Abstract
Purpose
Lately, the main technical innovations in the field of colorectal surgery have been the introduction of laparoscopic and robotic techniques; the aim of this study is to investigate the results and the advantages of these two surgical approaches.
Methods
Twenty-two studies including 1652 laparoscopic and 1120 robotic-assisted resections were analyzed and categorized into right, left, and pelvic resections of the middle/low rectum, aiming to the following outcomes: operating time, blood loss, bowel function recovery, return to oral intake, morbidity, hospital stay, and costs.
Results
The vast majority of the studies were non-randomized investigations (19/22 studies) enrolling small cohorts of patients (median 55.0 laparoscopic and 34.5 robotic-assisted group) with a mean age of 62.2–61.0 years. Funnel plot analysis documented heterogeneity in studies which combined cancers and benign diseases.
Our meta-analysis demonstrated a significant difference in favor of laparoscopic procedures regarding costs and operating time (standardized mean difference (SMD) 0.686 and 0.493) and in favor of robotic surgery concerning morbidity rate (odds ratio (OR) 0.763), although no benefits were documented when analyzing exclusively randomized trials. When we differentiated approaches by side of resections, a significant difference was found in favor of the laparoscopic group when analyzing operating time in left-sided and pelvic procedures (SMD 0.609 and 0.529) and blood loss in pelvic resections (SMD 0.339).
Conclusion
Laparoscopic techniques were documented as the shorter procedures, which provided lower blood loss in pelvic resections, while morbidity rate was more favorable in robotic surgery. However, these results could not be confirmed when we focused the analysis on randomized trials only.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colon and colorectal resections are some of the most common surgical procedures. They can be regarded as the standard of care for non-metastatic colon cancers and are often required for treating benign diseases [1–3].
Over the last few years, considerable progress has been made in improving survival and quality of life of patients undergoing colorectal surgery, the main technical innovations being the introduction of laparoscopy in 1991 [4] and the robotic DaVinci system 10 years later [5].
The introduction of laparoscopy was initially the cause for concern regarding the learning curve, and a possible development of port-site metastasis or inadequate oncologic resections [6–9]. All these problems were subsequently resolved, and a number of studies recognized the benefits of minimally invasive surgery. These advantages included lower blood loss, earlier recovery of bowel function, earlier return to oral intake, and shorter hospital stay [10].
Similarly, some authors recently reported the technical advantages of robotic surgery (e.g., 3D imaging and higher degrees of articulation and rotation) as particularly useful for dissecting intra-pelvic rectal cancers [11].
Nevertheless, there is little scientific literature available regarding studies that compare robotic versus laparoscopic colorectal resections, and they generally include heterogeneous types of research, with considerable variation in their design (randomized/non-randomized), the outcome measures considered (short/long-term results, costs analysis) and the selection criteria of patients (colon and/or rectal cancers/benign diseases). This, naturally, makes difficult to summarize results.
Thus, on the basis of this background and in order to highlight benefits and the short-term advantages of these techniques, we aimed this manuscript to systematic review and a comprehensive meta-analysis of studies which compare laparoscopic and robot-assisted colorectal resections.
Our primary objective was to highlight the designs and selection criteria and patients’ clinical and demographical features, along with surgical and functional postoperative results (operating time, blood loss, hospital stay, number of nodes harvested in the specimens), differentiating results whenever possible according to the side of resection. Of note, there have been few studies that have made this type of differentiation, and we feel that it might help readers in understanding the analyses. Moreover, we reviewed and meta-analyzed the costs related to these procedures.
Materials and methods
Data source and study design
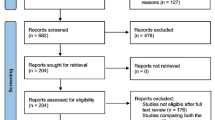
This investigation was conducted in accordance with the PRISMA Statements for review and meta-analysis (Fig. 1). We conducted a systematic review of literature by searching the PubMed, Ovid (Wolters Kluwer®), and ScienceDirect (Elsevier®) databases for all published series and trials which compared laparoscopic and robotic-assisted colorectal resections. Keywords “laparoscopic vs robotic colectomy” and “laparoscopic vs robotic rectal resection” of “English” language, limited to human, including clinical trials and comparative studies, were searched. We also included references from retrieved publications. Duplicate references were removed by manual search. The authors of this study were blinded to authors’ and journals’ names while reviewing the series and did not have any contact with the authors of the included papers. We did not consider any journal’s scores (e.g., journal’s Impact Factors) of published articles as exclusion criteria. Each paper retrieved was assessed for inclusion or exclusion in our study, by revision of titles and abstracts. Published laparoscopic series which lacked a robotic-assisted group or vice versa were excluded (see exclusion list, Fig. 1).
Systematic review
Patients from different studies were combined in a pooled analysis for statistical analyses, providing a systematic review of the literature. A first analysis was conducted in order to outline study designs (randomized/non-randomized studies) and the populations included in each group (age at the time of surgery, BMI, American Society of Anaesthesiologists (ASA) score, stage and rate of cancer patients). In order to assess a possible bias, those manuscripts which included a mixed series of cancer and benign diseases were evaluated using a funnel plot analysis.
The subsequent analyses focused on the surgical and functional results of studies that compare laparoscopic and robotic-assisted resections, overall or one single side at the time. In fact, whenever possible, we categorized studies according to the side of resection as follows: (a) right colon resections, (b) left colon resections, and (c) pelvic resections (if included a resection of the middle/low rectum).
Outcome measures
We considered as short-term outcome measures (a) operating time (measured in minutes), (b) blood loss (measured in milliliters), (c) bowel function recovery (defined by the passage of the first flatus/stool; measured in days), (d) return to oral intake (usually liquid diet; measured in days), (e) morbidity (defined by the rate of peri-operative complications), and (f) hospital stay (measured in days).
Of note, since complications were often reported using different modalities (e.g., major vs minor complications, and rate of adverse events), we extrapolated the overall morbidity rate in each group (laparoscopic and robotic-assisted surgery).
The oncologic adequateness was assessed as the mean number of lymph nodes harvested in surgical specimens. Cost analysis was conducted by recording the overall hospital costs for both procedures. These costs are expressed in US dollars in order to better compare results.
We did not consider conversion surgery or hospital volume, since they are seldom reported, even though the learning curves and the volume of patients might generate variations in short-term results and costs.
Statistics and meta-analysis
Categorical variables were analyzed using frequencies and percentages, and groups were compared using chi-square tests. Continuous variables were provided using means and medians. Moreover, standard deviations (SDs), standard errors (SEs), range, and 95 % confidence intervals (95%CIs) were also recorded.
Age at surgery and BMI were recorded, presented using a weighted mean analysis, and analyzed using a t test according to the formulas provided by Bland and Kerry [12].
A funnel plot was computed using the MetaXL v.2.2 meta-analysis tool (EpiGear, Brisbane, Australia), showing in the horizontal axis the ln odds ratio (OR) and in the vertical line the SE calculated using the inverse variance fixed effects model.
Meta-analyses were conducted when at least three studies provided computable variables. The Mantel–Haenszel method was used to calculate the weighted summary odds ratio. The heterogeneity statistic was then incorporated to calculate the summary odds ratio under the random effects model. The total odds ratio with 95%CI was given both for the fixed effects model and the random effects model, assuming that if the value 1 is not within the 95%CI, the odds ratio is statistically significant at the 5 % level (p < 0.05). For the meta-analysis of studies with a continuous measure (comparison of means between treated cases and controls), the Hedges g statistic was used for the formulation of the standardized mean difference (SMD). The heterogeneity statistic was then incorporated to calculate the summary standardized mean difference under the random effects model, assuming that if the value 0 is not within the 95%CI, the SMD is statistically significant at the 5 % level (p < 0.05). Statistical heterogeneity of results was assessed on the basis of a test of heterogeneity. Of note, if the test of heterogeneity is statistically significant (p < 0.05), more emphasis should be placed on the random effects model.
Statistical analysis was performed using MedCalc for Windows, version 10.2.0.0 (MedCalc Software®, MariaKerke, Belgium). All tests were two-tailed and a p < 0.05 was considered to be statistically significant.
Results
Study design and patients: systematic review
Figure 1 shows the 22 studies analyzed, which included 2772 patients: 1652 laparoscopic and 1120 robotic-assisted colorectal resections [13–34]. The vast majority of literature has been published over the last 3 years, with just seven papers published before 2010. Almost all the literature is based on case–control series except the papers by Park in 2012, Baik in 2009, and Jimenez Rodriguez in 2011 which are randomized trials [20, 26, 33].
The vast majority of papers focused on colorectal pelvic resections [13, 15–17, 19, 21, 24–27, 34], whereas two studies evaluated right colectomies [20, 23], one study exclusively left colectomies [30], and the remaining authors presented a mixed series of colorectal resections [14, 18, 28, 29, 31–33].
Table 1 outlines the features of the study populations. Overall, the mean number of patients enrolled in laparoscopic groups was of 75.1 versus a mean of 50.9 patients in robotic-assisted groups. The median number of patients enrolled was 55 in the laparoscopic resection group and just 34.5 in the robotic-resection group. Because of the great number of papers evaluating pelvic rectal cancers, pelvic resections were the most frequent procedures in robotic-assisted groups (59.7 % of laparoscopic resections vs 67.8 % of robotic-assisted procedures; chi-square test p < 0.0001).
Even though the vast majority of the literature referred to studies on cancer patients, the rate of benign diseases was higher in the robotic-assisted group compared to that in laparoscopic patients, although the difference was not statistically significant (respectively 20.5 vs 17.6 %; chi-square 0.06). Moreover, studies that combined cancer and benign patients were analyzed using a funnel plot (Fig. 2) [14, 18, 23, 28, 29, 31, 32], which indicated a mild asymmetry due to heterogeneity in studies conducted by Spinoglio [28] (SE 0.47; ln OR 0.63), Deutsch [18] (SE 0.43; ln OR −1.55), and D’Annibale [31] (SE 0.43; ln OR −1.68), p = 0.0015.
Both groups presented a high prevalence of patients scoring ASA 1/2 (reaching 82.3 % in the robotic-assisted group; Table 1, chi-square 0.002).
Similarly, the rate of stage I/II patients was high but comparable in both groups (laparoscopy vs robotic-assisted, respectively, 65.7 vs 68.2 %, p not significant (ns)). Weighted mean of ages at surgery has been included in Table 1. According to our analysis, the weighted mean of reported ages was 62.2 years in the laparoscopic group and 61.0 years in the robotic assisted group (t test, p ns).
Similarly, the BMI of patients was found to be comparable between groups (weighted mean laparoscopy vs robotic surgery, respectively, 24.0 vs 23.9; t test, p ns).
Outcome measures: systematic review
Table 2 shows the surgical and functional results of the two different procedures according to selected case series. The vast majority of studies reported a longer operating time for the robotic-assisted group, the only exception being Park in 2010 and 2012 [20, 24].
Even though the estimated mean of blood loss varied widely between studies, the vast majority of case series were homogeneous when considering the results between subgroups, with the sole exception of Erguner, who reported lower losses in the group treated with a robotic-assisted procedure [34].
Bowel function recovery was found to be similar between groups, with the sole exception of Miller who reported a shorter recovery time for patients undergoing proctocolectomy via laparoscopic procedures [19] and Lim who reported a shorter recovery for patients treated with robotic-assisted surgery [16].
Moreover, Lim and Baik reported a faster return to oral intake in the robotic-assisted group [16, 26].
Data regarding hospital stay ranged widely between studies. However, Miller alone [19] reported a shorter recovery for patients undergoing proctocolectomy via laparoscopic procedures, while Baik [26] reported a shorter hospitalization in the robotic-assisted group.
Even though the vast majority of studies recognized an adequate yield of nodes retrieved in specimens, the studies by Ielpo and Patritri reported a mean number of nodes <12 [13, 27].
Morbidity rate was found to range from 10.3 to 33.3 % in the laparoscopic group, and between 5.9 and 30.6 % in the robotic-assisted group. Of note, all the case series reported homogeneous results between the two approaches, but only a few of the reviewed studies used a standard classification in order to stratify adverse events (e.g., Clavien’s classification system) [35].
Meta-analysis: overall studies
Figure 3 and Table 3 show the results of the meta-analysis conducted on 21 studies [13, 15–34]. We considered operating time, blood loss, bowel function recovery, time to oral intake, lymph node harvest (LNH), hospital stay, costs, and morbidity rate variables. The standard mean difference (SMD) was significantly different between groups in favor of laparoscopic procedures when considering costs and operating time, and in favor of robotic-assisted group when considering morbidity rate (overall rate 19.4 % in robotic-assisted procedures vs 22.2 % in laparoscopic procedures; OR 0.736; 95%CI 0.607–0.959).
Meta-analysis of studies which compare laparoscopic and robotic assisted colectomies. Miller AT a: proctectomy; Miller AT b: proctectomy with ileal-pouch anal anastomosis; Rawlings Al a: right colectomy; Rawlings AL b: left colectomy; Deutsch GB a: right colectomy; Deutsch GB b: left colectomy; Casillas MA Jr a: right colectomy; Casillas MA Jr b: left colectomy; Casillas MA Jr c: pelvic resection. SMD: standard mean difference; OR: odds ratio
Table 3 shows also the results of the meta-analysis conducted focusing on randomized trials [20, 26, 33]: Although just three variables were computable (operating time, hospital stay, and morbidity rate), we could not document any benefit or advances related to the robotics or laparoscopic procedures.
Meta-analysis: right-sided colectomies and left-sided colectomies
Figure 4 shows the results of the meta-analysis of continuous measures conducted on five studies that reported right-sided colectomies [17, 19, 22, 28–31] and six studies concerning left-sided colectomies [16, 18, 29, 30, 32, 33]. The SMD was computable for operating time, blood losses, and hospital stay variables, and it did not indicate any significant differences between groups for all the variables investigated in right-sided colectomies, whereas operating time was found to be statistically in favor of laparoscopic procedures in left-sided resections (Table 3).
Meta-analysis: pelvic resections
Figure 5 shows the results of the meta-analysis of continuous measures conducted on seven studies which report pelvic resections [13, 17, 19, 21, 24, 26, 27]. The SMD was computable for operating time, blood loss, LNH, and hospital stay variables, and it indicated a significant difference between groups in favor of laparoscopic procedures which analyzed operating time and blood losses variables (Table 3).
Discussion
To date, a number of studies have provided evidence in support of laparoscopic procedures compared to open colorectal resections regarding several short-term outcomes [10], but there have been only a limited number of studies that have investigated the robotic approach, so literature is still at an early stage.
First, it seems important to highlight that we documented some issues concerning study design and power due to the non-randomization (19 out of 22 studies reviewed) and the small number of patients enrolled in each group (median 34.5–55 patients, Table 1). Moreover, funnel plot analysis indicated heterogeneity in studies which combined cancer and benign diseases. According to the recommendations provided by Sterne and co-authors [36], this asymmetry could be related to a possible bias, even though the visual interpretation alone might not be a sufficient criterion. Furthermore, this analysis was conducted on only seven studies. Nevertheless, both these issues (power and histology selection) should be considered in the design of future studies that aim to compare these approaches.
Finally, we found a prevalence of studies which focused on rectal resections and which enrolled low-risk patients (<65 years old, ASA 1/2, low BMI). This data mirrors the early stage of the literature in this field: Indeed, a certain bias of selection of less co-morbid patients can occur when introducing into practice a new procedure, like robotic resections. In order to validate the selection criteria, we compared the clinical data of a series of unselected patients that had undergone right-sided laparoscopic resections at our department between 2005 and 2012 (49 patients) with those of the right-sided colectomies herein reviewed. Even though a trend of prevalence of ASA 1/2 and stage I/II patients was registered in the robotic-assisted groups, subgroups were found to be homogeneous for these variables, with similar age and BMI (chi-square test and t test, p ns; data not shown). Therefore, we could consider these demographical features to be consistent with our clinical setting.
According to our results, a number of authors documented differences of statistical value in relation to some outcome measures, but the main findings regarded operating time, morbidity rate, and costs. Overall, operating time has been reported to be longer in robotic procedures by the vast majority of authors, and this trend was confirmed in our meta-analysis, possibly because of the early stage of learning curves. A possible explanation could be also related to the excursion of the robotic arms and the length of the surgical instruments that may limit the robotic system. Moreover, there have been reports regarding the difficulties the robotic instruments have in reaching splenic flexure and pelvis and in dissecting different abdominal areas which may require a repositioning of the surgical cast. In order to address these issues, a new generation of daVinci S robot has been recently introduced [37]. Furthermore, as documented by Lim, the progress of the learning curve has a significant impact on operating time, both on the docking and on the consol periods. The results for robotic total mesorectal excision are evidenced after the first 32 cases [38]. On this basis, we would expect a reduction in this difference in the near future, when the use of the robotic instruments becomes a regular practice.
Interestingly, the advantages documented in favor of the laparoscopic group (shorter operating time and minor blood losses) or those highlighted in the robotic patients (lower morbidity rate) could be replicated when we focused the analysis on randomized trials.
A recent meta-analysis which focused on rectal resections documented that conversion rate in the robotic group was significantly lower than in laparoscopic total mesorectal excisions, although no significant differences were reported when analyzing operating time, estimated blood loss, recovery outcome, postoperative morbidity and mortality, length of hospital stay, oncological accuracy of resection, and local recurrences between groups. Moreover, the positive rate of circumferential resection margins and the incidence of erectile dysfunction were found to be lower in robotic group compared to those for laparoscopy [39]. Consistent with these findings, we also found some divergent results, since our meta-analysis highlighted a lower morbidity rate in the robotic-assisted group and lower blood loss in laparoscopic pelvic resections.
Robotic procedures are emerging in gynecology, urology, and abdominal/pelvic surgery [37] and, according to Pigazzi, the most promising frontier regards the total mesorectal excision, since a 3D visualization and greater ability to retract in the deep and narrow pelvis may allow a more precise dissection and may reduce the risk of complications related to pelvic nerve injuries [40].
Costs, however, could remain an issue, since they, as is to be expected, were found to be higher in the robotic-assisted group.
On this basis, we should all wait with great interest for the results of the ROLARR trial, a world-based, prospective, randomized, controlled, un-blinded trial which compares robotic-assisted versus standard laparoscopic surgery for the curative treatment of rectal cancer. This trial commenced in the UK in 2011 and it aims to recruit 400 patients. It will investigate differences in terms of rate of conversion, rate of pathological involvement of circumferential resection margin, 3-year local recurrence, and disease-free and overall survival rates, and also operative morbidity and mortality, quality of life, and cost-effectiveness [41].
Conclusions
It is important to highlight the striking progress that has been made in the last 20 years mostly due to the introduction of these technologies in surgical practice. On the basis of our results, laparoscopic procedures were shorter, providing lower blood losses in pelvic resections. However, morbidity rate was better in robotic surgery. These findings could not be confirmed when we focused the analysis on randomized trials.
Future studies need to focus on (a) the design of powered trials to evaluate benefits, limitations, and cost-effectiveness of these procedures; (b) establishing a clear differentiation of results through histology and side of resection; and (c) a reduction of the training and learning curves which may result in comparable or shorter operating times.
References
National Comprehensive Cancer Network, NCCN Guidelines – Colon cancer, version 1.2013. www.nccn.org.
Bernell O, Lapidus A, Hellers G (2000) Risk factors for surgery and postoperative recurrence in Crohn’s disease. Ann Surg 231(1):38–45
Jafferji MS, Hyman N (2014) Surgeon, not disease severity, often determines the operation for acute complicated diverticulitis. J Am Coll Surg 218(6):1156–1161
Jacobs M, Verdeja JC, Goldstein HS (1991) Minimally invasive colon resection (laparoscopic colectomy). Surg Laparosc Endosc 1:144–150
Talamini M, Chapman S, Horgan S, Melvin W (2003) A prospective analysis of 211 robotic-assisted surgical procedures. Surg Endosc 17:1521–1524
Jacquet P, Averbach AM, Jacquet N (1995) Abdominal wall metastasis and peritoneal carcinomatosis after laparoscopic-assisted colectomy for colon cancer. Eur J Surg Oncol 21:568–570
Akle CA (1996) Early parietal recurrence of adenocarcinoma of the colon after laparoscopic colectomy. Port site metastasis after laparascopic colorectal surgery for cure of malignancy. Br J Surg 83:427
Wexner SD, Cohen SM (1995) Port site metastases after laparoscopic colorectal surgery for cure of malignancy. Br J Surg 82:295–298
Ramos JM, Gupta S, Anthone GJ, Ortega AE, Simons AJ, Beart RW (1994) Laparoscopy and colon cancer. Is the port site at risk? A preliminary report. Arch Surg 129:897–899
Lorenzon L, La Torre M, Ziparo V, Montebelli F, Mercantini P, Balducci G, Ferri M (2014) Evidence based medicine and surgical approaches for colon cancer: evidences, benefits and limitations of the laparoscopic vs open resection. World J Gastroenterol 20(13):3680–3692
Fung AK, Aly EH (2013) Robotic colonic surgery: is it advisable to commence a new learning curve? Dis Colon Rectum 56(6):786–796
Bland JM, Kerry SM (1998) Weighted comparison of means. BMJ 316:129
Ielpo B, Caruso R, Quijano Y, Duran H, Diaz E, Fabra I, Oliva C, Olivares S, Ferri V, Ceron R, Plaza C, Vicente E (2014) Robotic versus laparoscopic rectal resection: is there any real difference? A comparative single center study. Int J Med Robot. doi:10.1002/rcs.1583
Casillas MA Jr, Leichtle SW, Wahl WL, Lampman RM, Welch KB, Wellock T, Madden EB, Cleary RK (2013) Improved perioperative and short-term outcomes of robotic versus conventional laparoscopic colorectal operations. Am J Surg. doi:10.1016/j.amjsurg.2013.08.028
Fernandez R, Anaya DA, Linda TL, Orcutt ST, Balentine CJ, Awad SA, Berger DH, Albo DA, Artinyan AA (2013) Laparoscopic versus robotic rectal resection for rectal cancer in a veteran population. Am J Surg 206:509–517
Lim DR, Min BS, Kim MS, Alasari S, Kim G, Hur H, Baik SH, Lee KY, Kim NK (2013) Robotic versus laparoscopic anterior resection of sigmoid colon cancer: comparative study of long-term oncologic outcomes. Surg Endosc 27(4):1379–1385. doi:10.1007/s00464-012-2619-3
Kang J, Yoon KJ, Min BS, Hur H, Baik SH, Kim NK, Lee KY (2013) The impact of robotic surgery for mid and low rectal cancer: a case-matched analysis of a 3-arm comparison--open, laparoscopic, and robotic surgery. Ann Surg 257(1):95–101. doi:10.1097/SLA.0b013e3182686bbd
Deutsch GB, Sathyanarayana SA, Gunabushanam V, Mishra N, Rubach E, Zemon H, Klein JD, Denoto G 3rd (2012) Robotic vs laparoscopic colorectal surgery: an institutional experience. Surg Endosc 26(4):956–963
Miller AT, Berian JR, Rubin M, Hurst RD, Fichera A, Umanskiy K (2012) Robotic-assisted proctectomy for inflammatory bowel disease: a case-matched comparison of laparoscopic and robotic technique. J Gastrointest Surg 16(3):587–594
Park JS, Choi GS, Park SY, Kim HJ, Ryuk JP (2012) Randomized clinical trial of robot-assisted versus standard laparoscopic right colectomy. Br J Surg 99(9):1219–1226
Baek SJ, Kim SH, Cho JS, Shin JW, Kim J (2012) Robotic versus conventional laparoscopic surgery for rectal cancer: a cost analysis from a single institute in Korea. World J Surg 36(11):2722–2729. doi:10.1007/s00268-012-1728-4
Bertani E, Chiappa A, Biffi R, Bianchi PP, Radice D, Branchi V, Cenderelli E, Vetrano I, Cenciarelli S, Andreoni B (2011) Assessing appropriateness for elective colorectal cancer surgery: clinical, oncological, and quality-of-life short-term outcomes employing different treatment approaches. Int J Color Dis 26(10):1317–1327
deSouza AL, Prasad LM, Park JJ, Marecik SJ, Blumetti J, Abcarian H (2010) Robotic assistance in right hemicolectomy: is there a role? Dis Colon Rectum 53:1000–1006
Park JS, Choi GS, Lim KH, Jang YS, Jun SH (2010) Robotic-assisted versus laparoscopic surgery for low rectal cancer: case-matched analysis of short-term outcomes. Ann Surg Oncol 17(12):3195–3202. doi:10.1245/s10434-010-1162-5
Bianchi PP, Ceriani C, Locatelli A, Spinoglio G, Zampino MG, Sonzogni A, Crosta C, Andreoni B (2010) Robotic versus laparoscopic total mesorectal excision for rectal cancer: a comparative analysis of oncological safety and short-term outcomes. Surg Endosc 24(11):2888–2894. doi:10.1007/s00464-010-1134-7
Baik SH, Kwon HY, Kim JS, Hur H, Sohn SK, Cho CH, Kim H (2009) Robotic versus laparoscopic low anterior resection of rectal cancer: short-term outcome of a prospective comparative study. Ann Surg Oncol 16(6):1480–1487. doi:10.1245/s10434-009-0435-3
Patriti A, Ceccarelli G, Bartoli A, Spaziani A, Biancafarina A, Casciola L (2009) Short- and medium-term outcome of robot-assisted and traditional laparoscopic rectal resection. JSLS 13(2):176–183
Spinoglio G, Summa M, Priora F, Quarati R, Testa S (2008) Robotic colorectal surgery: first 50 cases experience. Dis Colon Rectum 51(11):1627–1632
Rawlings AL, Woodland JH, Vegunta RK, Crawford DL (2007) Robotic versus laparoscopic colectomy. Surg Endosc 21:1701–1708
Woeste G, Bechstein WO, Wullstein C (2005) Does telerobotic assistance improve laparoscopic colorectal surgery? Int J Color Dis 20(3):253–257
D'Annibale A, Morpurgo E, Fiscon V, Trevisan P, Sovernigo G, Orsini C, Guidolin D (2004) Robotic and laparoscopic surgery for treatment of colorectal diseases. Dis Colon Rectum 47(12):2162–2168
Delaney CP, Lynch AC, Senagore AJ, Fazio VW (2003) Comparison of robotically performed and traditional laparoscopic colorectal surgery. Dis Colon Rectum 46(12):1633–1639
Jiménez Rodríguez RM, Díaz Pavón JM, de La Portilla de Juan F, Prendes Sillero E, Hisnard Cadet Dussort JM, Padillo J (2011) Prospective randomised study: robotic-assisted versus conventional laparoscopic surgery in colorectal cancer resection. Cir Esp 89(7):432–438
Erguner I, Aytac E, Boler DE, Atalar B, Baca B, Karahasanoglu T, Hamzaoglu I, Uras C (2013) What have we gained by performing robotic rectal resection? Evaluation of 64 consecutive patients who underwent laparoscopic or robotic low anterior resection for rectal adenocarcinoma. Surg Laparosc Endosc Percutan Tech 23(3):316–319
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240(2):205–213
Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, Carpenter J, Rücker G, Harbord RM, Schmid CH, Tetzlaff J, Deeks JJ, Peters J, Macaskill P, Schwarzer G, Duval S, Altman DG, Moher D, Higgins JP (2011) Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 343:d4002
Ahmed K, Khan MS, Vats A, Nagpal K, Priest O, Patel V, Vecht JA, Ashrafian H, Yang GZ, Athanasiou T, Darzi A (2009) Current status of robotic assisted pelvic surgery and future developments. Int J Surg 7(5):431–440
Kim HJ, Choi GS, Park JS, Park SY (2014) Multidimensional analysis of the learning curve for robotic total mesorectal excision for rectal cancer: lessons from a single surgeon's experience. Dis Colon Rectum 57(9):1066–1074
Xiong B, Ma L, Zhang C, Cheng Y (2014) Robotic versus laparoscopic total mesorectal excision for rectal cancer: a meta-analysis. J Surg Res 188(2):404–414
Pigazzi A, Garcia-Aguilar J (2010) Robotic colorectal surgery: for whom and for what? Dis Colon Rectum 53(7):969–970
Collinson FJ, Jayne DG, Pigazzi A, Tsang C, Barrie JM, Edlin R, Garbett C, Guillou P, Holloway I, Howard H, Marshall H, McCabe C, Pavitt S, Quirke P, Rivers CS, Brown JM (2012) An international, multicentre, prospective, randomised, controlled, unblinded, parallel-group trial of robotic-assisted versus standard laparoscopic surgery for the curative treatment of rectal cancer. Int J Color Dis 27(2):233–241
Acknowledgments
The authors thank Mr. Michael Gerald Kenyon for his help in editing the manuscript.
Conflict of interest
None of the authors has any potential financial conflict of interest related to this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lorenzon, L., Bini, F., Balducci, G. et al. Laparoscopic versus robotic-assisted colectomy and rectal resection: a systematic review and meta-analysis. Int J Colorectal Dis 31, 161–173 (2016). https://doi.org/10.1007/s00384-015-2394-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-015-2394-4