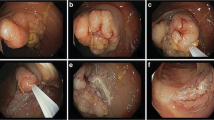
Abstract
Purpose
To evaluate the success and complication rates of endoscopic mucosal resections (EMR) for large flat adenomas and to identify risk factors for adenoma recurrence.
Methods
We evaluated all consecutive patients treated with EMR at our institution between 2003 and 2005 that fulfilled the following criteria: >10-mm diameter, Paris 0-Is and 0-IIa-c, and endoscopic follow-up. We conducted univariate analysis and multivariate analysis using a non-stratified logistic regression model to identify possible influencing factors.
Result
In a median follow-up period of 6 years, we analyzed 177 EMR procedures, with a mean size of 21 mm. The majority of the resections were in the right colon. Recurrence occurred in 29 patients. Further treatment of patients with recurrence was endoscopic in 27 patients, whereas 1 patient was treated with transanal endoscopic microsurgery and one underwent surgery. The variables influencing the multivariate model were resection technique, immediate complication age, and histology.
Conclusions
We show that EMR can achieve a long-term clearance of large flat adenomas. A recurrence after EMR does not equal to failed therapy. The possibility of recurrence has to be considered in the clinical implementation of EMR. An important part of the stratifying factors for follow-up is the procedural assessment of the effectiveness of the resection and the resection technique.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colonoscopy and endoscopic resection of adenomas leads to a reduced incidence and mortality of colorectal cancer [1]. Endoscopic mucosal resection (EMR) is an established technique for the resection of adenomas >1 cm. EMRs are used routinely to resect flat adenomas 10–20 mm in size and are more often used to treat flat adenomas >20 mm. Landmark studies have shown that polypectomy leads to a reduced incidence of colon cancer [1].
The goal of therapy is to spare the patient a surgical operation with a safe, less invasive, and an oncological-equivalent therapy. No patient should develop cancer at the resected area. Incomplete resection rates of large (10–20 mm) adenomas are described as being 17.3 % [2], and published recurrence rates after EMR for adenomas >20 mm are up to 55 % [3, 4]. This raises two issues: whether these recurrence rates with large adenomas equal failed therapy for those with a recurrence, and if there is a way to identify subjects at risk for recurrence. To address these issues, it is important to have long-term follow-up to capture late recurrences and the impact of recurrent endoscopies [3]. Therefore, we selected a cohort with a median follow-up time of 6 years. The intention of our study was to evaluate the success and complication rates of EMR for large 0-Is and 0-IIa-c adenomas, and to identify by univariate and multivariate analysis the risk factors for recurrence to develop tailored follow-up implementation.
Methods
EMR is described in detail in an earlier publication [5]. In brief, the lesions were subjected to submucosal needleless or needle injection at a pressure of 25 bar using a 0.9 % NaCl solution containing 0.1 % methylene blue. Resection was performed with a conventional resection snare 30 or 40 mm in diameter (medwork GmbH, Hoechstadt/Aisch, Germany) using ENDO CUT, Effekt 3,120 W (ICC 200; Erbe Elektromedizin, Tuebingen, Germany). The investigator assessed the completeness of the resection macroscopically.
We considered all consecutive patients for analysis in the time period from January 1, 2003 to December 31, 2005. All initial endoscopic procedures in 2003 were prospectively documented in an accessible database. Since January 1, 2004, all endoscopic procedures have been documented with the software Clinic WinData (E&L medical systems GmbH, Erlangen, Germany). The databases were searched for all patients with an EMR of a 0-Is to 0-IIa-c lesion, ≥10 mm in the longest diameter. Size was estimated intraprocedural by the examiner. All interventions were reassessed. In the case of external follow-up endoscopy, we assessed the colonoscopy report. In the analysis for recurrence, all patients with a follow-up colonoscopy were included, or in the case of rectal EMR, follow-up rectoscopy was performed and in the case of EMR in the sigmoid colon, follow-up sigmoidoscopy was performed. Patients were assessed for the development of a recurrence at the documented EMR site. Recurrence at the resection site was assumed if at least one of three criteria was fulfilled: the adenoma was localized at a scar, in the same anatomical position or if the location was initially described in centimeters from the anus in the same location, and the same colonoscopy phase, either while insertion or withdrawal phase. All patients that were not included in the analysis were documented separately.
Certain criteria were assessed in each intervention, i.e., localization of the EMR, EMR/patient, size, resection technique, histology, immediate complications (ICOM) consisting of immediate bleeding and perforation and delayed complications (DCOM) consisting of delayed bleeding and delayed perforation. Immediate bleeding was defined as intraprocedural bleeding needing an intervention either with 1:10000 diluted epinephrine, argon plasma coagulation (APC) or endoscopic clip application. Delayed bleeding was defined as bleeding after the end of the resection needing an additional endoscopy. Immediate perforation was defined as a visible lesion of the muscular layer.
Statistics
Continuous variables are expressed by using mean or median for skewed data. Age and size were calculated as continuous variables and were grouped in size and age groups. Frequencies (percentage) were used for categorical variables. The chi-square test or Fisher exact test and the odds ratio confidence interval of 95 % were used to analyze the association between categorical variables and recurrence. The Mann–Whitney or Student's t test was used for quantitative parameters and to compare the distribution of continuous variables by outcome. For the univariate analyses, statistical significance was stated with a significance level of α = 0.05.
For the multivariate analysis, we used a non-stratified logistic regression model to identify possible influencing factors. Starting with all factors, the binary target variable was recurrence vs. no recurrence of adenoma. The Hosmer–Lemeshow test was used as a goodness of fit level test. The impact of variables was measured by means of the Wald χ 2 test. Odds ratio estimates inclusive 95 % Wald confidence limits are given for those estimates.
For statistical analyses, the statistics software of CS9-Cytel Studio, Version 9.0.0 (StatXact-9 and LogXact-9, Cytel Inc. 675 Massachusetts Ave. Cambridge, MA 02139 USA2010), and SAS 9.2 (SAS Institute Inc., Cary, NC, USA, 2013) have been used.
Results
In the time period, from January 2003 to December 2005, 227 patients were treated with 282 EMR procedures in our institution. We acquired follow-up data on 209 patients (91.6 %, 209/227). Of these 209 patients, 52 patients had no surveillance colonoscopy, 41 patients declined a colonoscopy, and 11 patients died before the planned surveillance. Of the 11 patients, 2 died due to sepsis, 2 died due to acute-decompensated heart failure by existing chronic heart failure, 1 patient died due to a cerebral Non-Hodgkin's lymphoma, 1 patient died due to myocardial infarction not related to the colonoscopy, and in the remaining five cases, we only know the date of death, which was in all cases, >6 months from the initial endoscopy.
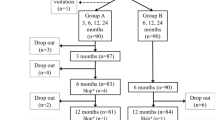
One hundred fifty-seven patients were left with surveillance colonoscopy, as shown in Fig. 1 corresponding to a re-colonoscopy rate of 75 % (157/209).
A total of 10 patients could not be evaluated because they did not develop a recurrence due to surgical resection of the part of the colon bearing the resected area. Of the 10 patients, 2 patients were operated due to low-risk T1 cancer because R0 couldn't be attested by the pathology, 2 patients with low-risk T1, R0 cancer were resected due to the patient's wishes, 2 patients were operated due to a synchronic colorectal cancer, 3 due to perforation, and 1 due to an ileus not related to colonoscopy.
The study population consisted of 147 patients with 177 EMRs translating into 65 and 63 % in relation to the screened population, respectively. The first follow-up surveillance was at a median of 12 months (mean 17 months; range 1–60 months). The median age was 66 years with a range of 37–87 years. The mean size of the EMR was 21.33 mm. There were two main locations, one in the right colon (48 % in the cecum and ascending colon) with a second peak in the rectum (16 %); see Table 1.
In 95 % of the patients, we achieved resection in one procedure. The resection technique was en bloc in 58 %, solely piecemeal in 24 %, piecemeal with adjunctive APC in 14 %, and the resection was done in two separate procedures in 5 %. The histology of the resected specimen is shown in Table 2. The main fraction (61 %) included adenomas with low-grade dysplasia (LGD); see Table 2.
The main immediate complication was bleeding in 14 %; bleeding could be stopped in all endoscopic cases. In 13 (36 %) of the 37 bleeding cases, a second colonoscopy or partial colonoscopy had to be done to stop recurrent bleeding. With regard to all endoscopies, a second interventional endoscopy for bleeding had to be performed in 21 cases (12 %). Perforation occurred in 13 cases. The perforation could be managed solely with clips in 85 % of cases. In two cases, the patients had to be operated on due to perforation; this represented 1.13 % of all patients.
In the surveillance of the 177 EMRs, there were 29 (16.4 %) recurrences, with a mean follow-up of 76 months (standard deviation 8 months, range 66–101 months). In five cases, the recurrence occurred in the second surveillance endoscopy, while all other recurrences were detected in the first control. Three patients with recurrence, had again a recurrence in further follow-up endoscopies after a recurrence-free endoscopy.
The treatment for recurrence was endoscopic for 27 patients (93.1 % of all recurrences); of the remaining two patients, one was treated with transanal endoscopic microsurgery and one underwent surgery; see Fig. 2. The operation was an ileocecal resection due to a perforation after APC of the recurrence in the first surveillance colonoscopy. All other recurring adenomas could be resected, as shown in Fig. 2. In the recurrence group, 16 patients had two interventions, 5 patients had three, 5 patients had four, 1 patient had five, 1 patient had six, and 1 patient had seven interventions; 93 % of patients had five or fewer interventions. In 16 patients, this could be validated with a least one negative control colonoscopy, with a mean of 14.5 months after the last intervention (range 1–75 months).
Because the focus was on the factors influencing a recurrence, for the univariate and multivariate analysis, we analyzed only EMRs with hyperplasia, LGD, high-grade dysplasia (HGD), and the group with lost specimens. The one serrated adenoma was grouped in the LGD group as it had no high-grade dysplasia.
In the univariate analysis, there was a statistical significance only for the absolute size, size group, histology, and resection technique; see Table 3. There was no statistical significance for the differences in age, gender, location of the EMR, ICOM, or DCOM. The latter are indicators that the patient characteristics are comparable in the two subgroups of recurrent and non-recurrent patients. In the univariate analyses, we also calculated the odds ratio for dichotomous variables and tested for trends in the variables with more than two characteristics. There were no significant results at the significance level of α = 0.05.
For the model (variable) selection in multivariate analysis in recurrence of disease we used in the first step all nine potential influencing variables, i.e., gender, age, resection technique, size group, ICOM, DCOM, histology, location, and binominal location. In the analysis of the logistic regression model, 160 observations were considered and 17 rejected. In the best subset selection, we finally ended with four variables, namely age, resection technique, ICOM, and histology which was all associated with some influence in developing recurrence disease in patients.
The logistic model with these four variables showed in the Hosmer–Lemeshow test for the goodness, a p value of 0.5781 indicating that the model is fitted fairly well with these variables. In the global fit statistics, all relevant tests (likelihood ratio-, score-, and Wald-test) had a p value in χ 2 test <0.0001. In the analysis of the maximum likelihood estimates (asymptotic procedure) the p values for age, resection technique, ICOM, and histology were 0.0420, 0.0006, 0.0092, and 0.0843, respectively. The model is shown in Table 4.
The point estimates of the odds ratios and their 95 % Wald confidence limits show that age, resection technique, and ICOM are associated with recurrence in this sample and should be taken as possible influencing factors. Of these three influencing factors, age is seen as the weakest. The confidence interval of histology indicates in this sample that it had no strong impact.
Discussion
No patient in the study cohort developed colorectal cancer after EMR. The main intention of EMR for adenoma is to stop the progression of an advanced adenoma to carcinoma. Advanced adenoma includes adenomas with a higher risk of recurrence and metachronous adenoma. Our collective consisted, per definition, of cases of advanced adenoma because we only included adenomas >1 cm. These lesions were challenging to resect because of the lateral spreading characteristic and the location in the colon.
In a large register-based study in Germany with 840,149 patients, in the same observation period, the annual transition rates from advanced adenoma to colorectal carcinoma varied age dependently from 2.6–5.6 % [6]. A large population-based case–control study showed a risk reduction for colorectal cancer after endoscopic resection for high-risk adenomas. The risk reduction was 60 % for the timeframe of up to 3 years after resection and 50 % in the timeframe of 3–5 years, and there was no risk reduction in the timeframe of 6–10 years in comparison with the control group without colonoscopy [7]. Our collective had a median follow-up period of 6 years and therefore compares to the crucial last time period such that the finding of no occurrence of cancer in this time period in our cohort is an important statement.
There is a relevant risk of developing a recurrence after EMR of large flat adenomas. As we could show, recurrence after EMR does not equal to failed therapy if there is a follow-up. The possibility of recurrence has to be considered in the clinical implementation of EMR.
We had a median recurrence rate of 16.4 % which is comparable to other published cohorts. The recurrence rate of adenomas after EMR in recently published western cohorts and in one multicenter prospective trial for large flat adenomas has been found in the range of 4.2–27 % [3, 4, 8–10].
While establishing a surveillance program, one has to take into consideration that there is a small portion of patients with recurrence beyond the first surveillance endoscopy. We have seen late recurrences in 5/177 (3 %) patients which is in line with published literature [3]. In our further follow-up endoscopies, we found three patients that had a recurrence-free interval and afterwards, a recurrence. Although 3 of 29 (10 %) of all recurrences is a small number which only represents 2 % of all EMRs, this is still a fact one has to be aware of.
Because of the variety of time points for the first surveillance endoscopy in our cohort, we can't conclude the most effective interval for the first surveillance. The fact that we could treat 93.1 % of the recurrence endoscopically with the first surveillance endoscopy after in median 12 months suggests that we had detected our recurrences in time. Taken together with the cases of late recurrence and the mentioned recurrences in case of a negative surveillance endoscopy, we think that a surveillance endoscopy too early is misleading and results in too many endoscopies per patient.
Within the context of the above mentioned, a first surveillance endoscopy after 12 months doesn't seem to be too late. This first surveillance endoscopy must be followed by repeated surveillance endoscopies due to the ongoing risk for recurrence and the risk for metachronous lesions.
To identify risk factors for recurrence, it is important to assure effective colorectal cancer risk reduction and cost-effectiveness of EMR. In our univariate analysis, there was a statistically significant difference in the development of a recurrence according to absolute size, size group, histology, and resection technique. In the multivariate analysis, the factors with a substantial impact on recurrence were ICOM and resection technique. The age and histology (p = 0.042 and p = 0.084 in the maximum likelihood, respectively) also had some impact, but the other factors could not be linked. While the univariate analysis seemed to imply that size is an important factor, size was no longer a significant factor in the multivariate model. For adenomas with a mean size of 21.3 mm and a predominant location in the cecum or ascending colon (48 %), our cohort is comparable to other specialized centers in the western world [8, 11]. Our procedural quality is comparably high as described in the literature. The en bloc resection rate in size and localization comparable cohorts has been found to be 0.54 [4]. The immediate (14 %) and delayed (12 %) bleeding rates seem to be higher than those described in the recent literature of 4–9 and 4–7 %, respectively [4, 8, 12–14]. This could be, in part, explained by the fact that some authors discriminate between intraprocedural bleeding and early bleeding while others do not report immediate bleeding at all [8, 14]. In our opinion, the immediate bleeding rate is noteworthy, as procedural bleeding can lead to a diminished view and a more complicated resection. While we cannot state how much ICOM influences the risk of recurrence, we can state that it is too early to neglect immediate bleeding as risk factor.
One of the strongest predictors in oncology for recurrence is the pathological assessment of R0. This is not applicable in piecemeal resection of adenomas, which occurred in 42 % of patients in our study. However, the resection technique has a substantial impact on recurrence. The assessment of the grade of the dysplasia and the distinction between LGD and HGD showed some association for recurrence in our univariate and multivariate analyses, but the influence in multivariate analysis was not as strong. This is in line with published analyses where there has been no correlation found between the presence of HGD and recurrence in multivariate analysis [4, 15].
While in some series age is not a risk factor for recurrence [4], in other studies, there has been a strong correlation between age and faster progression from adenoma to carcinoma, suggesting variable biology of adenomas in the elderly [6].
Our observation that size is not an independent factor has also been found in other cohorts with patients with EMR, i.e., in 222 patients in the study by Sakamoto et al. and 105 patients in the study by Mannath et al. [15, 16].
The factors with the strongest influence were interventional complications and resection technique. This stresses the importance of the assessment of the effectiveness of the resection by the endoscopist.
Our follow-up period is one of the longest published and we can therefore accurately estimate the true recurrence rate. A longer follow-up period and a larger cohort would be necessary to show an interpretable, may be significant, reduction rate in colorectal cancer incidence, even in the selected patient group with high-risk adenomas. A further limitation is the incomplete follow-up of our patients. This is, in our opinion and supported by the published literature, not uncommon in tertiary endoscopic centers, but should be seen as a limitation on the interpretation of our data. As a consequence from this finding, we intensified our efforts to achieve higher follow-up rates by improved integration of the patient and referring physician.
In our opinion, we show that EMR can achieve long-term clearance of large flat adenomas. The patient has to be informed about the possibility of recurrence, the consequential outcome of such a result, and the obligation for the adherence to follow-up endoscopies. Part of the stratifying factors for the follow-up will be the procedural assessment of the effectiveness of the resection and its technique. It seems to be feasible to start with the first follow-up endoscopy after 12 months, while it is important to repeat controls after the first surveillance endoscopy.
References
Zauber AG, Winawer SJ, O’Brien MJ, Lansdorp-Vogelaar I, van Ballegooijen M, Hankey BF, Shi W, Bond JH, Schapiro M, Panish JF, Stewart ET, Waye JD (2012) Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med 366(8):687–696. doi:10.1056/NEJMoa1100370
Pohl H, Srivastava A, Bensen SP, Anderson P, Rothstein RI, Gordon SR, Levy LC, Toor A, Mackenzie TA, Rosch T, Robertson DJ (2013) Incomplete polyp resection during colonoscopy—results of the Complete Adenoma Resection (CARE) study. YGAST 144(1):74–80. doi:10.1053/j.gastro.2012.09.043, e71
Khashab M, Eid E, Rusche M, Rex DK (2009) Incidence and predictors of “late” recurrences after endoscopic piecemeal resection of large sessile adenomas. YMGE 70(2):344–349. doi:10.1016/j.gie.2008.10.037
Buchner AM, Guarner-Argente C, Ginsberg GG (2012) Outcomes of EMR of defiant colorectal lesions directed to an endoscopy referral center. YMGE 76(2):255–263. doi:10.1016/j.gie.2012.02.060
Belle S, Collet PH, Szyrach M, Ströbel P, Post S, Enderle MD, Kähler G (2011) Selective tissue elevation by pressure for endoscopic mucosal resection of colorectal adenoma: first clinical trial. Surg Endosc 26(2):343–349. doi:10.1007/s00464-011-1873-0
Brenner H, Hoffmeister M, Stegmaier C, Brenner G, Altenhofen L, Haug U (2007) Risk of progression of advanced adenomas to colorectal cancer by age and sex: estimates based on 840,149 screening colonoscopies. Gut 56(11):1585–1589. doi:10.1136/gut.2007.122739
Brenner H, Chang-Claude J, Rickert A, Seiler CM, Hoffmeister M (2012) Risk of colorectal cancer after detection and removal of adenomas at colonoscopy: population-based case–control study. J Clin Oncol 30(24):2969–2976. doi:10.1200/JCO.2011.41.3377
Moss A, Bourke MJ, Williams SJ, Hourigan LF, Brown G, Tam W, Singh R, Zanati S, Chen RY, Byth K (2011) Endoscopic mucosal resection outcomes and prediction of submucosal cancer from advanced colonic mucosal neoplasia. YGAST 140(7):1909–1918. doi:10.1053/j.gastro.2011.02.062
Moss A, Williams SJ, Hourigan LF, Brown GJ, Zanati SA, Singh R, Tam W, Byth K, Bourke MJ (2012) 1143 Long term recurrence following wide field endoscopic mucosal resection (WF-EMR) for advanced colonic mucosal neoplasia—results of the Australian Colonic EMR (ACE) Multicenter prospective study of 940 patients. Gastrointestinal Endoscopy 75(4)
Swan MP, Bourke MJ, Alexander S, Moss A, Williams JS (2009) Large refractory colonic polyps: is it time to change our practice? A prospective study of the clinical and economic impact of a tertiary referral colonic mucosal resection and polypectomy service (with videos). YMGE 70(6):1128–1136. doi:10.1016/j.gie.2009.05.039
Conio M, Repici A, Demarquay JF, Blanchi S, Filiberti R (2004) EMR of large sessile colorectal polyps. Gastrointestinal Endoscopy 60(2):234–241
Luigiano C, Consolo P, Scaffidi M, Strangio G, Giacobbe G, Alibrandi A, Pallio S, Tortora A, Melita G, Familiari L (2009) Endoscopic mucosal resection for large and giant sessile and flat colorectal polyps: a single-center experience with long-term follow-up. Endoscopy 41(10):829–835. doi:10.1055/s-0029-1215091
Ah Soune P (2010) Large endoscopic mucosal resection for colorectal tumors exceeding 4 cm. World J Gastroenterol 16(5):588. doi:10.3748/wjg.v16.i5.588
Binmoeller KF, Weilert F, Shah J, Bhat Y, Kane S (2012) “Underwater” EMR without submucosal injection for large sessile colorectal polyps (with video). YMGE 75(5):1086–1091. doi:10.1016/j.gie.2011.12.022
Sakamoto T, Matsuda T, Otake Y, Nakajima T, Saito Y (2012) Predictive factors of local recurrence after endoscopic piecemeal mucosal resection. J Gastroenterol 47(6):635–640. doi:10.1007/s00535-011-0524-5
Mannath J, Subramanian V, Singh R, Telakis E, Ragunath K (2011) Polyp recurrence after endoscopic mucosal resection of sessile and flat colonic adenomas. Dig Dis Sci 56(8):2389–2395. doi:10.1007/s10620-011-1609-y
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Belle, S., Haase, L., Pilz, L.R. et al. Recurrence after endoscopic mucosal resection—therapy failure?. Int J Colorectal Dis 29, 209–215 (2014). https://doi.org/10.1007/s00384-013-1783-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-013-1783-9