion for the diagnosis of disabling CD [26]. After an initial intestinal resection, the relative risk of surgical recurrence for CD (according to a multivariate analysis) is significantly elevated in patients with perianal fistulae (HR = 1.4 (1.2–1.7); p = 0.0002) [27].
Some studies have suggested that preventive treatment (i.e., anti-TNF therapy before endoscopic recurrence) is more effective than post-recurrence treatment [28–30]. A recent review of the literature on preventing the recurrence of CD after surgery suggested that anti-TNFα treatment should always be provided after ileocolonic resection in high-risk patients (smokers and those with penetrating disease, previous surgery, or previous perianal disease) [30]. In the present study, few patients were treated immediately after DS. In fact, only seven patients initiated preventive treatment by infliximab (n = 4) or azathioprine (n = 3) within 3 months of DS (data not shown). Medical therapies have changed over time, especially with the development of biotherapies in the beginning of the 2000s. In any time period, none post-operative prophylactic treatment was recommended in patients with DS. Consequently, in our study, only seven patients among 83 received post-operative prophylactic treatment (within 3 months of DS) and therapeutic abstention after DS was the general attitude (n = 76). In view of the clinical recurrence rate after DS observed here (42%), we consider that preventive anti-TNF treatment should be initiated immediately after surgery—particularly in patients with APLs at the time of DS and when colostomy is used to create the DS. Further work is needed to establish whether immediate, aggressive drug therapy can improve outcomes.
Conclusion
After DS for CD, the risk of clinical recurrence is high and is synonymous with surgical recurrence. After DS, the patient should be provided with balanced information and careful clinical follow-up is warranted. Immediate post-DS prophylactic therapy should be considered, especially for patients with APLs and those having undergone colostomy. Further work is needed to establish whether immediate, aggressive drug therapy can improve outcomes in these patients.
References
Louis E, Collard A, Oger AF, Degroote E, Aboul Nasr El Yafi FA, Belaiche J (2001 Dec) Behaviour of Crohn’s disease according to the Vienna classification: changing pattern over the course of the disease. Gut 49(6):777–782
Cosnes J1, Cattan S, Blain A, Beaugerie L, Carbonnel F, Parc R, Gendre JP (2002) Long-term evolution of disease behavior of Crohn’s disease. Inflamm Bowel Dis 8(4):244–250
Etienney I, Bouhnik Y, Gendre JP et al (2004) Crohn’s disease over 20 years after diagnosis in a referral population. Gastroenterol Clin Biol 28(12):1233–1239
Bouguen G, Chevaux JB, Peyrin-Biroulet L (2011) Recent advances in cytokines: therapeutic implications for inflammatory bowel diseases. World J Gastroenterol 17(5):547–556
Rungoe C, Langholz E, Andersson M, Basit S, Nielsen NM, Wohlfahrt J et al (2014) Changes in medical treatment and surgery rates in inflammatory bowel disease: a nationwide cohort study 1979-2011. Gut 63(10):1607–1616
Post S, Herfarth C, Schumacher H, Golling M, Schurmann G, Timmermanns G (1995) Experience with ileostomy and colostomy in Crohn’s disease. Br J Surg 82(12):1629–1633
Coscia M, Gentilini L, Laureti S, Gionchetti P, Rizzello F, Campieri M et al (2013) Risk of permanent stoma in extensive Crohn’s colitis: the impact of biological drugs. Color Dis 15(9):1115–1122
Kasparek MS, Glatzle J, Temeltcheva T, Mueller MH, Koenigsrainer A, Kreis ME (2007) Long-term quality of life in patients with Crohn’s disease and perianal fistulas: influence of fecal diversion. Dis Colon rectum 50(12):2067–2074 15
Gooszen AW, Geelkerken RH, Hermans J, Lagaay MB, Gooszen HG (2000) Quality of life with a temporary stoma: ileostomy vs. colostomy. Dis Colon rectum 43(5):650–655
Nugent KP, Daniels P, Stewart B, Patankar R, Johnson CD (1999) Quality of life in stoma patients. Dis Colon rectum 42(12):1569–1574
Edwards CM, George BD, Jewell DP, Warren BF, Mortensen NJ, Kettlewell MG (2000) Role of a defunctioning stoma in the management of large bowel Crohn’s disease. Br J Surg 87(8):1063–1066
Glotzer DJ, Stone PA, Patterson JF (1967) Prognosis after surgical treatment of granulomatous colitis. N Engl J Med 277(6):273–279
Korelitz BI, Present DH, Alpert LI, Marshak RH, Janowitz HD (1972) Recurrent regional ileitis after ileostomy and colectomy for granulomatous colitis. N Engl J Med 287(3):110–115
Ritchie JK (1990) The results of surgery for large bowel Crohn’s disease. Ann R Coll Surg Engl 72(3):155–157
Vender RJ, Rickert RR, Spiro HM (1979) The outlook after total colectomy in patients with Crohn’s colitis and ulcerative colitis. J Clin Gastroenterol 1(3):209–217
Goligher JC (1985) The long-term results of excisional surgery for primary and recurrent Crohn’s disease of the large intestine. Dis Colon rectum 28(1):51–55
Scammell BE, Andrews H, Allan RN, Alexander-Williams J, Keighley MR (1987) Results of proctocolectomy for Crohn’s disease. Br J Surg 74(8):671–674
Yamamoto T, Allan RN, Keighley MR (2000) Audit of single-stage proctocolectomy for Crohn’s disease: postoperative complications and recurrence. Dis Colon rectum 43(2):249–256 16
Bernell O, Lapidus A, Hellers G (2001) Recurrence after colectomy in Crohn’s colitis. Dis Colon rectum 44(5):647–654
Amiot A, Gornet JM, Baudry C, Munoz-Bongrand N, Auger M, Simon M et al (2011) Crohn’s disease recurrence after total proctocolectomy with definitive ileostomy. Dig Liver Dis 43(9):698–702
Steinberg DM, Allan RN, Thompson H, Brooke BN, Alexander-Williams J, Cooke WT (1974) Excisional surgery with ileostomy for Crohn’s colitis with particular reference to factors affecting recurrence. Gut 15(11):845–851
Ho I, Greenstein AJ, Bodian CA, Janowitz HD (1995) Recurrence of Crohn’s disease in end ileostomies. Inflamm Bowel Dis 1(3):173–178
Ecker KW, Gierend M, Kreissler-Haag D, Feifel G (2001) Reoperations at the ileostomy in Crohn’s disease reflect inflammatory activity rather than surgical stoma complications alone. Int J Color Dis 16(2):76–80
Fichera A, McCormack R, Rubin MA, Hurst RD, Michelassi F (2005) Long-term outcome of surgically treated Crohn’s colitis: a prospective study. Dis Colon rectum 48(5):963–969
Satsangi J, Silverberg MS, Vermeire S, Colombel JF (2006) The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut 55(6):749–753
Beaugerie L, Seksik P, Nion-Larmurier I, Gendre JP, Cosnes J (2006) Predictors of Crohn’s disease. Gastroenterology 130(3):650–656
Bernell O, Lapidus A, Hellers G (2000) Risk factors for surgery and postoperative recurrence in Crohn’s disease. Ann Surg 231(1):38–45 17
Regueiro M, Schraut W, Baidoo L, Kip KE, Sepulveda AR, Pesci M et al (2009) Infliximab prevents Crohn’s disease recurrence after ileal resection. Gastroenterology 136(2):441–450
Sorrentino D, Paviotti A, Terrosu G, Avellini C, Geraci M, Zarifi D (2010) Low-dose maintenance therapy with infliximab prevents postsurgical recurrence of Crohn’s disease. Clin Gastroenterol Hepatol 8(7):591–599
Sorrentino D (2013) State-of-the-art medical prevention of postoperative recurrence of Crohn’s disease. Nat Rev Gastroenterol Hepatol 10(7):413–422
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Koriche, D., Gower-Rousseau, C., Chater, C. et al. Post-operative recurrence of Crohn’s disease after definitive stoma: an underestimated risk. Int J Colorectal Dis 32, 453–458 (2017). https://doi.org/10.1007/s00384-016-2707-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-016-2707-2