Abstract
Background and aims
Piecemeal endoscopic mucosal resection (EMR) of large nonpedunculated colorectal adenomas is associated with significant recurrence rates. After salvage endoscopic treatment of recurrences, there is a significant rate of second recurrences. There is a paucity of data on the efficacy and safety of continued endoscopic treatment after a second recurrence.
Methods
Consecutive patients with recurrent adenomas after initial piecemeal EMR of nonpedunculated colorectal adenomas >2 cm were reviewed. We assessed the feasibility, safety and efficacy of continued endoscopic treatment in these patients.
Results
Sixty-four patients with 70 recurrent lesions were identified. All were retreated endoscopically. Follow-up colonoscopy (mean interval 6.4 months) was performed on 62/70 lesions (89 %), and a second recurrence was found in 21/62 (34 %). One patient underwent surgery for a circumferential adenoma of the ileocecal valve. The other 20 lesions were treated endoscopically. Follow-up colonoscopy was performed on 15/20 (75 %) and demonstrated a third recurrence in 3/15 (20 %). One was a deep T1 cancer; curative surgery was performed. The other two patients each had one additional endoscopic treatment and both had no recurrence on subsequent colonoscopy. There were two complications: Both were delayed bleeds after treatment of the first recurrence. A mean of 1.3 endoscopic procedures was required to achieve a cure (range 1–3) for recurrent adenomas after piecemeal EMR.
Conclusion
Endoscopic treatment of patients with second recurrences is safe and effective, but is associated with a significant rate of additional recurrences. Continued endoscopic treatment of patients with multiple recurrences is associated with high cure rates, low complication rates and a low risk of progression to malignancy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Endoscopic removal of colorectal adenomas reduces the incidence of colorectal carcinoma (CRC) and CRC-related mortality [1–4]. Previous cohort and case–control studies have reported that colon polypectomy reduces the incidence of CRC by over 40 % [4, 5]. In spite of the advantage conferred by colonoscopy and polypectomy, the rate of CRC detected in the interval between scheduled surveillance colonoscopies varies from 3.7 to 9.0 % [6–8]. Local recurrences after piecemeal endoscopic mucosal resection (EMR) or incomplete endoscopic resection are likely responsible for a significant portion of these incident cancers [9].
Reported local recurrence rates after endoscopic resection of colorectal lesions vary widely, but are generally higher for lesions that are removed piecemeal rather than en bloc. Recurrence rates of 5–30 % are commonly observed after piecemeal EMR of nonpedunculated colorectal adenomas larger than 2 cm [10–15]. Recently, colorectal endoscopic submucosal dissection (ESD) has been demonstrated to have higher rates of en bloc resection and lower rates of recurrence than conventional EMR, but the technique is time-consuming, risky and not yet widely available outside of specialized centers in Asia [16, 17].
Recurrent lesions are difficult to treat endoscopically due to submucosal fibrosis that develops within a few weeks of endoscopic treatment. We previously reported that prior lesion manipulation including biopsy, partial snare resection and tattooing has significant deleterious effects on subsequent endoscopic treatment: lowering en bloc resection rates and raising recurrence rates [18]. In our prior study, we observed a recurrence rate (for endoscopic treatment of nonpedunculated lesions larger than 2 cm) of 7.7 % for lesions that had no prior manipulation, 41 % for lesions that had previously undergone biopsy (typically multiple large capacity biopsies performed during screening colonoscopy by the referring endoscopist) and 54 % for lesions that had previously undergone partial snare resection or tattoo placement within the lesion. Several endoscopic methods, including repeat conventional EMR, underwater EMR, ESD and argon plasma coagulation (APC), have been applied for treatment of recurrent or residual lesions after EMR [19–27]. ESD and underwater EMR appear to be superior to conventional EMR in this setting, with a higher en bloc resection rate and a lower rate of additional recurrences [21, 23, 26, 27]. However, previous studies of recurrent or residual lesions after EMR have generally focused on specific salvage methods or on the endoscopic treatment itself [19–27]; there is a paucity of data about clinical outcomes of lesions that recur after two or more endoscopic treatments. It is therefore unclear whether continued endoscopic treatment is safe and effective in this situation.
Methods
Patients and lesions
This two-center retrospective case series was approved by the Institutional Review Board of Stanford University and VA Palo Alto Health Care System. Electronic records of all patients referred to a single interventional colonoscopist (SF) who practices at both centers (Stanford University Hospital and VA Palo Alto) were reviewed. All patients with recurrence after piecemeal EMR of nonpedunculated colorectal adenomas (>2 cm) between January 2009 and September 2013 were identified and included in the analysis.
Procedures
All procedures were performed on an outpatient basis. The colonoscopies were performed by an endoscopist (SF) with extensive experience in EMR, who has performed more than 1000 EMR procedures. Colonoscopies were performed under conscious sedation with nurse-administered intravenous midazolam and fentanyl. Procedures were performed using a high-definition colonoscope (Pentax EC-3490L; Pentax, Montvale NJ or Olympus pcfH180 or CFH180; Olympus, Center Valley, Pa) with a high-definition processor (Pentax EPK-I HD or Olympus CV-180 Exera). A cap (Olympus D-201) was placed on the distal end of the colonoscope to facilitate endoscopic resection. Lesion size was estimated by opening a snare of known dimensions adjacent to the lesion. Location of the lesion was categorized as proximal (cecum to splenic flexure) or distal (descending colon to rectum). Conventional EMR was performed by using a stiff snare (Traxtion; US Endoscopy, Mentor, Ohio or SD-230 or SD-210; Olympus) after submucosal injection of saline solution or 1.5 % hyaluronate. Both solutions were mixed with a few drops of indigo carmine for staining. Cautery settings were not standardized.
Underwater EMR was performed in a uniform, standardized fashion according to the referential study [21, 28]. After reaching the recurrent adenoma, air was evacuated from the affected segment of lumen by suctioning through the colonoscope. Subsequently, between 500 mL and 1 L of water was infused until adequate luminal filling was achieved for lesion visualization, without over distension. The margins of the recurrent lesion were identified using high-definition narrow-band imaging or i-Scan. Underwater EMR was performed with a duckbill snare (15-mm AcuSnare; Cook Medical Inc, Bloomington, Ind) using blended current (DryCut, effect 2, 30 W, ERBE VIO 300 D, Marietta, Ga). In the underwater EMR group, suction was applied during snare closure if the lesion was difficult to snare due to fibrosis. En bloc resection was attempted if technically possible and piecemeal resection was performed when the entire lesion could not be removed in one piece. Areas of residual lesion that could not be successfully grasped and removed using the snare were removed with a hot biopsy forceps if they were less than 5 mm in size. Larger areas of residual adenomatous tissue were ablated using argon plasma coagulation (APC) at a setting of 40–60 W (ERBE VIO 300 D, Marietta, Ga).
Outcome measurements
The primary outcomes evaluated were the clinical course including second and third recurrence rates and the feasibility of endoscopic retreatment. Clinical courses were evaluated by several clinical indices including the cure rate by salvage endoscopic treatment, mean number of sessions of salvage treatment for cure, size and histology of recurrent or residual lesion of the each salvage session and complications. All patients were recommended to undergo follow-up colonoscopy within 6–12 months except patients whose life expectancy was estimated at less than 5 years. Recurrence during follow-up colonoscopy was defined as adenoma or cancer at the resection site on the follow-up colonoscopy. Biopsies were routinely obtained from the scar.
Statistics
When comparing the baseline characteristics of individuals with two different groups, a Chi-square test and Fisher’s exact test were used for categorical data, and the Student’s t test and Mann–Whitney U test were used for continuous variables, expressed as mean ± standard deviation. A value of P < 0.05 with a two-tailed test was considered significant. Data analysis was performed using SPSS 15.0 for Windows (Chicago, Illinois, USA).
Results
Clinical outcomes of treatment of the first recurrences
Sixty-four patients with 70 recurrent lesions after piecemeal EMR for large nonpedunculated adenomas were identified (Fig. 1). Thirty-six (56.3 %) were male. The mean age was 68.6 ± 9.7. Fifty-two lesions (74.2 %) were located in the proximal colon. The mean size of the first recurrence was 19.2 ± 12.1 mm. Conventional EMR (39/70, 55.7 %) or underwater EMR (31/70, 44.3 %) was performed in all patients. En bloc resection was achieved in 24.3 % (17/70) of the cases. Two complications (3 %) of delayed bleeding occurred; both were treated successfully with endoscopic hemoclipping. Follow-up colonoscopy (mean interval 6.4 ± 3.2 months) was performed on 62/70 lesions (89 %). On follow-up, 66 % (41/62) had no recurrence, while a second recurrence was observed in 34 % (21/61). The mean size of the second recurrences was 10.2 ± 4.8 mm. One of the second recurrences was a circumferential ileocecal valve adenoma; it was felt to be unresectable endoscopically and the patient underwent curative surgery. All of the other second recurrences (n = 20) were treated endoscopically. Table 1 compares the lesion and procedural characteristics of first recurrences that were successfully cured with endoscopic treatment to those that developed a second recurrence. Underwater EMR and en bloc resection were more frequent in the successfully treated lesions.
Clinical outcomes of treatment of the second recurrences
Additional endoscopic treatment was performed on 20 of 21 lesions that developed a second recurrence (Table 2). The endoscopic treatment consisted of: underwater EMR (11/20, 55 %), conventional EMR (6/20, 30 %), hot forceps biopsy (2/20, 10 %) and APC (2/20, 10 %). There were no complications from any of these procedures. After treatment, follow-up colonoscopy was performed on 15 (75 %) lesions. Three of the 15 (20 %) had a third recurrence. All three lesions were located in the ascending colon. One patient was found to have a deep T1 cancer and underwent curative surgery. The two other patients were successfully treated endoscopically without complications and had no further recurrence on subsequent colonoscopy.
Overall outcomes
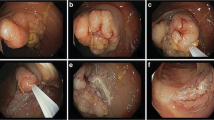
Among 70 lesions that underwent endoscopic treatment for a first recurrence after piecemeal EMR, a second endoscopic treatment was performed on 32 % (20/62) that underwent follow-up colonoscopy and a third endoscopic treatment was performed on 13 % (2/15) who returned for follow-up. The cure rate for endoscopic treatment of the first recurrence (no adenoma on the follow-up colonoscopy) was 66 % (41/62). The cure rate for endoscopic treatment of the second recurrence was 80 % (12/15). Surgery was performed on 2 of the 70 lesions (2.9 %). In all, 55/70 lesions (78.6 %) had a demonstrated cure by endoscopic treatment with mean of 1.3 (range 1–3) procedures. The lesion size was progressively smaller as the treatment progressed: Prior to any treatment, the mean lesion size was 34.5 ± 8.6 mm. The mean size of first recurrences was 22.2 ± 11.7 mm. The mean size of second recurrences was 10.2 ± 4.8 mm (P < 0.001). Figure 2 shows a representative lesion, a 35-mm villous adenoma that was successfully eradicated after a second recurrence.
A 35-mm villous adenoma in the ascending colon of a 72-year-old patient. B There is extensive involvement of the ileocecal valve, seen in the lower left of the image. The appendiceal orifice is uninvolved (arrow). C On the first follow-up colonoscopy, no residual adenoma is seen distal to the ileocecal valve. D However, recurrent adenoma is seen inside the ileocecal valve (first recurrence). It was treated by repeat EMR. E Residual adenoma was found at the ileocecal valve on the second follow-up (second recurrence). Injection of blue-stained saline was performed on the ileal side of the recurrence (arrow), which facilitates snare resection by displacing the adenoma toward the cecum. F The adenoma was successfully resected, and argon plasma was used to cauterize potential microscopic residual after snare resection. Subsequent follow-up revealed no further recurrence
Discussion
For retreatment of recurrent lesions after piecemeal EMR of large (>2 cm) nonpedunculated colorectal lesions, various endoscopic treatment methods have been utilized depending on lesion size, morphology, location and institutional expertise. Recently, colorectal ESD and underwater EMR have demonstrated good results as salvage treatment for recurrent lesions with encouraging clinical outcomes [21, 23, 26, 27].
As a salvage treatment modality for recurrent lesions after EMR, ESD has shown excellent outcomes: Only 0–12.5 % of patients develop a second recurrence when ESD is performed on the first recurrence [23, 26, 27, 29]. En bloc resection rates have varied widely, ranging from 56 to 93.8 % [23, 26, 27, 29]. However, colorectal ESD requires highly specialized expertise and is still rarely performed outside of Asia. In contrast, underwater EMR is a modified EMR technique that utilizes a snare underwater and is therefore more accessible to Western endoscopists who have no ESD training. We recently reported that underwater EMR as a salvage modality for recurrent lesions had a higher en bloc resection rate (47.2 %) and lower recurrence rate (10 %) than conventional EMR [21]. However, underwater EMR is a relatively new technique and has not yet achieved widespread adoption. The most well-established modality for salvage endoscopic treatment for recurrent adenomas after piecemeal EMR in the USA is still repeat conventional EMR [30, 31]. Further recurrence after initial salvage endoscopic treatment is therefore likely to continue to be an important clinical problem.
Endoscopic retreatment of recurrent lesions can be technically challenging. Although the size of the lesion is often smaller with each incomplete treatment, fibrosis and scarring induced by cautery can be more severe after each treatment, making the lesion more difficult to grasp with a snare. Published data on endoscopic retreatment of second or third recurrences are very limited. Most prior studies concentrated on salvage endoscopic treatment of the first recurrence, with a focus on the potential advantages of ESD over EMR [23, 26, 27, 29]. The clinical outcome of recurrent lesions treated by repeated application of EMR is therefore not well established.
The present study, with 70 lesions from two centers, is larger than most prior studies of recurrent lesions [23, 26, 27]. In the current study, the second recurrence rate after initial salvage endoscopic treatment was 34 % and the third recurrence rate was 20 %. The second recurrence rate of 34 % is relatively high, but it is difficult to compare rates between different studies because multiple factors such as lesion size, morphology and tattoo placement involving the lesion itself can influence recurrence rates. In addition, the use of ancillary techniques such as APC and hot biopsy removal could affect the recurrence rates. It is difficult to evaluate the precise role of these ancillary techniques, which were typically applied in our study after exhaustive attempts at snare excision of small remaining areas during EMR. More aggressive use of hot biopsy or APC could theoretically reduce recurrence rate but may also raise the risk of perforation or bleeding.
Our mean follow-up interval after the initial salvage procedure was 6.4 months (maximal interval was 12 months). It is already known that surveillance colonoscopy should be performed within 2–6 months in patients with sessile adenomas that are removed piecemeal. However, subsequent surveillance is recommended to be individualized, based on the endoscopist’s judgment [32]. Our study suggests that a second surveillance within 6 months after the initial salvage procedure is appropriate.
In spite of our short-term follow-up colonoscopy intervals, one T1 cancer was found after endoscopic treatment of a second recurrence. The lesion was diagnosed as a tubular adenoma on the initial colonoscopy. High-grade dysplasia was found on subsequent endoscopic procedures, but the lesion was not cured by these repeated endoscopic treatments and advanced to T1 cancer on follow-up 4 months after treatment of the second recurrence. This case highlights the need for continued surveillance of recurrent lesions.
Up to 6 % of recurrent or residual lesions after EMR have been treated surgically in previous studies [23–27]. In the current study, a total of 2.9 % of lesions eventually underwent surgery—one because of progression to T1 cancer and one because of inability to resect a large adenoma with circumferential involvement of the ileocecal valve. Prior studies have also reported higher complication rates: bleeding rates of up to 16 % and perforation rates of up to 15 % during salvage ESD [23, 26, 27, 29]. Even in the salvage EMR method, a 2 % perforation rate has been reported. However, in the current study, only two cases (3 %) of delayed bleeding occurred, both during treatment of a first recurrence. There were no perforations. Overall, our data suggest that recurrent lesions can be treated safely and effectively, with a low incidence of complications and only rare cases of endoscopic failure requiring surgery.
There are some limitations of our study. First, this study was a small-scaled retrospective case series based on our experience. Although we enrolled consecutive cases of recurrent adenoma over a 4-year period, the number of cases is relatively small to generalize our outcomes. Furthermore, all of our data were based on single expert endoscopist’s experience. Follow-up data were incomplete, and there are still some patients awaiting surveillance colonoscopy; a considerable portion of patients (18.6 %, 13/70) did not undergo complete follow-up. To generalize our results, a larger study involving multiple endoscopists with more complete follow-up is required.
In conclusion, second recurrences after endoscopic treatment of recurrent large nonpedunculated colorectal adenomas can be treated endoscopically with high cure rates, low complication rates and a low risk of progression to malignancy. Continued endoscopic surveillance should be performed until eradication of the lesion is demonstrated.
References
Winawer SJ, Zauber AG, Ho MN et al (1993) Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med 329:1977–1981
Zauber AG, Winawer SJ, O’Brien MJ et al (2012) Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med 366:687–696
Brenner H, Chang-Claude J, Rickert A et al (2012) Risk of colorectal cancer after detection and removal of adenomas at colonoscopy: population-based case-control study. J Clin Oncol 30:2969–2976
Muller AD, Sonnenberg A (1995) Prevention of colorectal cancer by flexible endoscopy and polypectomy. A case-control study of 32,702 veterans. Ann Intern Med 123:904–910
Nishihara R, Wu K, Lochhead P et al (2013) Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med 369:1095–1105
Singh S, Singh PP, Murad MH et al (2014) Prevalence, risk factors, and outcomes of interval colorectal cancers: a systematic review and meta-analysis. Am J Gastroenterol 109:1375–1389
Cooper GS, Xu F, Barnholtz-Sloan JS et al (2012) Prevalence and predictors of interval colorectal cancers in medicare beneficiaries. Cancer 118:3044–3052
Baxter NN, Sutradhar R, Forbes SS et al (2011) Analysis of administrative data finds endoscopist quality measures associated with postcolonoscopy colorectal cancer. Gastroenterology 140:65–72
Rex DK, Eid E (2008) Considerations regarding the present and future roles of colonoscopy in colorectal cancer prevention. Clin Gastroenterol Hepatol 6:506–514
Katsinelos P, Kountouras J, Paroutoglou G et al (2006) Endoscopic mucosal resection of large sessile colorectal polyps with submucosal injection of hypertonic 50 percent dextrose-epinephrine solution. Dis Colon Rectum 49:1384–1392
Belderbos TD, Leenders M, Moons LM et al (2014) Local recurrence after endoscopic mucosal resection of nonpedunculated colorectal lesions: systematic review and meta-analysis. Endoscopy 46:388–402
Hurlstone DP, Sanders DS, Cross SS et al (2004) Colonoscopic resection of lateral spreading tumours: a prospective analysis of endoscopic mucosal resection. Gut 53:1334–1339
Iishi H, Tatsuta M, Iseki K et al (2000) Endoscopic piecemeal resection with submucosal saline injection of large sessile colorectal polyps. Gastrointest Endosc 51:697–700
Kobayashi N, Yoshitake N, Hirahara Y et al (2012) Matched case-control study comparing endoscopic submucosal dissection and endoscopic mucosal resection for colorectal tumors. J Gastroenterol Hepatol 27:728–733
Lee EJ, Lee JB, Lee SH et al (2012) Endoscopic treatment of large colorectal tumors: comparison of endoscopic mucosal resection, endoscopic mucosal resection-precutting, and endoscopic submucosal dissection. Surg Endosc 26:2220–2230
Niimi K, Fujishiro M, Kodashima S et al (2010) Long-term outcomes of endoscopic submucosal dissection for colorectal epithelial neoplasms. Endoscopy 42:723–729
Lee EJ, Lee JB, Lee SH et al (2013) Endoscopic submucosal dissection for colorectal tumors–1000 colorectal ESD cases: one specialized institute’s experiences. Surg Endosc 27:31–39
Kim HG, Thosani N, Banerjee S et al (2015) Effect of prior biopsy sampling, tattoo placement, and snare sampling on endoscopic resection of large nonpedunculated colorectal lesions. Gastrointest Endosc 81:204–213
Mannath J, Subramanian V, Singh R et al (2011) Polyp recurrence after endoscopic mucosal resection of sessile and flat colonic adenomas. Dig Dis Sci 56:2389–2395
Tsiamoulos ZP, Bourikas LA, Saunders BP (2012) Endoscopic mucosal ablation: a new argon plasma coagulation/injection technique to assist complete resection of recurrent, fibrotic colon polyps (with video). Gastrointest Endosc 75:400–404
Kim HG, Thosani N, Banerjee S et al (2014) Underwater endoscopic mucosal resection for recurrences after previous piecemeal resection of colorectal polyps (with video). Gastrointest Endosc 80:1094–1102
Regula J, Wronska E, Polkowski M et al (2003) Argon plasma coagulation after piecemeal polypectomy of sessile colorectal adenomas: long-term follow-up study. Endoscopy 35:212–218
Zhou P, Yao L, Qin X et al (2009) Endoscopic submucosal dissection for locally recurrent colorectal lesions after previous endoscopic mucosal resection. Dis Colon Rectum 52:305–310
Hotta K, Fujii T, Saito Y et al (2009) Local recurrence after endoscopic resection of colorectal tumors. Int J Colorectal Dis 24:225–230
Moss A, Williams SJ, Hourigan LF et al (2015) Long-term adenoma recurrence following wide-field endoscopic mucosal resection (WF-EMR) for advanced colonic mucosal neoplasia is infrequent: results and risk factors in 1000 cases from the Australian Colonic EMR (ACE) study. Gut 64:57–65
Hurlstone DP, Shorthouse AJ, Brown SR et al (2008) Salvage endoscopic submucosal dissection for residual or local recurrent intraepithelial neoplasia in the colorectum: a prospective analysis. Colorectal Dis 10:891–897
Sakamoto T, Saito Y, Matsuda T et al (2011) Treatment strategy for recurrent or residual colorectal tumors after endoscopic resection. Surg Endosc 25:255–260
Binmoeller KF, Weilert F, Shah J et al (2012) “Underwater” EMR without submucosal injection for large sessile colorectal polyps (with video). Gastrointest Endosc 75:1086–1091
Kuroki Y, Hoteya S, Mitani T et al (2010) Endoscopic submucosal dissection for residual/locally recurrent lesions after endoscopic therapy for colorectal tumors. J Gastroenterol Hepatol 25:1747–1753
Moss A, Bourke MJ, Williams SJ et al (2011) Endoscopic mucosal resection outcomes and prediction of submucosal cancer from advanced colonic mucosal neoplasia. Gastroenterology 140:1909–1918
Tanaka S, Oka S, Nagata S, Ito M, Haruma K, Chayama K (2003) Endoscopic diagnosis and treatment of local residual/recurrent lesions after endoscopic mucosal resection for early colorectal carcinoma. Dig Endosc 15:S36–S38
Winawer SJ, Zauber AG, Fletcher RH et al (2006) Guidelines for colonoscopy surveillance after polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer and the American Cancer Society. Gastroenterology 130:1872–1885
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Drs. Kim, Sethi, Banerjee and Friedland have no conflicts of interest or financial ties to disclose.
Rights and permissions
About this article
Cite this article
Kim, H.G., Sethi, S., Banerjee, S. et al. Outcomes of endoscopic treatment of second recurrences of large nonpedunculated colorectal adenomas. Surg Endosc 30, 2457–2464 (2016). https://doi.org/10.1007/s00464-015-4497-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-015-4497-y