Abstract
Background and Aims
Ulcerative colitis (UC) patients with progression of their disease despite optimized medical therapy may warrant “curative” proctocolectomy with end ileostomy or ileo-anal pouch (IPAA) anastomosis. The aim of our study was to assess the incidence of later recurrent ileitis that lead to altering the initial diagnosis to Crohn’s disease (CD).
Methods
A retrospective analysis was conducted on the inflammatory bowel disease database at Lenox Hill Hospital. The database consisted of patients that were diagnosed with UC or CD based on clinical assessment, endoscopic appearance, gross and histological examination, and imaging between 1960 and 2015. The post-colectomy follow-up period was at least 10 years. Recurrent disease was classified by evidence of transmural inflammation in the distal ileum, fistulizing disease, or stricturing disease.
Results
From our IBD database, we identified 128 patients who underwent elective or urgent colectomy with the preoperative diagnosis of UC. Thirty-two (25%) had either an IPAA or end ileostomy with documented recurrence of inflammation in the small bowel mucosa consistent with CD. There was no significant difference between the type of surgical approach and the chance of recurrent disease (p = .20). The average time to clinically significant recurrence was 5 years.
Conclusion
The incidence of recurrent CD following colectomy for ulcerative colitis, when followed postoperatively for an average of 20 years, was 25%, considerably more than previously reported. Patients who come to colectomy for ulcerative colitis and are followed for at least 10 years show a high incidence of recurrent Crohn’s disease in the ileostomy or ileo-anal pouch. Extended follow-up should be included in patients coming to colectomy for ulcerative colitis before they should be considered cured of their disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ulcerative colitis (UC) is a chronic inflammatory bowel disease (IBD) characterized by confluent mucosal ulceration of the colon from the rectum to various extents proximally producing bloody diarrhea and cramping abdominal pain. The disease is of uncertain etiology, resulting from interaction of environmental, genetic, immunological and microbial factors and is characterized by periods of quiescence and flares. In 2012, the estimated prevalence of UC in the USA using insurance information was 790,000 cases [1]. Patients with progression of disease course despite optimized medical therapy may warrant “curative” proctocolectomy with either ileo-anal pouch anastomosis (IPAA) or end ileostomy [2]. Of patients with colitis, 7% will be labeled as indeterminate colitis at diagnosis due to having some characteristics of both UC and Crohn’s disease (CD) [3].
Up until the last 15 years, approximately 25% of patients diagnosed with UC eventually underwent colectomy. The indications for colectomy varied from urgent (perforation, toxic megacolon and uncontrolled bleeding) to elective (persistent disease or its complications not responding to medical management). The percentage of patients currently requiring colectomy has decreased due to the advent of immunosuppressive drugs and then biological therapies [2].
Since its introduction in 1978, total proctocolectomy with IPAA has been the definitive operation of choice for patients with confirmed ulcerative colitis (UC) [4]. Prior to 1978, subtotal colectomy combined with end ileostomy, followed by abdomino-perineal resection in some cases, was the most common surgical approach. Of patients diagnosed with UC preoperatively, 2 to 8% have at some point developed recurrent inflammation in the ileum, and then the diagnosis has been changed to Crohn’s disease [3]. Recurrence of disease as small bowel inflammation, stricturing disease, or fistulizing disease has been documented with variable incidence complicating both surgical approaches. The incidence of these recurrences is not well documented largely because long-term follow-up data are lacking for patients initially considered to have UC.
Recurrent CD after IPAA has been described as: (1) debilitating inflammation of the pouch resistant to antibiotic management, (2) stricturing or inflammation of the afferent limb and/or more proximal small bowel, and/or (3) fistulizing disease involving the perineum, the small bowel, or the pouch itself [5,6,7].
While surgical treatment has been considered “curative” in patients with UC, patients with CD who proceed to surgery will have a 10% risk of an additional operation in the first postoperative year and a 70% chance of a surgery in the ensuing 10 years [8]. Follow-up in the UC population is scarce due to the prevalent conclusion that the patient is cured following total proctocolectomy.
The aim of our study was to assess the incidence of postoperative recurrence in UC patients that undergo subtotal colectomy that lead to altering the initial diagnosis to CD.
Methods
This study and the consent procedure were approved by the Institutional Review Board of Northwell Health Systems.
Data Set
A retrospective analysis was conducted on medical records from our inflammatory bowel disease database of over 3000 IBD patients collected over a 55-year period at Lenox Hill Hospital (IBDLH) in which 128 patients diagnosed with UC came to colectomy and were followed for at least 10 years. These records contain data that include time of symptom onset, date of initial diagnosis, severity, therapeutic history, indications for surgery, radiologic and endoscopic procedures, time and nature of surgical procedures and pathologic results. The diagnoses of UC and CD were based on clinical history, physical examination, and endoscopic, histological, and radiologic findings. Ileocolonoscopy once available was performed in all cases, and ileoscopy or pouch endoscopy was performed in patients following the occurrence of postoperative symptoms or other suggestions of recurrence. All data were collected between January 1960 and June 2015 (a 55-year period).
Data included demographic information (age, gender, and ethnicity), IBD subtype (before and after surgery), and symptoms. The nature of symptoms and severity was scored by physician global assessment (PGA) and defined as quiescent, mild, moderate, and severe. Medical therapies, including immunosuppressives, biologics (infliximab or adalimumab), intravenous or oral corticosteroids (CS), and aminosalicylates (5-ASA), were recorded at each visit until operative intervention. The types of operation including ileocolectomy, subtotal (STC) and total colectomy, abdomino-perineal resection subsequent to STC, end ileostomy, and IPAA were recorded for each patient. Any endoscopic findings and pathology were recorded throughout the postoperative period and subsequent follow-up.
Eligible subjects included all patients with isolated colonic involvement consistent with UC who had undergone colectomy for either emergency or elective indication. Included patients had to have a follow-up period of at least 10 years from the operation. Recurrent disease was classified as: (1) development of inflammation and some evidence of transmural inflammation in the neo-terminal ileum, (2) fistulizing disease, (3) stricturing disease, and (4) pouchitis with (a) granulomas on pathology and (b) radiological evidence of disease extending to the afferent loop [6].
Statistical Methods
Data were analyzed using Excel software. The incidence of altered diagnosis (from the pre-op diagnosis) within the colectomy population was assessed. Statistics were calculated from the overall data set and the postoperative follow-up that met the criteria for changing diagnosis from UC to CD. Subgroup analysis was then performed within the IBD group. Patients were divided into those in whom the diagnosis was changed from UC to CD and those which remained UC by using the appropriate International Classification of Disease, 10th Revision codes at diagnosis [9].
Results
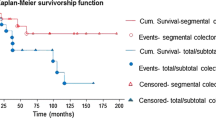
From the IBDLH database, we identified 128 patients who underwent elective or urgent colectomy with the preoperative diagnosis of UC between 1960 and 2013. Overall 99 (77.3%) patients underwent elective colectomy, mainly due to progression of disease despite optimal therapy, and 29 (22.6%) underwent emergency colectomy due to perforation (9), toxic megacolon (7), or uncontrollable bleeding (13). Thirty-two (25%) patients that underwent colectomy with either end ileostomy or IPAA later had documented recurrence of inflammation in the ileostomy or IPAA with characteristics of CD (Fig. 1).
Of the 32 patients with recurrent CD, 16 (50%) had an end ileostomy, and 16 (50%) had an IPAA (Fig. 1). There was no significant difference between the type of surgical approach and the chance of recurrent disease (p = .20). The criteria for altering diagnosis in the end ileostomy and IPAA patients are shown in Table 1. Reference images of endoscopic manifestations are shown in Table 2. Of the 16 ileostomy patients, eight (50%) manifested the recurrent CD as ileitis with linear deep ulcerations, six (38%) with fistulizing Crohn’s disease, and two (12%) with stricturing disease. In the IPAA group, four (25%) had stricturing disease proximal to the pouch, seven (43%) had deep linear ulcerations in the efferent loop of the proximal small bowel supportive of transmural inflammation, and five (31%) had perineal fistulae developing more than 2 years postoperative.
The average time to clinically significant recurrence, defined as recurrent clinical disease that meets criteria for Crohn’s disease, was 5 years. One exception had manifestations of a clinical flare 16 years post-colectomy. This patient with an IPAA developed small bowel inflammation with linear deep ulcerations at that time. The time of follow-up to time of recurrence is shown in Fig. 2. The average follow-up of all 128 patients was 20 years.
Discussion
This study serves to document the incidence of disease recurrence or change in diagnosis to CD in 25% of patients who underwent colectomy with either end ileostomy or IPAA formation with the preoperative diagnosis of ulcerative colitis after a long follow-up period averaging 20 years. This study has demonstrated that the incidence of recurrent Crohn’s disease in patients that had undergone a curative surgical approach may be far higher than the 8% previously reported [8].
Surgery has been considered the only curative option in the treatment of ulcerative colitis. Advances in medical management have served to decrease the need for surgery, and only 5–7% of UC patients still require surgery [10]. Due to the UC surgical curative expectation, patient follow-up is sparse in this population. On the other hand, 80% of patients with Crohn’s disease will need surgery in their lifetime and have a 70% chance of reoperation in the next 10 years [11]. CD patients are thus followed closely and monitored for recurrence, while the UC post-colectomy patients are not. The data in this paper suggest that patients initially diagnosed with UC should undergo follow-up for the first 10 years postoperatively since greater than 90% of patients in whom the diagnosis was changed to CD occurred within that period.
Increasing utilization of video capsule endoscopy (VCE) and magnetic resonance enterography (MRE) in the inflammatory bowel disease population over the past decade has increased our ability to diagnose CD by identifying subtle inflammatory changes in the small bowel [12, 13]. Most of our colitis population that developed disease recurrence was initially labeled as UC prior to 1999. Had these emerging technologies been available perhaps the diagnosis of CD may have been made earlier. Furthermore, specific emerging serological testing used postoperatively whether for the purpose of recognizing residual inflammation or recurrent inflammation may help to identify the change in diagnosis sooner. With new innovations, there is more opportunity for the small bowel to be evaluated prior to settling on the differential diagnosis of CD versus UC [12,13,14]. With the advent of biologic therapy and as more non-operative options become available, the application rate of colectomy for non-acute severe UC has been reduced significantly from 10–15% to 5–7% at 5 years [15].
The senior author of this study and other gastroenterologists who have had a special interest in inflammatory bowel disease have long attempted to define the relationship between ulcerative colitis and Crohn’s disease. An early study revealed that what seemed to be UC with initial sparing of the rectum often proved to be CD [16]. In response to two studies that concluded that CD limited to the colon was cured by ileostomy and colectomy [17, 18], our later follow-up showed that recurrent CD in the ileostomy was almost as prevalent as with an anastomosis [19]. Recently, it has been reported that there is a significant prevalence of non-granulomatous ileitis in patients with mild ulcerative colitis who do not come to surgery, considered to be an extra-intestinal manifestation of UC [20], but given our experience in this study, we raise the possibility of many of these cases representing CD [21].
One of the strengths of our study was the 55-year extended longitudinal follow-up, which allowed us to elucidate the true disease course for post-colectomy UC patients. With this decrease in surgical intervention, UC patients should be followed longitudinally for a longer period of time allowing for greater follow-up and proper classification of the exact disease process.
This study has limitations related to the nature of its retrospective design, as well as any data collection errors inherent in database registries. Another limitation is that most of the patients surveyed underwent colectomy prior to the biologic era; however, this limitation enables us to comment on the overall decreasing need for surgery in the new treatment era as well as the time factor enhancing the conversion of diagnosis from UC to CD.
Conclusion
Our retrospective cohort study has demonstrated that there is a significantly higher incidence of the mistaken diagnosis of ulcerative colitis rather than Crohn’s disease in patients that have progressed to colectomy than previously reported. We conclude that even though UC patients may be considered cured after resection, follow-up may enable the unmasking of recurrent CD and successful medical management once recognized. Prolonged observation suggests that time should be a factor in aiding the differential diagnosis of Crohn’s disease from ulcerative colitis.
This study is the longest survey and follow-up of ulcerative colitis patients that have undergone colectomy with a search for later conversion of diagnosis to Crohn’s disease; therefore, the factor of time and long period of observation should be added to location, complications, imaging, and pathology in the evaluation of the nature and type of IBD.
Key Messages
Current Knowledge
-
Colectomy has been considered “curative” for ulcerative colitis.
-
Accordingly, extended follow-up has usually not been recommended after colonic resection for ulcerative colitis.
New
-
From a database at Lenox Hill Hospital, long term follow-up of post-colectomy ulcerative colitis patients has revealed recurrent ileitis in the ileostomy or in the ileo-anal pouch in 25% of patients when followed for at least 10 years.
-
These findings warrant extended follow-up following colectomy for ulcerative colitis.
-
Anatomical alterations of ileostomy and ileo-anal pouch anastomosis may predispose to the development of Crohn’s disease or unmask the diagnosis of Crohn’s disease previously unrecognized at the time of colectomy.
References
Kappelman MD, Moore KR, Allen JK, Cook SF. Recent trends in the prevalence of Crohn’s disease and ulcerative colitis in a commercially insured US population. Dig Dis Sci. 2013;58:519–525. https://doi.org/10.1007/s10620-012-2371-5.
Samuel S, Ingle SB, Dhillon S, et al. Cumulative incidence and risk factors for hospitalization and surgery in a population-based cohort of ulcerative colitis. Inflamm Bowel Dis. 2013;19:1858–1866. https://doi.org/10.1097/MIB.0b013e31828c84c5.
Geboes K, Colombel JF, Greenstein A, et al. Indeterminate colitis: a review of the concept—what’s in a name? Inflamm Bowel Dis. 2008;14:850–857. https://doi.org/10.1002/ibd.20361.
Parks AG, Nicholls RJ. Proctocolectomy without ileostomy for ulcerative colitis. Br Med J. 1978;2:85–88.
Keighley MR. The final diagnosis in pouch patients for presumed ulcerative colitis may change to Crohn’s disease: patients should be warned of the consequences. Acta Chir Iugosl. 2000;47:27–31.
Deutsch AA, McLeod RS, Cullen J, et al. Results of the pelvic-pouch procedure in patients with Crohn’s disease. Dis Colon Rectum. 1991;34:475–477.
Shen B. Crohn’s disease of the ileal pouch: reality, diagnosis and management. Inflamm Bowel Dis. 2009;15:284–294. https://doi.org/10.1002/ibd.20661.Review.
Rutgeerts P. Protagonist: Crohn’s disease recurrence can be prevented after ileal resection. Gut. 2002;51:152–153.
ICD-10 codes retrieved from https://www.cms.gov/medicare/Coding/ICD10/index.html
Chardavoyne R, Flint GW, Pollack S, et al. Factors affecting recurrence following resection for Crohn’s disease. Dis Colon Rectum. 1986;29:495–502.
Fornario R, Caratto E, Caratto M, et al. Post-operative recurrence in Crohn’s disease. Critical analysis of potential risk factors: an update. Surgeon. 2015;13:330–347. https://doi.org/10.1016/j.surge.2015.04.002.
Leighton JA, Helper DJ, Grainek IM, et al. Comparing diagnostic yield of a novel pan-enteric video capsule endoscope with ileoclonoscopy in patients with active Crohn’s disease: a feasibility study. Gastrointest Endosc. 2017;85:196–205. https://doi.org/10.1016/j.gie.2016.09.009. (Epub 08/19/2016).
Jensen MD, Nathan T, Rafaelsen SR, et al. Diagnostic accuracy of capsule endoscopy for small bowel Crohn’s disease is superior to that of MR enterography or CT enterography. Clin Gastroenterol Hepatol. 2011;9:124–129. https://doi.org/10.1016/j.cgh.2010.10.019.
Kopylov U, Yablecovitch D, Lahat A, et al. Detection of small bowel mucosal healing and deep remission in patients with known small bowel Crohn’s disease using biomarkers, capsule endoscopy, and imaging. Am J Gastroenterol. 2015;110:1316–1323. https://doi.org/10.1038/ajg.2015.221.
De Cruz P, Kamm M, Prideaux L, et al. Post-operative recurrent luminal Crohn’s disease: a systematic review. Inflamm Bowel Dis. 2012;18:758–777.
Korelitz BI. Clinical course, late results and pathological nature of inflammatory bowel disease of the colon initially sparing the rectum. Gut. 1967;8:281–290.
Glotzer DJ, Gardner RC, Goldman H, et al. Comparative features and course of ulcerative and granulomatous colitis. N Engl J Med. 1970;282:582–587.
Nugent FW, Veidenheimer MC, Meissner WA, Haggitt RC. Prognosis after colonic resection in Crohn’s Disease of the colon. Gastroenterology. 1973;65:398–402.
Korelitz BI, Present DH, Alpert LI, et al. Recurrent regional ileitis after ileostomy and colectomy for granulomatous colitis. N Engl J Med. 1972;287:110–115. https://doi.org/10.1056/NEJM197207202870302.
Hamilton MJ, Makrauer FM, Golden K, et al. Prospective evaluation of terminal ileitis in a surveillance population of patients with ulcerative colitis. Inflamm Bowel Dis. 2016;22:2448–2455. https://doi.org/10.1097/MIB.0000000000000911.
Korelitz BI, Shamah S. The ileitis of ulcerative colitis: Why is it not Crohn’s disease? Inflamm Bowel Dis. 2017;23:e7. https://doi.org/10.1097/MIB.0000000000001006.
Acknowledgments
This research in inflammatory bowel disease is supported by the New York Crohn’s Foundation.
Author information
Authors and Affiliations
Contributions
SS—senior fellow in gastroenterology—has been the primary researcher who analyzed the data and assimilated the results. JS is our database research coordinator who provided Dr. Shamah and Dr. Korelitz with the cases who fulfilled the requirements for entry. BIK is the senior investigator with a long experience in the research of inflammatory bowel disease who provided the concept, the database, and the interpretation of the data.
Corresponding author
Ethics declarations
Conflict of interest
None of the above authors have financial disclosures or conflicts of interest.
Institutional review board
This study was reviewed and approved by the Northwell Health Systems Institutional Review Board.
Informed consent
All study participants or their legal guardian, provided informed written consent prior to study enrollment.
Rights and permissions
About this article
Cite this article
Shamah, S., Schneider, J. & Korelitz, B.I. High Incidence of Recurrent Crohn’s Disease Following Colectomy for Ulcerative Colitis Revealed with Long Follow-Up. Dig Dis Sci 63, 446–451 (2018). https://doi.org/10.1007/s10620-017-4873-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-017-4873-7