Abstract
Purpose
The aim of this study is to compare surgical parameters and the costs of robotic surgery with those of laparoscopic approach in rectal cancer based on a single surgeon’s early robotic experience.
Methods
Data from 25 laparoscopic (LapTME) and the first 50 robotic (RobTME) rectal resections performed at our institution by an experienced laparoscopic surgeon (>100 procedures) between 2009 and 2014 were retrospectively analyzed and compared. Patient demographic, procedure, and outcome data were gathered. Costs of the two procedures were collected, differentiated into fixed and variable costs, and analyzed against the robotic learning curve according to the cumulative sum (CUSUM) method.
Results
Based on CUSUM analysis, RobTME group was divided into three phases (Rob1: 1–19; Rob2: 20–40; Rob3: 41–50). Overall median operative time (OT) was significantly lower in LapTME than in RobTME (270 vs 312.5 min, p = 0.006). A statistically significant change in OT by phase of robotic experience was detected in the RobTME group (p = 0.010). Overall mean costs associated with LapTME procedures were significantly lower than with RobTME (p < 0.001). Statistically significant reductions in variable and overall costs were found between robotic phases (p < 0.009 for both). With fixed costs excluded, the difference between laparoscopic and Rob3 was no longer statistically significant.
Conclusions
Our results suggest a significant optimization of robotic rectal surgery’s costs with experience. Efforts to reduce the dominant fixed cost are recommended to maintain the sustainability of the system and benefit from the technical advantages offered by the robot.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Over the last 30 years, we have witnessed a progressive improvement of colorectal disease treatment with the application of total mesorectal excision (TME), neoadjuvant therapies, and minimally invasive techniques. Nevertheless, rectal resection for cancer remains a challenging procedure, especially when using a minimally invasive approach. Conventional laparoscopy has been shown to have several advantages over open surgery for the treatment of colorectal cancer, particularly in terms of early postoperative outcomes [1–4]. However, unlike colon cancer, there is relatively limited evidence regarding the safety and efficacy of laparoscopic surgery for rectal cancer, mainly because of the intrinsic drawbacks of laparoscopic instruments used during rectal dissection in the confined space of the pelvis [5]. The critical issues include high rates of conversion to open surgery and circumferential resection margin (CRM) involvement of the specimen [1]. The recently published COLOR II study confirmed that these issues remain regardless of the experience and learning curve of the operator [6]. Moreover, the laparoscopic approach failed to show a clear improvement in the early postoperative outcomes over time, partly because of the inherently high morbidity associated with rectal surgery and partly because of the limitations of current laparoscopic instruments [7]. The introduction of robotic systems in surgery was intended to overcome the known limitations of conventional laparoscopic surgery, while preserving the advantages of the minimally invasive surgery [8, 9]. This result may be attributed to the robot’s wristed instrument ability to overcome the fulcrum effect created by the trocars, maximizing the workspace when applied in narrow anatomical space such as the pelvis [10, 11]. This ability justifies the growing interest in robotic technology for rectal surgery worldwide. Since the first prospective randomized trial comparing robotic and laparoscopic low anterior resection performed by Baik et al. in 2006 [8], several studies have been published that have focused primarily on feasibility and safety. These studies have reported, with broad accord, an increased rate of uninvolved CRM [12, 13] and a lower rate of conversion to laparotomy with robotic surgery [14–16], especially for subgroups of patients with unfavorable anatomy. However, the costs of robotic surgery represent a critical issue for its widespread use. Increased costs associated with robotic surgery are mainly because of high purchase and maintenance costs for the robot, surgical consumables, and—to a lesser extent—the longer times required in the operating room. To date, only a few studies have reported a cost analysis on this approach for treatment of rectal cancer, with poor level of evidence.
The aim of this study is to compare the surgical parameters and costs of robotic surgery with those of the laparoscopic approach in rectal cancer and to evaluate the effect of the learning curve on these parameters. All analyzed procedures were performed by a single surgeon with laparoscopic experience (>500 laparoscopic procedures performed).
Materials and methods
Patients and data
This retrospective study was based on prospectively collected surgical data from 75 patients who underwent minimally invasive rectal resection with TME performed by a single surgeon from January 2009 to December 2014 at our institution. The first consecutive 50 robotic rectal resections (RobTME) were compared with 25 laparoscopic rectal resections (LapTME) performed within the same period by the same experienced laparoscopic surgeon (>100 procedures). All robotic operations were performed with the da Vinci Surgical System Si (Intuitive Surgical, Sunnyvale, CA, USA). The study was approved by the Institutional Review Board of our hospital.
The preoperative workup included colonoscopy with biopsy, abdominal and transrectal ultrasonography, chest radiography, abdomen and pelvic CT scan, and/or magnetic resonance imaging. Patients with clinical stage I cancer were referred for prompt surgical treatment. Patients with T3 or N-positive cancer received neoadjuvant chemoradiation followed by surgical resection within 8 weeks. T4 lesions were operated upon through an open approach and excluded from the current series.
Obesity (BMI >30) or previous abdominal or pelvic surgical procedures were not considered contraindications for a minimally invasive approach. An anterior resection of the rectum (ARR) was proposed for lesions whose caudal margin was located at least 3 cm above the dentate line, whereas intersphincteric resection (ISR) with coloanal anastomosis was considered for lesions located between 3 and 0.5 cm above the dentate line. Abdominoperineal resection (APR) was indicated for lesions invading the dental line only. A diverting ileostomy was performed in all low ARRs.
Operative technique
For LapTME, patients were placed in a modified lithotomy position with a 30° Trendelenburg and tilted to the right side. After induction of general anesthesia, the pneumoperitoneum was established. The first 11-mm trocar was placed above the navel and used at the beginning as a 30° 10-mm telescope. The second 11-mm trocar was placed in the right lower quadrant and used for the camera during the entire operation, and a 12-mm trocar was placed in the right iliac fossa/suprapubic area. A 5-mm trocar was placed in the left quadrant and used by an assistant to expose the structures as well as for splenic flexure mobilization. Dissection and coagulation were always performed using Ultracision® Harmonic Scalpel (Ethicon Endo-Surgery Inc., Cincinnati, OH, USA).
The RobTME procedures were carried out using the four-arm da Vinci Surgical System Si (Intuitive Surgical, Sunnyvale, CA, USA). We used both the hybrid and the single docking full robotic technique depending on tumor and patient characteristics. Patients were placed in a modified lithotomy position with a 30° Trendelenburg and tilted to the right side. In the hybrid technique, the first part of the operation (inferior mesenteric vessel ligation, left colon and splenic flexure mobilization) was carried out laparoscopically. An 8-mm robotic trocar replaced the 5-mm one in the left flank, and a 12-mm trocar for assistant use was placed in the right flank. For the robotic step of the intervention (which is specifically the TME), the cart was positioned to the patient’s left side along the imaginary line between the anterior superior iliac spine and the umbilical scar at a 60° angle. In this phase, the surgeon used the two 8-mm robotic trocars and the 12-mm suprapubic trocar by inserting the 8-mm trocar into the 12-mm one (trocar in trocar). This avoids the need to introduce another robotic trocar and maintains the possibility of introducing an articulated stapler for the rectal resection. In the full robotic technique, the robotic cart had the same position, and the full operation was carried out robotically with the single docking technique [17]. A 12-mm optical trocar for the camera was inserted 3 cm right lateral to the umbilicus. Two 8-mm trocars for the robotic arms were placed under direct vision, at the point of intersection between the midclavicular line and the line between the umbilicus and the superior iliac spine, one in the left side and one in the right side. A third robotic trocar was inserted 5 cm below the xiphoid process on the right side of the falciform ligament. Another 12-mm trocar was inserted into the right flank for the use of the assistant surgeon. For robotic dissection and coagulation, we used the Ultracision® Harmonic Scalpel, robotic monopolar curved scissors and/or cautery hook in the right hand, and the bipolar fenestrated grasp in the left hand. Dissection was carried out in the same way in both the robotic-assisted and the pure laparoscopic procedure; we always used the medial-to-lateral approach. Dissection of the peritoneum was initiated at the level of the sacral promontory and directed cranially to the origin of the inferior mesenteric artery and vein. The inferior mesenteric vessels were divided between Weck® Hem-o-lok® Ligation System clips (Teleflex, Morrisville, NC, USA). Then, the left colon was mobilized along the Toldt fascia and the splenic flexure was mobilized using a lateral-to-medial approach. The mesorectal dissection is initiated at the level of the sacral promontory where the cleavage plane between the presacral fascia and the visceral layer of the mesorectum was identified. The hypogastric nerves, the autonomic branches of the sacral nerves, and the pelvic autonomic nerve plexus were identified and preserved. The dissection continued anteriorly and then laterally down to levator ani plane. The rectum was divided with the Echelon Flex™ 60-mm stapler (Ethicon, Cincinnati, OH, USA). The colon resection was completed through a Pfannenstiel suprapubic minilaparotomy. The anastomosis was performed with a CDH29 circular stapler (Ethicon, Cincinnati, OH, USA) inserted transanally, according to the Knight–Griffin technique [18]. In the case of ISR, the dissection continued until the intersphincteric space, and then the left mesocolon is prepared intracorporeally to arrive up to the pelvic floor. Then, the operation was completed with a perineal phase in which we performed an incision at the dentate line to join with the intersphincteric plane, the specimen was extracted, and the anastomosis was performed transanally by hand. In the case of APR, we did not mobilize the splenic flexure and the peritoneum was always closed after the pelvic phase. Conversion to hand-assisted laparoscopy (HALS) was always considered, as an intermediate step, before proceeding with open surgery when a conversion was necessary. In this case, we performed a supraumbilical 8-cm incision to insert the laparoscopic device, and we used the other trocars to insert the camera and the right-hand instruments.
Surgical parameters and costs
Patient demographics, the American Society of Anesthesiologists (ASA) scores, body mass index (BMI), neoadjuvant treatment, and distance of the tumor from the anal verge were the demographic variables analyzed. The perioperative results were operative time (OT), blood transfusions, and conversion to open or hand-assisted laparoscopic surgery. Postoperative data included length of stay and postoperative complication according to the Clavien–Dindo classification [19]. Cost data were obtained from the accounting department of the hospital and were divided into fixed and variable items. The fixed costs included amortized costs of the robot and laparoscopic instruments, purchasing the robotic system, and the amortized cost of robot maintenance. Variable cost items were those related to disposable instruments, operating room personnel, and length of stay. All costs were expressed in euros and referred to the most recent available year.
All data regarding clinical and surgical parameters and postoperative course were prospectively collected in a dedicated database. Economic data were collected retrospectively from a health technology assessment (HTA) report of our hospital.
For the purpose of this study, we compared the parameters and costs between the two groups (LapTME and RobTME). Furthermore, to compare different phases of these first 50 robotic cases with the experienced phase of the LapTME group, we stratified patients in the RobTME according to surgeon experience, evaluating the learning curve using the cumulative sum (CUSUM) method.
The CUSUM method [20] was used to analyze the learning curve of robotic surgery considering OT as an indicator of the learning process. This method comprises running the total of the differences between the individual data points and the mean of all data points. First, the cases were ordered chronologically, from the earliest to the latest date of surgery. The value of CUSUMoperative time (CUSUMOT) of the first case was then obtained as the difference between the OT for the first case and the mean OT for all the cases (mOT). The CUSUMOT of the second case was the CUSUMOT of the previous case added to the difference between the OT of the second case and mOT. The same procedure was repeated for each of the patients except for the last one, which was calculated as 0.
Statistical analysis
Categorical variables were reported as number of cases and percentage, while continuous variables were expressed as mean ± (standard deviation) or median [25–75 percentile], depending on their distribution. The chi-square test and Fisher test were used to compare the distribution of categorical variables. For continuous variables, paired comparisons were made using an independent t test or Mann–Whitney test, while multiple comparisons were performed by means of an analysis of variance or Kruskal–Wallis test. The Bonferroni and Mann–Whitney test with Bonferroni correction were considered for post hoc test.
Patient characteristics and perioperative data among RobTME subgroups identified through the CUSUM analysis were compared.
Generalized linear models were used to estimate costs associated with the different surgical techniques and phases, adjusting for clinical characteristics and operative parameters. Variables with a p value <0.10 during univariate analysis were included in the multivariable analysis. A p value <0.05 was considered statistically significant. All analyses were performed using R v3.0.2 (R, Vienna, Austria) and Stata version 12 (StataCorp, College Station, TX, USA).
Results
Demographic characteristics and preoperative conditions are summarized in Table 1. We did not detect significant differences in clinical features between the two whole groups (LapTME vs RobTME), except for the median distance from the dentate line: 6 [5–8] cm for LapTME patients and 4 [1–5] cm for the RobTME group (p = 0.006). Nine patients (36 %) in the LapTME group and 23 patients (46 %) in the RobTME group underwent surgery after neoadjuvant chemoradiation. Of the 25 LapTME cases, 21 (84 %) were ARRs, 2 (8 %) were APRs, and 2 (8 %) were ISRs. In comparison, 32 of the 50 RobTME were ARRs (64 %), 7 APRs (14 %), and 11 ISRs (22 %). Operative data and postoperative course are summarized in Table 2. The mean OT in the LapTME group was significantly lower than in the RobTME group (270 vs 313 min, docking time included, respectively; p = 0.006). Conversion (to HALS) in the LapTME group was performed in eight patients vs one patient (to HALS and then to open surgery) in the RobTME group because of visceral obesity that made it impossible to obtain adequate exposure of the operative field (p < 0.001). In the RobTME group, 22 procedures (44 %) were performed using the hybrid technique; the remaining 28 (56 %) of procedures were performed with a full robotic technique. In the LapTME group, patients received blood transfusions in four cases (16 %), whereas this occurred in seven cases (14 %) in the RobTME group (p > 0.5). The mean length of hospital stay was 11.1 ± 5.9 days for the LapTME group vs 10.4 ± 4.7 days for the RobTME group (p > 0.5). Three patients in the LapTME group vs four patients in the RobTME group (p > 0.5) experienced transient small bowel obstruction, which resolved with insertion of a 24-F Foley catheter in the ileostomy. Four patients (16 %) in the LapTME group and six (12 %) in the RobTME group, all with diverting ileostomy, had anastomotic leakages or pelvic abscesses, which were treated conservatively. One patient that developed a pelvic abscess after RobTME required surgical treatment. One patient and two patients in the LapTME and RobTME groups, respectively, developed wound infections that resolved with medical treatment. Medical complications were observed in five patients in the LapTME group (two cases of grade II and three cases of grade III according to the Clavien–Dindo classification) vs four in the RobTME group (three cases of grade III and one case of grade V according to the Clavien–Dindo classification) (p > 0.5). One patient in the LapTME group died because of cardiovascular complications compared with none in the RobTME group.
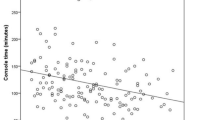
The evaluation of the RobTME group according to learning curve and based on the CUSUM analysis identified three phases: the Rob1 phase (cases 1–19), corresponding to the initial learning curve, the Rob2 phase (cases 20–40) which showed stabilization of OT, and the Rob3 phase (cases 21–50), representing the experienced phase (Figs. 1 and 2).
The three robotic phases were characterized by different median OTs (Rob1 325 [310–330] minutes, Rob2 290 [255–320] minutes, and Rob3 315 [299–330] minutes) with statistically significant changes across robotic experience levels (p = 0.010).
The comparison of patient characteristics and perioperative data by the CUSUM learning phases are summarized in Table 3. Age, ASA score, and BMI showed no significant differences among the learning phases. Although not statistically significant, we observed a higher percentage of distally located tumors in phase 3 compared with phase 1, leading to a greater number of ISRs among patients treated during phase 3 (5 [50 %] in phase 3 vs 2 [10 %] in phase 1). We also observed more patients in phase 3 who underwent preoperative chemoradiation than in phase 1 (six cases (30 %) vs six cases (60 %), respectively), and patients were more frequently male in phase 3 than in phase 1 (80 vs 50 %). Moreover, in phase 3 a hybrid approach was used in two cases only (20 %), while in phase 1 it was used in 11 cases (55 %). Even without significant differences between phase 1 and phase 3 for all these parameters, we can see a trend towards more complex operations after the learning curve phase.
The comparison of costs associated to the LapTME and RobTME groups is summarized in Table 4. Overall median costs associated with LapTME procedures were significant lower than with RobTME: 7620 euros and 12,284 euros, respectively (p < 0.001). Costs related to the hospital stay only were similar in the two groups (p = 0.910), while the cost of consumables and personnel were significantly higher (p < 0.001 and p = 0.006, respectively) for RobTME. These findings were also confirmed in multivariate regression analysis; when adjusting for age and neoadjuvant therapy, overall costs were significantly higher in the RobTME group (Table 5).
Costs were higher for Rob1 as compared with Rob3 (p < 0.009), with both univariate and multivariate analyses (Table 6 and Table 7). The reduction of OT with an increase of robotic experience is awarded with the reduction of fixed and variable costs. A statistically significant reduction in variable and overall costs was found among the different robotic phases (p < 0.009 for both). Furthermore, excluding fixed costs, the difference between LapTME and the last robotic phase (Rob3) is no longer significantly different (Table 8).
Discussion
Even though laparoscopy in colorectal surgery was first introduced in 1991 [21], this technique has not yet achieved wide application as a standard approach for patients with rectal cancer because of the technical complexity of laparoscopic TME, a steep learning curve, and a high conversion rate [22, 23]. Therefore, the advent of robotic system with its technological improvements has been welcomed by many surgeons as a way to increase the use of the minimally invasive approach in colorectal surgery and to improve postoperative outcomes, expanding the number of patients who could benefit from a minimally invasive approach. Effectively, studies published in literature suggest that the da Vinci Si system could contribute to reducing the conversion rate with respect to laparoscopy, obtaining good functional outcomes without compromising the oncologic outcomes [12, 15, 16, 24–27].
However, many authors criticize the use of robotic technology, because its advantages are not overwhelming when compared with the laparoscopic approach, and claim that the systems do not justify the cost of the robotic technology. In support of these criticisms, the few reports available in the literature regarding the costs of robotic surgery in rectal surgery almost invariably conclude that it is more time-consuming and more expensive compared with open or conventional laparoscopy [28–30].
The two main drawbacks of robotic surgery remain the longer OTs and costs. However, most of the published data included small robotic case series performed by surgeons inexperienced with the new technology, and the comparisons were made against a steady method such as laparoscopy, with laparoscopic rectal resection series, performed by surgeons out of the learning curve phase of this procedure. We think that such comparisons introduce an important bias that may influence both OT and costs and that the reported conclusions could be critically reviewed. In support of the above, some authors have recently observed a trend towards cost and OT reduction with experience in robotic technique [31] and a recent HTA evaluation demonstrated that the greater clinical benefits of the robotic technique might justify the higher costs [32].
For this reason, we decided to conduct a cost analysis of a single surgeon’s first 50 robotic rectal resections with the da Vinci Si, while also comparing different phases of learning curve of the robotic experience, with laparoscopic cases considered out of the learning curve and performed in the same period. Importantly, the specific experience and competence of the operating surgeon is an essential prerequisite for reducing OT and highlighting the utility of robotic assistance in rectal resection. Evidence is accumulating that the learning curve in this setting passes through several phases in which surgeons first gain robotic skills and then, with increasing familiarity with this technique, decreases in OT and hospital costs over time [33–36]. In our study, we decided to use the CUSUM method to analyze the learning curve for our single surgeon’s first 50 cases of robot-assisted rectal cancer resection. The main advantages of this method are its independence from sample size, its effectiveness in detecting small shifts in the system, and its ability to allow for a continuous analysis in time and rapid evaluation of data. Therefore, CUSUM learning curves are used as indicators of satisfactory outcome in relation to the acquisition of clinical skills [34]. In our study, and in good agreement with the other manuscripts, the learning curve of robotic surgery for rectal cancer was divided into three stages: the initial learning period (1st–19th case) in which there is a rapid decrease of OT, the competent period (20th–40th case) showing stabilization of OT, and finally the challenging period (40th–50th case) comprising the most difficult cases. The surgeons’ console times have commonly been regarded as a surrogate marker to reflect the learning curve, but after the first phase of training could reflect the technical difficulty of the specific case. In our study, we reported a different median OT for each of these three phases and a statistically significant reduction with robotic experience and primary technical competence in reducing the operation time achieved after the initial learning period. Focusing on phase 3, the surgeon dealt with more complex operations because he had acquired self-confidence in performing robotic rectal resection. In fact, as we have shown in Table 3, there were more cases that underwent ISR in the Rob3 period after the learning curve; ISR is a more demanding procedure than standard ARR. This challenging operation and difficult anatomy group were more frequently enrolled in phase 3, justifying the longer OT in this period. Moreover, in phase 3 compared with phase 1 we also noted higher rates of preoperative chemoradiation, more cases performed with a totally robotic approach, and a tendency towards lower incidence of complications, although none of these variables were statistically significant. Furthermore, while the hybrid approach was extensively used during the entire Rob1 phase, its use tended to be reduced in subsequent phases and was reserved only for cases such as obese, tall patients with difficult splenic flexure mobilization using the single docking robotic technique. However, despite the increased OT and the presence of difficult cases recruited in phase 3, we noted that costs were significantly higher in Rob1 compared with Rob3, at both univariate and multivariate analyses. These higher Rob1 costs may be associated with costs related to laparoscopic energy devices and laparoscopic instruments used in hybrid laparoscopic-robotic rectal resection, which was performed more frequently in Rob1. Excluding fixed cost, however, Rob3 costs were not statistically different from those of the laparoscopic group. Therefore, the reduction of OT with the increase of robotic experience is rewarded by the reduction of fixed and variable costs. Furthermore, in the Rob1 phase, the surgeon aimed at adapting his laparoscopic experience to the novel robotic technique; for this reason, the following instruments were used alternatively on the right robotic arm: the Ultracision® Harmonic Scalpel, the robotic monopolar curved scissors, and the cautery hook for dissection. Subsequently, the surgeon changed used only the monopolar curved scissors on the right robotic arm, and this may be associated with the cost reduction in the last phase of the learning curve. Even if the overall median costs associated to laparoscopic procedures were statistically significantly lower than robotic colorectal procedures, the costs related only to hospital stay were similar in the two groups throughout a similar postoperative course in both groups, while consumable costs were the principal cause of higher costs related to robotic colorectal surgery. Furthermore, the most important finding of our study was that, excluding fixed costs, the difference between LapTME and the last robotic phase (Rob3) was not statistically significant, suggesting a significant optimization of costs with robotic experience. In fact, direct hospital costs were significantly improved over time, and the reasons for this decrease in cost were likely multifactorial, but optimizations of instrument use and reduction in OT are likely two major contributors.
The main cost item, which is more difficult to reduce, is represented by the fixed costs of purchasing and maintaining the robotic system. Therefore, the amortization of fixed costs of the robot plays a crucial role in the dynamics of costs, and it can be achieved through the use of robots in high-volume and multidisciplinary robotic centers. In our series, we also reported a reduction of overall costs in the Rob3 phase with respect to the initial experience with robotic technology because of a better organization of the use of the da Vinci system. This organization is largely due to the creation of a multidisciplinary center of robotic surgery that has led to an intensive use of da Vinci. Our results support the idea that all the costs and OT data reported up to now may be affected by the initial experience of a few years of robotic colorectal surgery. Moreover, the relationship between surgeon experience and organizational issues needs to be considered as it strongly influences economic concerns and quality of care, as well as the overall planning of training and hospital staffing [37].
A further consideration is that, while the presented data refer to operations performed with the da Vinci Si, we have already published our preliminary surgical results on the use of the new Xi [38, 39], which suggest the potential of further improvements in OT, surgical results, and the ability to perform full robotic rectal resections; these findings also hint at further possible improvements in terms of cost reduction and sustainability.
In our experience, robotic rectal surgery is more expensive than conventional laparoscopic surgery. However, excluding the fixed costs and comparing the experienced phase of RobTME and LapTME, the variable costs are not statistically different. We reported also a reduction of overall costs with better organization and more intensive use of the da Vinci, suggesting a possible optimization of robotic surgery with experience. The reduction of costs with experience could be maximized by optimizing the depreciation of fixed costs within a high-volume and multidisciplinary center.
In conclusion, our results suggest a significant optimization of robotic rectal surgery’s costs with experience. Efforts to reduce the dominant fixed cost are recommended to maintain the sustainability of the system and benefit from the technical advantages offered by the robot.
References
Guillou PJ, Quirke P, Thorpe H, Walker J, Jayne DG, Smith AM, Heath RM, Brown JM, MRC CLASICC trial group (2005) Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomized controlled trial. Lancet 365:1718–1726
Buunen M, Veldkamp R, Hop WC, Kuhry E, Jeekel J, Haglind E, Påhlman L, Cuesta MA, Msika S, Morino M, Lacy A, Bonjer HJ (2009) Survival after laparoscopic surgery versus open surgery for colon cancer: long-term outcome of a randomized clinical trial. Lancet Oncol 10(1):44–52
Jayne DG, Guillou PJ, Thorpe H, Quirke P, Copeland J, Smith AM, Heath RM, Brown JM, UK MRC CLASICC Trial Group (2007) Randomized trial of laparoscopic-assisted resection of colorectal carcinoma: 3-year results of the UK MRC CLASICC Trial Group. J Clin Oncol 25(21):3061–3068
Clinical Outcome of Surgical Therapy Study Group (2004) A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med 350(20):2050–2059
Ishihara S, Otani K, Yasuda K, Nishikawa T, Tanaka J, Tanaka T, Kiyomatsu T, Hata K, Kawai K, Nozawa H, Kazama S, Yamaguchi H, Sunami E, Kitayama J, Watanabe T (2015) Recent advances in robotic surgery for rectal cancer. Int J Clin Oncol 20(4):633–640
Van der Pas MH, Haglind E, Cuesta MA, Fürst A, Lacy AM, Hop WC, Bonjer HJ, Colorectal cancer Laparoscopic or Open Resection II (COLOR II) Study Group (2013) Laparoscopic versus open surgery for rectal cancer (COLORII): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol 14(3):210–218
Shearer R, Gale M, Aly OE, Aly EH (2013) Have early postoperative complications from laparoscopic rectal cancer surgery improved over the past 20 years? Color Dis 15(10):1211–1226
Baik SH, Ko YT, Kang CM, Lee WJ, et al. (2008) Robotic tumor-specific mesorectal excision of rectal cancer: short-term outcome of a pilot randomized trial. Surg Endosc 22(7):1601–1608
Aly EH (2014) Robotic colorectal surgery: summery of the current evidence. Int J Color Dis 29:1–8
Szold A, Bergamaschi R, Broeders I, Dankelman J, Forgione A, Langø T, Melzer A, Mintz Y, Morales-Conde S, Rhodes M, Satava R, Tang CN, Vilallonga R, European Association of Endoscopic Surgeons (2015) European Association of Endoscopic Surgeons (EAES) consensus statement on the use of robotics in general surgery. Surg Endosc 29(2):253–288
Freschi C, Ferrari V, Melfi F, Ferrari M, Mosca F, Cuschieri A (2013) Technical review of the da Vinci surgical telemanipulator. Int J Med Robot 9(4):396–406
D’Annibale A, Pernazza G, Monsellato I, Pende V, Lucandri G, Mazzocchi P, Alfano G (2013) Total mesorectal excision: a comparison of oncological and functional outcomes between robotic and laparoscopic surgery for rectal cancer. Surg Endosc 27(6):1887–1895
Kang JYK, Min BS, Hur H, Baik SH, Kim NK, Lee KY (2013) The impact of robotic surgery for mid and low rectal cancer: a case-matched analysis of a 3-arm comparison-open, laparoscopic and robotic surgery. Ann Surg 257(1):95–101
Baik SH, Kwon HY, Kim JS, Hur H, Sohn SK, Cho CH, Kim H (2009) Robotic versus laparoscopic low anterior resection of rectal cancer: short-term outcome of a prospective comparative study. Ann Surg Oncol 16(6):1480–1487
Bianchi PP, Ceriani C, Locatelli A, Spinoglio G, Zampino MG, Sonzogni A, Crosta C, Andreoni B (2010) Robotic versus laparoscopic total mesorectal excision for rectal cancer: a comparative analysis of oncological safety and short-term outcomes. Surg Endosc 24(11):2888–2894
Baek JH, Pastor C, Pigazzi A (2011) Robotic and laparoscopic total mesorectal excision for rectal cancer: a case-matched study. Surg Endosc 25(2):521–525
Luca F, Cenciarelli S, Valvo M, Pozzi S, Faso FL, Ravizza D, Zampino G, Sonzogni A, Biffi R (2009) Full robotic left colon and rectal resection: technique and early outcome. Ann Surg Oncol 16(5):1274–1278
Griffen FD, Knight CD Sr, Whitaker JM, Knight CD Jr (1990) The double stapling technique for low anterior resection. Results, modifications, and observations. Ann Surg 211(6):745–751
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240(2):205–213
Yamaguchi T, Kinugasa Y, Shiomi A, Sato S, Yamakawa Y, Kagawa H, Tomioka H, Mori K (2015) Learning curve for robotic-assisted surgery for rectal cancer: use of the cumulative sum method. Surg Endosc 29(7):1679–1685
Jacobs M, Verdeja JC, Goldstein HS (1991) Minimally invasive colon resection (laparoscopic colectomy). Surg Laparosc Endosc 1:144–150
Young M, Pigazzi A (2014) Total mesorectal excision: open, laparoscopic or robotic. Recent Results Cancer Res 203:47–55
Caputo D, Caricato M, La Vaccara V, Capolupo GT, Coppola R (2014) Conversion in mini-invasive colorectal surgery: the effect of timing on short term outcome. Int J Surg 12(8):805–809
Morelli L, Ceccarelli C, Di Franco G, Guadagni S, Palmeri M, Caprili G, D’Isidoro C, Marciano E, Pollina L, Campani D, Massimetti G, Di Candio G, Mosca F (2015) Sexual and urinary functions after robot-assisted versus pure laparoscopic total mesorectal excision for rectal cancer. Int J Colorectal Dis Jul 7, 2015
Morelli L, Guadagni S, Di Franco G, Palmeri M, Caprili G, D’Isidoro C, Pisano R, Marciano E, Moglia A, Di Candio G, Mosca F (2015) Short-term clinical outcomes of robot-assisted intersphincteric resection and low rectal resection with double-stapling technique for cancer: a case-matched study. Int J Colorectal Dis, May 16, 2015
D’Annibale A, Morpurgo E, Fiscon V, Trevisan P, Sovernigo G, Orsini C, Guidolin D (2004) Robotic and laparoscopic surgery for treatment of colorectal diseases. Dis Colon Rectum 47:2162–2168
Luca F, Valvo M, Ghezzi TL, Zuccaro M, Cenciarelli S, Trovato C, Sonzogni A, Biffi R (2013) Impact of robotic surgery on sexual and urinary functions after fully robotic nerve-sparing total mesorectal excision for rectal cancer. Ann Surg 257:672–678
Baek SJ, Kim SH, Cho JS, Shin JW, Kim J (2012) Robotic versus conventional laparoscopic surgery for rectal cancer: a cost analysis from a single institute in Korea. World J Surg 36(11):2722–2729
Yang Y, Zhang P, Shi C, Zou Y, Qin H, Ma Y (2012) Robot-assisted versus conventional laparoscopic surgery for colorectal disease, focusing on rectal cancer: a meta-analysis. Ann Surg Oncol 19(12):3727–3736
Turchetti G, Palla I, Pierotti F, Cuschieri A (2012) Economic evaluation of da Vinci-assisted robotic surgery: a systematic review. Surg Endosc 26(3):598–606
Byrn JC, Hrabe JE, Charlton ME (2014) An initial experience with 85 consecutive robotic-assisted rectal dissections: improved operating times and lower costs with experience. Surg Endosc 28(11):3101–3107
Turchetti G, Pierotti F, Palla I, Manetti S, Freschi C, Ferrari V, Cuschieri A (2016) Comparative Health Technology Assessment of robotic-assisted, direct manual laparoscopic and open surgery: a prospective study. Surg Endosc 2016 [in press]
Bokhari MB, Patel CB, Ramos-Valadez DI, Ragupathi M, Haas EM (2011) Learning curve for robotic-assisted laparoscopic colorectal surgery. Surg Endosc 25(3):855–860
Jiménez-Rodríguez RM, Díaz-Pavón JM, de la Portilla de Juan F, Prendes-Sillero E, Dussort HC, Padillo J (2013) Learning curve for robotic-assisted laparoscopic rectal cancer surgery. Int J of Colorectal Dis 28(6):815–821
Park EJ, Cho MS, Baik SH, Kim DW, Min BS, Lee KY, Kim NK (2014) Multidimensional analyses of the learning curve of robotic low anterior resection for rectal cancer: 3-phase learning process comparison. Surg Endosc 28(10):2821–2831
Sng KK, Hara M, Shin JW, Yoo BE, Yang KS, Kim SH (2013) The multiphasic learning curve for robot-assisted rectal surgery. Surg Endosc 27(9):3297–3307
Cuschieri A, Turchetti G (2011) Change in the hospital care following European Working Time Directory with special reference to the craft specialties. Int J Healthcare Technology and Management 12:215–229
Morelli L, Guadagni S, Di Franco G, Palmeri M, Caprili G, D’Isidoro C, Pisano R, Moglia A, Ferrari V, Di Candio G, Mosca F (2015) Use of the new Da Vinci Xi® during robotic rectal resection for cancer: technical considerations and early experience. Int J Color Dis 30(9):1281–1283
Morelli L, Guadagni S, Di Franco G, Palmeri M, Caprili G, D’Isidoro C, Cobuccio L, Marciano E, Di Candio G, Mosca F (2016) Use of the new da Vinci Xi® during robotic rectal resection for cancer: a pilot matched-case comparison with the da Vinci Si®. Int J Med Robot Jan 25, 2016
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The authors declare that no funding support was received for this study.
Disclosures
Professor Luca Morelli, Dr. Simone Guadagni, Dr. Valentina Lorenzoni, Dr. Gregorio Di Franco, Dr. Luigi Cobuccio, Dr. Matteo Palmeri, Dr. Giovanni Caprili, Dr. Cristiano D’Isidoro, Dr. Andrea Moglia, Dr. Vincenzo Ferrari, Professor Giulio Di Candio, Professor Franco Mosca, and Professor Giuseppe Turchetti have no conflicts of interest or financial ties to disclose.
Additional information
Study supported by ARPA foundation, www.fondazionearpa.it
Rights and permissions
About this article
Cite this article
Morelli, L., Guadagni, S., Lorenzoni, V. et al. Robot-assisted versus laparoscopic rectal resection for cancer in a single surgeon’s experience: a cost analysis covering the initial 50 robotic cases with the da Vinci Si. Int J Colorectal Dis 31, 1639–1648 (2016). https://doi.org/10.1007/s00384-016-2631-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-016-2631-5