Abstract
Purpose
Current guidelines suggest that adjuvant chemotherapy (AC) be administered to all locally advanced (clinically T3–4 or N-positivity) rectal cancer patients undergoing neoadjuvant chemoradiotherapy (nCRT) and radical surgical resection regardless of the final pathological staging (yp staging). This study aimed to evaluate the necessity of AC for ypT0-2N0 rectal cancer.
Methods
Patients with ypT0-2N0 rectal cancer, who received nCRT and radical surgical resection, were recruited retrospectively at a university hospital. The main outcome was to evaluate the 5-year overall survival (OS) and disease-free survival (DFS) between ypT0-2N0 rectal cancer patients with AC and those without AC. We also identified potential independent prognostic factors associated with poor outcomes.
Results
One hundred and ten ypT0-2N0 rectal cancer patients (ypT0: n = 6; ypT1: n = 44; ypT2: n = 60) were followed up for a median of 60 months. No significant difference was observed in DFS and 5-year OS between patients with AC and those without AC. The risk of recurrence was associated with the postoperative pathological staging (0% with ypT0, 2.4% with ypT1, and 10% with ypT2). In the multivariate analysis, retrieval of < 12 lymph nodes was an independent favorable prognostic factor, which correlated with a higher OS (HR: 2.263; 95% CI: 1.093–4.687, P = 0.028). Intra-tumor lymphovascular and perineural invasion were poor prognostic markers for shorter DFS (HR: 5.940; 95% CI: 1.150–30.696, P = 0.033).
Conclusion
Postoperative AC is not required for patients with ypT0-2N0 rectal cancer downstaged by nCRT, especially in those without poor prognostic factors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neoadjuvant chemoradiation therapy (nCRT) followed by radical surgical resection is the current mainstay treatment for locally advanced (clinical stage of T3–4 or N-positivity) rectal cancer [1, 2]. Compared with postoperative radiotherapy, nCRT decreases the local recurrence rate [3] and reduces toxicity [4]. Furthermore, nCRT may enhance the magnitude of tumor shrinkage and increases the possibility of performing sphincter-sparing surgery, which may help achieve better functional outcomes [5].
According to the National Comprehensive Cancer Network® (NCCN®) guidelines of 2020, a 6-month perioperative adjuvant chemotherapy (AC) should be universally administered irrespective of the postoperative pathological staging for locally advanced rectal cancer patients undergoing nCRT and radical surgery [2]. The rationale of these guidelines adopted the concept before the era of preoperative radiation, which indicated that adjuvant 5-fluorouracil (5-FU) would improve the overall survival (OS) in operated patients with Dukes’ B and C rectal cancer [6]. Furthermore, the rationale of guidelines is based on a speculation taken for granted that the benefits of postoperative AC of stage III colon cancer patients may extrapolate to stage III rectal cancer patients [7, 8].
However, for locally advanced rectal cancer patients pretreated with nCRT and surgery, there is controversy surrounding the undifferentiated use of AC without specifying the final pathological stage (i.e., yp stage). To date, the absence of robust evidence makes AC in pretreated rectal cancer precarious and exposes the patients to additional toxicity [9]. It seems that it should be the final pathological stage, not pre-nCRT clinical stage, that accurately predicts the outcomes. For example, patients with pathologically complete response to nCRT demonstrated excellent outcomes and did not require AC [10, 11]. Moreover, in some patients who achieved a certain degree of downstaging, such as those with stage ypT1–2 or ypN0, some previous studies failed to observe the survival benefits of AC in this patient group [12]. Therefore, it makes no sense to give AC to such people unless robust evidence is presented.
Hence, we hypothesize that the need for administering AC should be based on the pathological staging (yp staging) rather than the clinical staging. As it is difficult to verify this hypothesis by conducting a randomized controlled study based on the considerations in the current practice guidelines, this retrospective study aimed to evaluate the necessity of AC in the ypT0-2N0 subgroup of rectal cancer patients using a prospectively collected database. Further, we aimed to identify the potential prognostic factors for recurrence and mortality in stage ypT0-2N0 rectal cancer patients.
Materials and methods
Patient selection
Between January 2006 and December 2011, 389 patients, who were diagnosed with locally advanced (cT3–4 or nodal positivity on radiological images), non-metastatic, middle, and lower rectal cancer (less than 12 cm from the anal verge), were treated with nCRT plus radical surgical resection at our hospital. Among them, 110 patients with a pathological stage of T0–T2, without nodal involvement, and without distant metastasis (ypT0-2N0M0) were recruited for this study. All data collected in this study were retrieved retrospectively from a prospectively collected colorectal database of a single institution (National Taiwan University Hospital, NTUH), and the study was approved by the Institutional Review Board, which waived the need for obtaining an informed consent. Data on the patient’s demographic characteristics as well as the intraoperative and postoperative parameters such as age, sex, performance status (Eastern Cooperative Oncology Group, ECOG), tumor location, type of surgery, pathological stage, the number of retrieved lymph nodes (LNs), and tumor differentiation were obtained for the analysis.
Treatment
All patients underwent nCRT based on the standard protocol. The treatment included two courses of preoperative chemotherapy: (1) 5-FU 2000 mg/m2 and leucovorin (LV) 200 mg/m2 IV infusion for 24 h, every 2 weeks for a total of 8 weeks, and (2) oral capecitabine (Xeloda, Roche), capecitabine 800 mg/m2 twice daily for a total of 8 weeks. In addition, the radiation dose was 180 cGy per day delivered in 25 fractions. The patient received a total radiation dose of 4,500–5,040 cGy over 5 weeks. Radiotherapy was administered to the whole pelvis. Radiotherapy was delivered using a three-dimensional conformal radiotherapy treatment planning system. The upper border of the tumor bed field was the L5/S1 junction, while the lower part was the inferior border of the ischial tuberosity. The lateral borders of the radiotherapy were located 1.5 cm lateral to the bony pelvis, and the posterior border encompassed the whole sacrum.
Radical surgery for rectal cancer was performed 6–8 weeks after the completion of nCRT. The type of surgery included low anterior resection, abdominoperineal resection, or sphincter-preserving surgery (coloanal anastomosis). All the total mesorectal excision (TME) and the LN retrieval abided by the following principles: (1) high/low ligation of the inferior mesenteric artery (IMA) and removal of the LN along the course of the vessel and (2) complete resection of the mesorectal envelope containing the rectum and adjacent lymphovascular tissue.
The following AC regimens were used during the study: (1) 5-FU 2000 mg/m2 and leucovorin (LV) 200 mg/m2 IV infusion for 24 h, every 2 weeks for a total of 16 weeks, and (2) oral capecitabine (Xeloda, Roche) 800 mg/m2 twice daily for a total of 16 weeks.
Surveillance
All patients underwent regular follow-up, including physical examinations, blood tests such as complete blood cell count and serum carcinoembryonic antigen (CEA) level, and colonofibroscopy. The patient also underwent imaging studies such as abdominal ultrasonography and chest X-ray. Computed tomography (CT) scan or magnetic resonance imaging (MRI) was performed when there was a suspicion of recurrence.
Statistical methods
The chi-square test was used for the comparison of categorical variables. Disease-free survival (DFS) was defined by measuring between the date of the primary surgery and the date of recurrence. The OS time was defined by measuring between the date of primary surgery and the time of the last visit or death. The last follow-up date was January 2017. Survival was demonstrated using the Kaplan-Meier curve. We calculated the significance of differences between subgroups using the log-rank test. A multivariate Cox regression analysis with stepwise selection was applied to identify the independent prognostic factors which were associated with survival. A probability value of less than 0.05 was considered significant. All tests were two sided. The analyses were carried out using SPSS version 16.0 for Windows.
Results
During a median follow-up period of 60 months, a total of 110 rectal cancer patients with stage ypT0-2N0M0 were treated at NTUH. Among them, 34 (30.9%) underwent total mesorectal excision (TME) alone, while 76 (69.1%) underwent TME plus 5-FU-based AC. Table 1 summarizes the clinicopathological features of the analyzed patients stratified by postoperative treatments. Notably, age was still an important determinant for choosing postoperative treatment. Patients who did not receive postoperative AC were significantly older than those who received AC (P = 0.005). The tumor location (defined by the distance above the anal verge) in patients receiving AC was higher than that in patients without AC (P = 0.033). The other important variables, including sex, pathological T stage, differentiation, intra-tumor invasion (i.e., if the pathological report showed venous, lympho-vessel, or perineural invasion), type of surgery, number of LN sampling, and ECOG performance, were not significantly different between the two groups.
A total of 17 rectal cancer patients with cN1 showed nodal-staging migration to pN0. Among them, 11 patients presented LN retrieved < 12, while 6 patients presented LN retrieved ≥ 12. Among the 92 cN0 rectal cancer patients, 57 patients presented LN retrieved < 12, while 35 patients presented LN retrieved ≥ 12. No association was found between pretreated nodal status (cN staging) and the number of retrieved LNs (P = 0.854).
R0 resection with a negative circumferential resection margin was achieved, which was examined and confirmed by the pathologists, and there were only two cases with local recurrence in this series (recurrence rate = 1.8%). AC was administered in 76 patients with the 5-FU based regimens. Finally, 38 patients failed to receive the treatment of AC because of other comorbidities, older age, or patients’ refusal of AC.
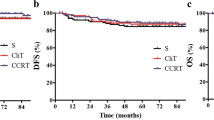
Interestingly, our results found that the administration of AC influenced neither the patients’ 5-year DFS nor the 5-year OS (P = 0.372 and P = 0.529, respectively, Table 2, Fig. 1). After further exploring the potential prognostic factors that may affect patient outcomes, we found that the 5-year OS rates in patients with < 12 retrieved LNs and in those with ≥ 12 retrieved LNs were 95.6% and 90.7%, respectively (Fig. 2, P = 0.014). The retrieved LNs < 12 was a favorable prognostic factor for 5-year OS (Table 2). Moreover, the 5-year DFS rates of patients with intra-tumor invasion and those without intra-tumor invasion were 68.6% and 93.5%, respectively (P = 0.015, Fig. 3). Intra-tumor invasion was inversely associated with 5-year DFS (Table 2).
In the multivariate analysis, retrieval of > 12 LNs was the most unfavorable factor associated with lower OS (hazard ratio [HR]: 2.263; 95% confidence interval [CI]: 1.093–4.687, Table 3). Intra-tumor lymphovascular or perineural invasion was another significant unfavorable factor for shorter DFS (HR: 5.940; 95% CI: 1.150–30.696; Table 3).
Discussion
The benefit of AC for downstaged ypT0-2N0 rectal cancer after nCRT remains controversial. Our study demonstrated no survival benefits of postoperative AC in downstaged ypT0-2N0 rectal cancer patients. The results suggest that the universal use of AC in downstaged ypT0-2N0 rectal cancer patients should be reconsidered, especially in those without adverse prognostic factors.
Level I studies did not provide a strong evidence regarding the need for AC in locally advanced rectal cancer patients pretreated with nCRT and radical surgery, although this was proposed in the NCCN guidelines [2]. The randomized controlled study EORTC 22921 also failed to support the benefits of adjuvant 5-FU in the 5-year OS [13]. Furthermore, three other randomized prospective trials (PROCTOR/SCRIPT [14], CHRONICLE [15], and I-CNR-RT [16]) showed that AC had no survival benefits. The PORCTOR/SCRIPT [14] and CHRONICLE [15] studies used 5-FU/LV (PROCTOR) or capecitabine (SCRIPT and CHRONICLE) as AC regimens. Despite incomplete patient accrual, these two studies showed that AC did not provide benefits in DFS, OS, or recurrence rate. The I-CNR-RT trial [16] used 5-FU/LV as AC with a reduced dose (5-FU 350 mg/m2 and folinic acid 20 mg/m2) and reported that AC did not improve the DFS, OS, or distant metastatic rate. The same findings were repeatedly reported in other retrospective studies conducted on patients with locally advanced rectal cancer [17,18,19].
However, the data that support the benefits of AC in pretreated locally advanced rectal cancer were obtained from some retrospective cohort studies [20,21,22]. Garlipp et al. performed a propensity score matching to analyze 1040 pretreated rectal cancer patients receiving 5-FU/capecitabine/oxaliplatin-based AC and showed an improvement in DFS [22]. Tiselieus et al. retrospectively recruited 436 patients with stage III rectal cancer pretreated with nCRT and surgery and receiving 5-FU/LV as AC. This study showed that AC improved the OS [23]. Moreover, the pathological complete response (pCR) rate after nCRP plus surgery was reported to be 20% [24], and recent cohort studies in patients with pCR demonstrated the OS benefits of FU-based AC, compared with surveillance alone [20, 21]. Overall, these contradictions in previous studies posed a predicament for AC in rectal cancer patients pretreated with nCRT and surgery. Therefore, it is necessary to assess the necessity of AC in downstaged rectal cancer and identify the specific group that will benefit from this treatment.
Tumor response to nCRT is difficult to predict in patients with locally advanced rectal cancer; therefore, postoperative pathological staging rather than preoperative clinical staging might be a reliable predictor and can be used a basis for determining the necessity of AC [11, 25]. In light of the abovementioned concept and conflicting data on the universal use of AC, the following questions were raised: what subgroups of patients can benefit from AC and what subgroups of patients require supportive care alone without AC. Our results showed that pre-nCRT patients with downstaged ypT0-2N0 did not benefit from AC. The same observation was reported in the previous studies conducted by Govindarajan et al. [17] and Yu et al. [18]. Govindarajan et al. performed a retrospective cohort study enrolling 203 ypT0-2N0 patients and showed that adding AC (N = 173) had no effect on the 5-year DFS. Yu et. al.’s retrospective study, enrolling 91 ypT0-2 patients, indicated that AC (N = 65) did not improve the OS or DFS. Our results are similar to those of previous studies.
In our study, retrieval of < 12 LNs was associated with a better 5-year OS compared with the retrieval of ≥ 12 LNs. The number of LNs retrieved is conventionally viewed as an indicator of surgical radicality as well as an indicator of proper staging [25, 26]. According to the NCCN guidelines, retrieval of at least 12 LNs meet the criteria for adequate staging. However, some studies have doubted this view based on the observation that the number of LNs harvested in patients after nCRT appeared to be less compared with those harvested from patients who did not receive nCRT. The studies also found that retrieval of < 12 LNs was associated with a favorable DFS or OS. The decreased number of LNs harvested may be regarded as an individual’s response to the chemoradiation therapy rather than an indication of surgical insufficiency [27, 28]. The LNs in the mesorectum are vulnerable to irradiation. Therefore, radiotherapy can cause lymphocyte apoptosis or atrophy of the stroma [29]. Additionally, from the anatomical view, the total number and size of LNs were lesser and smaller in rectal specimens than in colon specimens [30]. Thus, the anatomical characteristics and irradiation effects attributed to the decreased number of LNs harvested in patients who received nCRT. In this context, the overall effect of sampling a smaller number of LNs could not be considered as understaging, which is associated with poor prognosis [27].
One of the prognostic factors corresponding to poor 5-year DFS in our study was lymphovascular or perineural invasion. Microscopic lymphovascular or perineural invasion indicates a higher invasive propensity of the tumor and is a high-risk feature of stage II colon cancer [31]. Lymphovascular or perineural invasion was also identified as an independent unfavorable prognostic factor for DFS in the study published by Park et al. [32] and an independent poor prognostic factor of shortened OS and DFS in the study by Leonard et al. [33].
This current study had several limitations. First, age was still unavoidably an important determinant and was a potential selection bias during the selection of the postoperative treatment modality. In our study, among the patients pretreated with nCRT and surgery, a significant imbalance was observed in the age distribution between patients with AC and those without AC. In addition, AC was intended to be administered younger patients with fewer comorbidities and better physical performance. The second limitation was the retrospective nature of data collection, which weakened the strength of the interpretation. For example, the tumor locations (distance above the anal verge) were different between patients receiving AC and those not receiving AC. Moreover, the impact of prognostic factors (retrieved LNs < 12 and the presence of lymphovascular invasion) should be examined in a larger study sample in order to consolidate the findings. Third, the study was a single-institution, retrospective cohort study, which may also have led to a potential selection bias. Further large-scale, prospective, randomized studies are warranted and may overcome these weaknesses. Nevertheless, we believe that the current results provide important information regarding the effectiveness of AC in the ypT0-T2N0 patient subgroup for clinical judgment.
Conclusion
Our study revisited the need for the universal use of AC irrespective of the final pathological stage in patients with locally advanced rectal cancer pretreated with nCRT and radical surgical resection. Additionally, we suggest that patients with ypT0-2N0 rectal cancer may not need AC, especially those without adverse prognostic factors.
References
Sauer R, Liersch T, Merkel S et al (2012) Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol 30:1926–1933. https://doi.org/10.1200/JCO.2011.40.1836
National Comprehensive Cancer Network (2020) Rectal cancer (Version 2.2020). https://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf. Accessed 3 March 2020
van Gijn W, Marijnen CA, Nagtegaal IDT et al (2011) Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol 12:575–582. https://doi.org/10.1016/S1470-2045(11)70097-3
Bosset JF, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, Daban A, Bardet E, Beny A, Ollier JC, EORTC Radiotherapy Group Trial 22921 (2006) Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med 55:1114–1123. https://doi.org/10.1056/NEJMoa060829
Madbouly KM, Hussein AM (2015) Changing operative strategy from abdominoperineal resection to sphincter preservation in T3 low rectal cancer after downstaging by neoadjuvant chemoradiation: a preliminary report. World J Surg 39:1248–1256. https://doi.org/10.1007/s00268-014-2930-3
Fisher B, Wolmark N, Rockette H, Redmond C, Deutsch M, Wickerham DL, Fisher ER, Caplan R, Jones J, Lerner H, Gordon P, Feldman M, Cruz A, Legault-Poisson S, Wexler M, Lawrence W, Robidoux A, Other NSABP Investigators (1988) Postoperative adjuvant chemotherapy or radiation therapy for rectal cancer: results from NSABP protocol R-01. J Natl Cancer Inst 80:21–29. https://doi.org/10.1093/jnci/80.1.21
Wolpin BM, Mayer RJ (2008) Systemic treatment of colorectal cancer. Gastroenterology 134:1296–1310. https://doi.org/10.1053/j.gastro.2008.02.098
Andre T, Boni C, Navarro M et al (2009) Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol 27:3109–3116. https://doi.org/10.1200/JCO.2008.20.6771
Hess V, Winterhalder R, von Moos R et al (2017) Capecitabine and oxaliplatin prior and concurrent to preoperative pelvic radiotherapy in patients with locally advanced rectal cancer: long-term outcome. Clin Colorectal Cancer 16:240–245. https://doi.org/10.1016/j.clcc.2016.07.008
Maas M, Nelemans PJ, Valentini V, Das P, Rödel C, Kuo LJ, Calvo FA, García-Aguilar J, Glynne-Jones R, Haustermans K, Mohiuddin M, Pucciarelli S, Small W Jr, Suárez J, Theodoropoulos G, Biondo S, Beets-Tan RGH, Beets GL (2010) Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol 11:835–844. https://doi.org/10.1016/S1470-2045(10)70172-8
Gamaleldin M, Church JM, Stocchi L, Kalady M, Liska D, Gorgun E (2017) Is routine use of adjuvant chemotherapy for rectal cancer with complete pathological response justified. Am J Surg 213:478–483. https://doi.org/10.1016/j.amjsurg.2016.11.028
Collette L, Bosset JF, den Dulk M, Nguyen F, Mineur L, Maingon P, Radosevic-Jelic L, Piérart M, Calais G (2007) Patients with curative resection of cT3-4 rectal cancer after preoperative radiotherapy or radiochemotherapy: does anybody benefit from adjuvant fluorouracil-based chemotherapy? A trial of the European Organisation for Research and Treatment of Cancer Radiation Oncology Group. J Clin Oncol 25:4379–4386. https://doi.org/10.1200/JCO.2007.11.9685
Bosset JF, Calais G, Mineur L, Maingon P, Stojanovic-Rundic S, Bensadoun RJ, Bardet E, Beny A, Ollier JC, Bolla M, Marchal D, van Laethem JL, Klein V, Giralt J, Clavère P, Glanzmann C, Cellier P, Collette L (2014) Fluorouracil-based adjuvant chemotherapy after preoperative chemoradiotherapy in rectal cancer: long-term results of the EORTC 22921 randomised study. Lancet Oncol 15:184–190. https://doi.org/10.1016/S1470-2045(13)70599-0
Breugom AJ, van Gijn W, Muller EW, Berglund Å, van den Broek CBM, Fokstuen T, Gelderblom H, Kapiteijn E, Leer JWH, Marijnen CAM, Martijn H, Meershoek-Klein Kranenbarg E, Nagtegaal ID, Påhlman L, Punt CJA, Putter H, Roodvoets AGH, Rutten HJT, Steup WH, Glimelius B, van de Velde CJH (2015) Adjuvant chemotherapy for rectal cancer patients treated with preoperative (chemo) radiotherapy and total mesorectal excision: a Dutch Colorectal Cancer Group (DCCG) randomized phase III trial. Ann Oncol 26:696–701. https://doi.org/10.1093/annonc/mdu560
Glynne-Jones R, Counsell N, Quirke P et al (2014) Chronicle: results of a randomised phase III trial in locally advanced rectal cancer after neoadjuvant chemoradiation randomising postoperative adjuvant capecitabine plus oxaliplatin (XELOX) versus control. Ann Oncol 251:356–1362. https://doi.org/10.1093/annonc/mdu147
Sainato A, Cernusco Luna Nunzia V, Valentini V, de Paoli A, Maurizi ER, Lupattelli M, Aristei C, Vidali C, Conti M, Galardi A, Ponticelli P, Friso ML, Iannone T, Osti FM, Manfredi B, Coppola M, Orlandini C, Cionini L (2014) No benefit of adjuvant fluorouracil leucovorin chemotherapy after neoadjuvant chemoradiotherapy in locally advanced cancer of the rectum (LARC): long term results of a randomized trial (I-CNR-RT). Radiother Onco 113:223–229. https://doi.org/10.1016/j.radonc.2014.10.006
Govindarajan A, Reidy D, Weiser MR, Paty PB, Temple LK, Guillem JG, Saltz LB, Wong WD, Nash GM (2011) Recurrence rates and prognostic factors in ypN0 rectal cancer after neoadjuvant chemoradiation and total mesorectal excision. Ann Surg Oncol 18:3666–3672. https://doi.org/10.1245/s10434-011-1788-y
You KY, Huang R, Ding PR, Qiu B, Zhou GQ, Chang H, Xiao WW, Zeng ZF, Pan ZZ, Gao YH (2014) Selective use of adjuvant chemotherapy for rectal cancer patients with ypN0. Int J Colorectal Dis 29:529–538. https://doi.org/10.1007/s00384-014-1831-0
Bujko K, Glynne-Jones R, Bujko M (2010) Does adjuvant fluoropyrimidine-based chemotherapy provide a benefit for patients with resected rectal cancer who have already received neoadjuvant radiochemotherapy? A systematic review of randomised trials. Ann Oncol 21:1743–1750. https://doi.org/10.1093/annonc/mdq054
Dossa F, Acuna SA, Rickles AS, Berho M, Wexner SD, Quereshy FA, Baxter NN, Chadi SA (2018) Association between adjuvant chemotherapy and overall survival in patients with rectal cancer and pathological complete response after neoadjuvant chemotherapy and resection. JAMA Oncol 4:930–937. https://doi.org/10.1001/jamaoncol.2017.5597
Polanco P, Huerta S (2018) Omitting adjuvant chemotherapy in patients with rectal cancer who received neoadjuvant chemoradiation followed by total mesorectal excision and achieved a pathological complete response. Am J Surg 216:387–388. https://doi.org/10.1016/j.amjsurg.2017.03.019
Garlipp B, Ptok H, Benedix F, Otto R, Popp F, Ridwelski K, Gastinger I, Benckert C, Lippert H, Bruns C (2016) Adjuvant treatment for resected rectal cancer: impact of standard and intensified postoperative chemotherapy on disease-free survival in patients undergoing preoperative chemoradiation-a propensity score-matched analysis of an observational database. Langenbecks Arch Surg 401:1179–1190. https://doi.org/10.1007/s00423-016-1530-0
Tiselius C, Gunnarsson U, Smedh K, Glimelius B, Pahlman L (2013) Patients with rectal cancer receiving adjuvant chemotherapy have an increased survival: a population-based longitudinal study. Ann Oncol 24:160–165. https://doi.org/10.1093/annonc/mds278
Martin ST, Heneghan HM, Winter DC (2012) Systematic review and meta-analysis of outcomes following pathological complete response to neoadjuvant chemoradiotherapy for rectal cancer. Br J Surg 99:918–928. https://doi.org/10.1002/bjs.8702
Kuo LJ, Liu MC, Jian JJ et al (2007) Is final TNM staging a predictor for survival in locally advanced rectal cancer after preoperative chemoradiation therapy. Ann Surg Oncol 14:2766–2772. https://doi.org/10.1245/s10434-007-9471-z
Kim YW, Kim NK, Min BS, Lee KY, Sohn SK, Cho CH (2009) The influence of the number of retrieved lymph nodes on staging and survival in patients with stage II and III rectal cancer undergoing tumor-specific mesorectal excision. Ann Surg 249:965–972. https://doi.org/10.1097/SLA.0b013e3181a6cc25
Tsai CJ, Crane CH, Skibber JM, Rodriguez-Bigas MA, Chang GJ, Feig BW, Eng C, Krishnan S, Maru DM, Das P (2011) Number of lymph nodes examined and prognosis among pathologically lymph node-negative patients after preoperative chemoradiation therapy for rectal adenocarcinoma. Cancer 117:3713–3722. https://doi.org/10.1002/cncr.25973
de Campos-Lobato LF, Stocchi L, de Sousa JB, Buta M, Lavery IC, Fazio VW, Dietz DW, Kalady MF (2013) Less than 12 nodes in the surgical specimen after total mesorectal excision following neoadjuvant chemoradiation: it means more than you think. Ann Surg Oncol 20:3398–3406. https://doi.org/10.1245/s10434-013-3010-x
Persiani R, Biondi A, Gambacorta MA, Bertucci Zoccali M, Vecchio FM, Tufo A, Coco C, Valentini V, Doglietto GB, D'Ugo D (2014) Prognostic implications of the lymph node count after neoadjuvant treatment for rectal cancer. Br J Surg 101:133–142. https://doi.org/10.1002/bjs.9341
Fajardo LF (1994) Effects of ionizing radiation on lymph nodes. A review. Front Radiat Ther Oncol 28:37–45. https://doi.org/10.1159/000423371
Yao YF, Wang L, Liu YQ, Li JY, Gu J (2011) Lymph node distribution and pattern of metastases in the mesorectum following total mesorectal excision using the modified fat clearing technique. J Clin Pathol 64:1073–1077. https://doi.org/10.1136/jclinpath-2011-200190
Lin BR, Lai HS, Chang TC, Lee PH, Chang KJ, Liang JT (2011) Long-term survival results of surgery alone versus surgery plus UFT (Uracil and Tegafur)-based adjuvant therapy in patients with stage II colon cancer. J Gastrointest Surg 15:2239–22345. https://doi.org/10.1007/s11605-011-1722-4
Park IJ, Yu CS, Lim SB, Yoon YS, Kim CW, Kim TW, Kim JH, Kim JC (2014) Prognostic implications of the number of retrieved lymph nodes of patients with rectal cancer treated with preoperative chemoradiotherapy. J Gastrointest Surg 18:1845–18451. https://doi.org/10.1007/s11605-014-2509-1
Acknowledgments
The authors thank Professor Jin-Tung Liang for his comments that have led to the improvement of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liao, YT., Lin, YL., Huang, J. et al. Downstaged ypT0-2N0 rectal cancer after neoadjuvant chemoradiation therapy may not need adjuvant chemotherapy: a retrospective cohort study. Int J Colorectal Dis 36, 509–516 (2021). https://doi.org/10.1007/s00384-020-03787-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-020-03787-5