Abstract
Purpose
The aim of this study was to clarify whether a surgical-specific risk scoring system estimating the physiologic ability and surgical stress (E-PASS) score was useful for prediction of postoperative morbidity and mortality.
Methods
The E-PASS score consists of the preoperative risk score (PRS), surgical stress score (SSS), and the comprehensive risk score (CRS). Conventional scoring systems [colorectal physiologic and operative severity score for the enumeration of mortality (CR-POSSUM) and the prognostic nutritional index (PNI)] were also examined. We retrospectively compared these scores in patients with or without postoperative complications. We assessed the relationship between these scores, clinicopathological features and postoperative mortality.
Results
Postoperative complications developed in 78 patients (33 %). American Society of Anesthesiologists score, performance status, PNI score, PRS, SSS, and CRS were significantly higher in patients with postoperative complications than in those without postoperative complications (p < 0.05). The area under the receiver operating characteristic curve (AUC) was highest for E-PASS [E-PASS (PRS, 0.74; SSS, 0.62; CRS, 0.78), PNI (0.62), CR-POSSUM (PS, 0.57; OSS, 0.52)]. Multivariate logistic analysis identified CRS ≥ 0.2 as a significant determinant of postoperative complications (p < 0.01; hazard ratio, 4.84). Overall survival was significantly better in the CRS < 0.2 group than in the CRS > 0.2 group (p < 0.01).
Conclusions
The E-PASS score system was a useful predictor of postoperative complications and mortality, especially in patients with advanced age.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The mean age of Japanese individuals has increased to 79.94 years for men and 86.41 years for women, and the proportion of the Japanese population over 75 years was 11.7 % according to the latest report of the Ministry of Welfare in 2014 (http://www.mhlw.go.jp). Similar trends are noted worldwide [1]. In colorectal cancer patients, perioperative mortality ranges from 1 to 12 % around the world and occurs in less than 1 % in Japan [2]. However, previous reports indicated that more than 10 % of patients of advanced age have protracted postoperative disability that could cause serious postoperative complications [3, 4]. In particular, elderly patients often have comorbidities, such as cardiovascular disease and pulmonary disease that can pose difficulties with recovery from unexpected perioperative events [5–8]. Postoperative complications are significantly associated with increased rates of distant recurrence and long-term outcomes in colorectal cancer patients [9–11]. Therefore, clinicians should pay greater attention to perioperative conditions, including performance status, biological age, and physical presentation [3, 12–14].
Recently, several scoring systems have been developed to predict postoperative morbidity and mortality, such as the estimation of physiologic ability and surgical stress (E-PASS) score, the colorectal physiologic and operative severity score for the enumeration of mortality (CR-POSSUM), and the prognostic nutritional index (PNI) [15, 16]. The E-PASS scoring system is a useful and simple strategy to predict postoperative mortality and morbidity [3, 17, 18]. It evaluates the patient’s physiological condition and surgical invasion and precisely reflects the patient’s general condition in the perioperative setting. CR-POSSUM can predict outcomes, especially in colorectal cancer patients [19–21]. However, this score overestimates mortality and morbidity. Onodera et al. advocated the use of the prognostic nutrition index (PNI) to assess immune-nutritional status of patients with gastrointestinal tumors [15]. A large study reported that PNI can predict outcomes in patients with malignancy, regardless of the site of origin of the malignancy [22, 23]. This index is calculated based on two simple laboratory parameters (serum albumin and blood lymphocyte count), but the index does not include surgical factors.
In this study, we aimed to identify risk factors that correlate with postoperative morbidity and mortality in 239 elderly colorectal cancer patients who underwent colectomy in our hospital. Specifically, we assessed the utility of the E-PASS, CR-POSSUM, and PNI scores in this regard.
Methods
An Institutional Review Board approved this retrospective observational study. Informed consent was obtained from all patients before surgery.
Patients
Between January 2009 and August 2013, 519 patients were diagnosed with colorectal cancer and underwent curative colorectal resection at the Department of Surgical Oncology of Nagasaki University Graduate School of Biological Sciences. This retrospective study collected consecutive data from 239 (46 %) patients over 70 years of age. The median follow-up time was 25.7 months (range, 0.2–69.2 months).
Before surgery, we evaluated the indication for resections by abdominal computed tomography (CT). Performance status, American Society of Anesthesiologists score (ASA score), and comorbidity were evaluated as an assessment of general status preoperatively. Curative colectomy, anterior resection, and abdominoperineal resection accompanied by regional lymph node resection were performed according to the guidelines of the Japanese Society for Cancer of the Colon and Rectum (JSCCR) [24]. We performed either end-to-end anastomosis using the double stapling technique or using a hand sewing technique as reconstruction according to tumor location.
Postoperative complications were graded according to the Clavien-Dindo classification, categorizing surgical complications from grade 1 to 5 based on the invasiveness of the treatment required [25, 26]. In the present study, we defined complications as conditions that required treatment at the time of first discharge from the hospital (Clavien-Dindo classification Grade 2–5). We usually administered 5-fluorouracil-based adjuvant chemotherapy to patients with stage III or high-risk stage II disease (inadequately sampled nodes, T4 lesions, perforation, or poorly differentiated histology) patients. However, in elderly patients with low performance status level or severe comorbidities, we eliminated the indications for adjuvant therapies. Mortality and morbidity data were collected from the database of our department and from that of a collaborating hospital.
Examined scoring systems
The E-PASS score consists of three parts for estimation of physiologic ability (PRS), surgical stress (SSS) and their comprehensive score (CRS), as proposed by Haga et al. [17]. The formula for each score was as follows:
X1, age; X2, absence (0) or presence (1) of severe heart disease; X3, absence (0) or presence (1) of severe pulmonary disease; X4, absence (0) or presence (1) of diabetes mellitus; X5, performance status (0–4); X6, ASA physiological status classification (1–5)
X1, blood loss/body weight (g/kg); X2, operation time (hours); X3, extent of skin incision (0: minor incision, 1: laparotomy or thoracotomy alone, 2: both laparotomy and thoracotomy).
We calculated PRS using data recorded at the time of admission, and then added SSS data. The CRS score was calculated postoperatively.
CR-POSSUM has two main components: physiological severity (PS includes age, cardiac sign, systolic blood pressure, pulse, hemoglobin, and urea) and operative severity (OS includes operative severity, peritoneal soiling, malignancy status, and mode of surgery).
where PS is the total physiological score and OSS is the total operative severity score.
The PNI is established using the following formula: 10 × serum albumin (g/dL) + 0.005 × total lymphocyte count (per mm3).
Statistical analysis
The primary outcomes of our retrospective cohort study were the incidences of postoperative complications, and secondary outcomes were survival rates. In the primary analysis, multivariate logistic analysis was used to determine significant factors affecting the presence of complications. Data from different groups were compared using Student’s t test. Continuous data are expressed as mean ± standard deviation (SD). In univariate analysis, the comparison of categorical variables was performed using the chi-square test or Fisher’s exact test. P < 0.05 was considered to indicate statistical significance.
Receiver operating characteristic (ROC) curves were plotted to identify a cutoff value of postoperative complication for the E-PASS score, and the area under the ROC curve (AUC) was used to assess the power of a model to discriminate patients who experienced postoperative complications. The AUC value ranged from 0.5 to 1.0; and the greater the AUC, the better the model. Overall survival and disease-free survival (DFS) were calculated according to the Kaplan-Meier method. The differences between groups were tested for statistical significance using the log-rank test. Statistical analysis was performed using SPSS ver. 14 (Chicago, Illinois, USA).
Results
Postoperative complications
Among a total of 239 patients, postoperative complications developed in 78 patients (Table 1). Among those patients, 21 had surgical site infections, 18 had sub-ileus, six had minor or major leakage, five had urinary tract infections and five had delirium. Cardiopulmonary disorders developed in nine patients (12 %). Severe postoperative complications (Clavien-Dindo classification Grade 3–5) occurred in 11 cases, and four patients died due to their complications (sepsis in two patients, major leakage in one, heart failure in one).
Postoperative complication and associated parameters
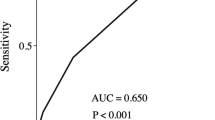
Patients who developed complications were assessed (Table 2). Postoperative complications occurred more often in male patients than in female patients. Univariate analysis showed that comorbidity, performance status, ASA score, albumin level, operation time, and blood loss were significantly associated with postoperative complications. All patients who experienced hospital death were in the complication group. The duration of hospital stay was longer in the complication group than in the no-complication group (p < 0.05). With regard to surgical severity score, PNI (p < 0.05), and all E-PASS scores, including PRS (p < 0.01), SSS (p < 0.01), and CRS (p < 0.01), significantly correlated with systemic complications. Factors of the CR-POSSUM score, PS (p = 0.13) and OSS (p = 0.11), were not significantly different when comparing the complication group and the no-complication group. ROC curve analysis calculated CRS = 0.2 as a cutoff value for the E-PASS score. The sensitivity and specificity of E-PASS (CRS = 0.2) was 0.71 and 0.79, respectively. The AUCs of each model for the detection of postoperative complications were as follows: E-PASS (PRS, 0.74; SSS, 0.62; CRS, 0.78), PNI (0.62), and CR-POSSUM (PS, 0.57; OSS, 0.52) (Fig. 1). Multivariate analysis using the clinicopathological factors that were selected according to the backward elimination method identified CRS ≥ 0.2 (hazard ratio [HR], 4.84; 95 % confidence interval [CI], 2.46–9.56; p < 0.01) as a significant determinant of postoperative complications (p < 0.01).
Receiver operating curve analysis for postoperative complications Area under the curve (AUC) of E-PASS (PRS/SSS/CRS) (a), PNI (b), and CR-POSSUM (PS/OSS) (c) is 0.74/0.62/0.78, 0.62, and 0.57/0.52, respectively. a E-PASS estimation of physiologic ability and surgical stress, b PRS preoperative risk score, c SSS surgical stress score, d CRS comprehensive risk score, e PNI prognostic nutritional index, f CR-POSSUM colorectal physiologic and operative severity score for the enumeration of mortality, g PS physiological score, h OSS operative severity score
Relationship between postoperative complications and survival after colectomy
Tumor recurrence was diagnosed after colectomy in 33 patients (14 %), and 28 patients (12 %) died during the observation period. The Kaplan-Meier method and log-rank testing indicated that both disease-free survival and overall survival in patients with complications tended to be lower than in those without complications (Fig. 2a, b), but these differences did not reach the level of statistical significance (p = 0.06 and p = 0.26, respectively). Elderly patients were further subdivided into two groups according to the CRS score (CRS < 0.2, and >0.2), and disease-free survival and overall survival were evaluated (Fig. 2c, d). There were significant differences in the survival rate between the two groups (p < 0.01). The patients were further subdivided into two groups according to whether patient did or did not receive perioperative chemotherapy, and postoperative outcomes were examined (Fig. 2e–h). When comparing these two groups, there were no significant differences in disease-free survival. In the chemotherapy-negative group, overall survival was significantly better in the CRS < 0.2 group (HR, 15.73; 95 % CI, 4.01–61.62; p < 0.01) (Fig. 2h).
Disease-free survival (DFS) (a) and overall survival (OS) (b) after colorectal resection, according to the presence or absence of complications. There are no significant differences between the two groups (p = 0.09 and p = 0.08, respectively). The patients are subdivided into two groups according to the CRS score (CRS < 0.2, CRS > 0.2), and DFS (c) and OS (d) were evaluated. OS is significantly better in the CRS < 0.2 group (p < 0.01). The patients are further subdivided into two groups according to whether or not they received anticancer drugs perioperatively (e–h). In the chemotherapy-negative group, OS is significantly better in the CRS < 0.2 group (p < 0.01). Numbers under the plots are patients at risk. HR hazard ratio, CI confidence interval
Discussion
The present study revealed that postoperative complications occurred in 78 patients (33 %) and that four patients (1.7 %) died within 30 days after surgery. These results support those from previous reports that postoperative morbidity and mortality rates were higher in elderly patients than in younger patients [2, 8, 27].
Various scoring systems have been developed to predict postoperative morbidity and mortality. Hattori and colleagues described the effectiveness of E-PASS scores for predicting postoperative complications in colorectal patients [9]. However, few studies have assessed this system in elderly patients [28, 29]. We focused on elderly patients with colorectal cancer who were considered to have a relatively high risk of morbidity when compared with younger patients.
The CRS was calculated from the PRS (which includes perioperative patient condition factors) and the SSS (including surgical condition factors) [17, 30]. In this study, CRS was higher in the complication group than in the no-complication group (0.18 vs. −0.03; p < 0.01). Past studies have suggested that CRS > 0.5 was associated with a higher probability of postoperative morbidity and mortality [30, 31]. However, in our cases, most patients who experienced postoperative complications had CRS < 0.5; only patients with severe complications (Clavien-Dindo classification Grade >3) had a high CRS score (mean CRS = 0.55). There are several possible reasons to account for why our cases showed lower CRS levels. First, SSS has three components, including blood loss, operation time, and the extent of skin incision [17]. In general colorectal cancer surgery, operation time, and average blood loss was lower when compared with that in hepatocellular carcinoma or pancreatic cancer, as previously reported [28, 29]. Second, the use of laparoscopic-assisted colectomy continues to increase. In regard to the extent of skin incision, laparoscopic surgery is assigned 0 points when compared with 1 point for open surgery, and this difference ultimately accounted for 0.352 points in the SSS score. Therefore, depending on the characteristics of the malignancy, laparoscopic surgery may be preferred, as it is less invasive from the view of the E-PASS score.
In 2004, Tekkis et al. developed the colorectal cancer-specific POSSUM (CR-POSSUM) in an effort to resolve discrepancies from previous models, and several studies have reported that this score is valid in colorectal patients [19, 32, 33]. In our study, no significant differences could be seen in the two components, including physiological score (p = 0.13) and operative severity score (p = 0.11), between the complication group and the no-complication group. Both the E-PASS and CR-POSSUM score consist of two main factors: perioperative patient conditions and operative conditions. In comparison with the E-PASS score, CR-POSSUM does not take into account the presence of pulmonary disease, diabetes mellitus, ASA score, performance status, blood loss, or operation time. Results from the present study suggest that these factors are crucial for the prediction of postoperative morbidity.
Good nutritional status is needed to avoid postoperative complications in patients with malignant cancers [34]. Low levels of serum albumin, a nutritional marker, and preoperative hypoalbuminemia are major risk factors for adverse postoperative outcomes in patients with colorectal cancer [35]. Total peripheral blood lymphocyte (TLC) count is another parameter, and several scores for predicting mortality and morbidity have been developed that include the TLC count [36–39]. Onodera and colleagues reported that PNI was useful to assess immune-nutritional status of patients with gastrointestinal tumors [9]. This index is calculated by combining the serum albumin and total peripheral blood lymphocyte count and is, therefore, more easily calculated when compared with the E-PASS and CR-POSSUM. Lower PNI score is a poor prognostic marker in patients with colorectal cancer patients [18]. In the present study, the PNI score was significantly lower in patients with complications than in patients without complications (44.7 and 47, respectively; p < 0.05); however, multivariate analysis identified only CRS of E-PASS (and not PNI) as an independent risk factor for postoperative complications. This may be attributed to the lack of operation factors within the PNI scoring system. Furthermore, in this study, the AUC was highest for the E-PASS, which suggests that the E-PASS is better than the other models (e.g., CR-POSSUM and PNI) for prediction of postoperative complications (Fig. 1).
Postoperative complications, such as anastomotic leakage and surgical site infection, are associated with poor outcomes in patients with different types of cancer [40–42]. Another report revealed that advanced age and comorbidities were associated with a higher risk of death when complications occurred [43]. In the present study, patients with postoperative complications were older, had a lower performance status, more rectal resection, longer operative time, and more blood loss (Table. 2). However these factors, which were components of CRS (except for rectal resection), did not correlate with disease-free survival or overall survival (Table. 3). Both the disease-free survival and the overall survival for the no-complication group tended to be better than that of the complication group (p = 0.09 and p = 0.08, respectively), and there was significant differences between CRS < 0.2 group and CRS > 0.2 group with regard to overall survival (p < 0.01). These results might indicate that CRS, which is a completely enclosed scoring system, is a more useful predictor of postoperative mortality when compared with other single factors described in previous reports [30, 31]. We also considered the effect of perioperative chemotherapy on the survival rate; patients were subdivided according to whether or not they received perioperative anticancer agents. In the patients who did not receive chemotherapy, overall survival was significantly better in the CRS < 0.2 group than in the CRS > 0.2 group (p < 0.01). Perioperative chemotherapy tends to be contraindicated in elderly patients due to age, performance status, and the risk of severe toxicity [44]. In this regard, E-PASS is a reasonable scoring system as a predictor of mortality in elderly patients, especially for those patients who are not eligible for chemotherapy. However, in patients who did receive perioperative chemotherapy, there were no significant differences in overall survival between the high CRS group and the low CRS group (p = 0.36). The varying nature of these results might reflect that there were several types of chemotherapy administered, such as intravenous drugs or oral drugs, depending on patient characteristics. In addition, in this study, the number of patients who received perioperative chemotherapy was relatively small. Further large-scale studies are needed to clarify the effect of perioperative chemotherapy on disease-free survival and overall survival.
Conclusions
The E-PASS score was the most useful predictor of postoperative morbidity and mortality in elderly colorectal cancer patients. Use of this score may help guide appropriate management and therefore improve outcomes in elderly colorectal cancer patients.
References
Teriokhin AT, Budilova EV, Thomas F, Guegan JF (2004) World wide variation in life-span sexual dimorphism and sex-specific environmental mortality rates. Hum Biol 76:623–641
Bottino V, Esposite MG, Mottola A et al (2012) Early outcomes of colon laparoscopic resection in the elderly patients compared with the younger. BMG Surg 12:58–61. doi:10.1186/1471-2482-12-S1-S8
Amemiya T, Oda K, Ando M et al (2007) Activities of daily living and quality of life of elderly patients after elective surgery for gastric and colorectal cancer. Ann Surg 246:222–228. doi:10.1097/SLA.0b013e3180caa3fb
Alves A, Panis Y, Mathieu P, Mantion G, Kwiatkowski F, Slim K (2005) Postoperative mortality and morbidity in French patients undergoing colorectal surgery: result of a prospective multicenter study. Arch Surg 140:278–283. doi:10.1001/archsurg.140.3.278
Neuman HB, O’Connor ES, Weiss J, Loconte NK, Greenberg CC, Smith MA (2013) Surgical treatment of colon cancer in patients aged 80 years and older. Cancer 1:639–647. doi:10.1002/cncr.27765
Fielding LP, Phillips RK, Hittinger R (1989) Factors influencing mortality after curative resection for large bowel cancer in elderly patients. Lancet 1:595–597. doi:10.1016/S0140-6736(89)91618-8
Turrentine FE, Wang H, Simpson VB, Jones RS (2006) Surgical risk factors morbidity, and mortality in elderly patients. J Am Coll Surg 203:865–877. doi:10.1016/j.jamcollsurg.2006.08.026
Savlovschi C, Serban D, Trotea T, Borcan R, Dumitrescu D (2013) Post-surgery morbidity and mortality in colorectal cancer in elderly subjects. Chirurgia 108:177–179
Ariake K, Ueno T, Takahashi M et al (2014) E-PASS comprehensive risk score is a good predictor of postsurgical mortality from comorbid disease in elderly gastric cancer patients. J Surg Oncol 109:586–592. doi:10.1002/jso.23542
Farid SG, Aldouri A, Morris-Stiff G et al (2010) Correlation between postoperative infective complications and long-term outcomes after hepatic resection for colorectal liver metastasis. Ann Surg 251:91–100. doi:10.1097/SLA.0b013e3181bfda3c
Krarup PM, Nordholm-Carstensen A, Jorgensen LN, Harling H (2014) Anastomotic leak increases distant recurrence and long-term mortality after curative resection for colonic cancer: a nationwide cohort study. Ann Surg 259:930–938. doi:10.1097/SLA.0b013e3182a6f2fc
Saif MW, Makrilia N, Zalonis A, Merikas M, Syrigos K (2010) Gastric cancer in elderly: an overview. Eur J Surg Oncol 36:709–717. doi:10.1016/j.ejso.2010.05.023
Seo SH, Hur H, An CW (2011) Operative risk factors in gastric cancer surgery for elderly patients. J Gastric Cancer 11:116–121
Eguchi T, Fujii M, Takayama T (2013) Mortality for gastric cancer in elderly patients. J Surg Oncol 84:132–136. doi:10.5230/jgc.2011.11.2.116
Tekkis PP, Prytherch DR, Kocher HM et al (2004) Development of dedicated risk-adjustment scoring system for colorectal surgery. Br J Surg 115:1174–1182
Onodera I, Goseki N, Kosaki G (1984) Prognostic nutrition index in gastrointestinal surgery of malnourished cancer patients. Nohon Geka Gakkai Zassi 105:1001–1005
Haga Y, Ikei S, Ogawa M (1999) Estimation of Physiologic Ability and Surgical Stress (E-PASS) as a new prediction scoring system for postoperative morbidity and mortality following elective gastrointestinal surgery. Surg Today 29:219–225. doi:10.1007/s005950050392
Hattori H, Kamiya J, Shimada H et al (2009) Assessment of the risk of postoperative delirium in elderly patients using E-PASS and the NEECHAM Confusion Scale. Int J Gariatr Psychiatry 24:1304–1310. doi:10.1002/gps.2262
Copeland GP, Jones D, Walters M (1991) POSSUM: a scoring system of surgical audit. Br J Surg 78:355–360. doi:10.1002/bjs.1800780327
Ren L, Upadhyay AM, Wang L, Li L, Lu J, Fu W (2009) Mortality rate prediction by Physiological and Operative Severity Score for the Enumeration of Mortality and Morbidity (POSSUM), Portsmouth POSSUM and Colorectal POSSUM and the development of new scoring systems in Chinese colorectal cancer patients. Clin Surg Int 198:31–38. doi:10.1016/j.amjsurg.2008.06.044
Prytherch DR, Whiteley MS, Higgins B, Weaver PC, Prout WG, Powell SJ (1998) POSSUM and Portsmouth POSSUM for predicting mortality. Physiological and operative severity score for the enumeration of mortality and morbidity. Br J Surg 85:1217–1220
Proctor MJ, Morrison DS, Talwar D et al (2011) A comparison of inflammation-based prognostic scores in patients with cancer. A Glasgow inflammation outcome study. Eur J Cancer 47:2633–2641. doi:10.1016/j.ejca.2011.03.028
Nozoe T, Kohno M, Iguchi T et al (2012) The prognostic nutritional index can be a prognostic indicator in colorectal carcinoma. Surg Today 42:532–535
Watanabe T, Itabashi M, Shimada Y et al (2012) Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2010 for the treatment of colorectal cancer. Int J Clin Oncol 17:1–29
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a surgery. Ann Surg 240:205–213
Clavien PA, Barkun J, de Oliveira ML et al (2009) The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 250:187–196. doi:10.1097/SLA.0b013e3181b13ca2
Ulander K, Jappsson B, Gragn G (1997) Quality of life and independence in activities of daily living preoperatively and at follow-up patients with colorectal cancer. Support Care Cancer 5:402–409. doi:10.1007/s005200050099
Nanashima A, Abo T, Nonaka T et al (2011) Prognosis of patients with hepatocellular carcinoma after hepatic resection: are elderly patients suitable for surgery? J Surg Oncol 104:284–291. doi:10.1002/jso.21932
Hashimoto D, Takamori H, Hirotaz M, Baba H (2013) Prediction of operative morbidity after pancreatic resection. Hepatogastroenterology 60:1577–1582
Haga Y, Wada Y, Takeuchi H et al (2004) Estimation of physiologic ability and surgical stress (E-PASS) for a surgical audit in elective digestive surgery. Surgery 135:586–594. doi:10.1016/j.surg.2003.11.012
Haga Y, Yagi Y, Ogawa M (1999) Less-invasive surgery for gastric cancer prolongs survival in patients over 80 years of age. Surg Today 29:842–848
Stephen JB, William JC (2006) Validation of the CR-POSSUM risk-adjusted scoring system for major colorectal cancer surgery in a single center. Dis Colon Rectum 50:192–196
Senagore AJ, Warmuth AJ, Delaney CP, Tekkis PP, Fazio VW (2004) POSSUM, p-POSSUM, and Cr-POSSUM: implementation issues in a United States health care system for prediction of outcome for colon cancer resection. Dis Colon Rectum 47:1435–1441. doi:10.1007/s10350-004-0604-1
Nozoe T, Kimura Y, Ishida M, Saeki H, Korenaga D, Sugimachi K (2002) Correlation of pre-operative nutritional condition with post-operative complications in surgical treatment for esophageal carcinoma. Eur J Surg 28:396–400
Ionescu D, Tibrea C, Puia C (2013) Pre-operative hypoalbuminemia in colorectal cancer patients undergoing elective surgery-a major risk factor for postoperative outcome. Chirurgia 108:822–828
Blackburn GL, Bistrian BR, Maini BS, Schlamm HT, Smith MF (1977) Nutritional and metabolic assessment of the hospitalized patient. JPEN J Parenter Enteral Nutr 1:11–22. doi:10.1177/014860717700100111
Ji FF, Ying H, Jin SL (2013) Combination of neutrophil lymphocyte ratio and platelet lymphocyte ratio is a useful predictor of postoperative survival in patients with esophageal squamous cell carcinoma. Onco Targets Ther 6:1605–1612
He W, Yin C, Guo G et al (2013) Initial neutrophil lymphocyte ratio is superior to platelet lymphocyte ratio as an adverse prognostic and predictive factor in metastatic colorectal cancer. Med Oncol 30:439–445. doi:10.1007/s12032-012-0439-x
Smith RA, Bosonnet L, Reraty M et al (2009) Preoperative platelet-lymphocyte ratio is an independent significant prognostic marker in resected pancreatic ductal adenocarcinoma. Am J Surg 197:466–472. doi:10.1016/j.amjsurg.2007.12.057
Walker KG, Bell SW, Rickard MJ et al (2004) Anastomotic leakage is predictive of diminished survival after potentially curative resection for colorectal cancer. Ann Surg 240:255–259. doi:10.1097/01.sla.0000133186.81222.08
Lagarde SM, de Boer JD, ten Kate FJ, Busch OR, Obertop H, van Lanschot JJ (2008) Postoperative complications after esophagectomy for adenocarcinoma of the esophagus are related to timing of death due to recurrence. Ann Surg 247:71–76. doi:10.1097/SLA.0b013e31815b695e
Tsujimoto H, Ichikura T, Ono S et al (2009) Impact of postoperative infection on lomg term survival after potentially curative resection for gastric cancer. Ann Surg Oncol 16:311–318
Bakker IS, Grossmann I, Henneman D, Havendga K, Wiggers T (2014) Risk factors for anastomotic leakage and leak-related mortality after colonic cancer surgery in a nationwide audit. Br J Surg 101:424–432. doi:10.1002/bjs.9395
Stein BN, Petrelli NJ, Douglass HO, Discoll DL, Arcangeli G, Meropol NJ (1995) Age and sex are independent predictors of 5-fluorouracil toxicity. Analysis of a large scale phase III trial. Cancer 75:11–17. doi:10.1002/1097-0142(19950101)75:1%3C11::AID-CNCR2820750104%3E3.0.CO;2-N
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
For this type of study, formal consent is not required.
Conflict of interest
The authors declare that they have no competing of interests.
Rights and permissions
About this article
Cite this article
Tominaga, T., Takeshita, H., Takagi, K. et al. E-PASS score as a useful predictor of postoperative complications and mortality after colorectal surgery in elderly patients. Int J Colorectal Dis 31, 217–225 (2016). https://doi.org/10.1007/s00384-015-2456-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-015-2456-7