Abstract
Purpose
Although thrombocytosis has been reported in patients with various cancers including the colorectal one, the impact of elevated platelet counts on the response to chemoradiotherapy (CRT) for rectal cancer has not been fully investigated. We investigated the clinical significance of pre- and post-CRT platelet counts in patients with rectal cancer.
Methods
The medical records of 101 patients with rectal cancer, who had received CRT followed by surgical resection, were retrospectively reviewed. The correlations between the clinicopathological variables and the pre- or post-CRT platelet counts were analyzed. The correlations between tumor regression rate induced by CRT, as evaluated by barium enema and pathological examination, and the pre- or post-CRT platelet counts were also evaluated. Finally, the impact of pre-CRT thrombocytosis on the prognosis of these patients was assessed.
Results
The pre-CRT platelet count correlated with venous invasion and tumor size, and it strongly correlated with the response rate evaluated by barium enema and the grade of pathological tumor regression. Furthermore, patients with pre-CRT thrombocytosis had significantly shorter local recurrence-free survival.
Conclusion
Platelet count before CRT should be a promising biomarker for predicting the efficacy of CRT and the risk of local recurrence in rectal cancer patients after CRT.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Clinically evident coagulation disorders may be detected as the first sign of malignancy. It is reported that patients without an evident cancer, who develop symptomatic idiopathic thromboembolism, have an approximately 10 % risk of subsequently being diagnosed as cancer [1]. Furthermore, various associations of coagulation abnormalities with malignancies have been documented. Elevated plasma D-dimer level correlates with poor prognosis in lung cancer, colorectal cancer, and sarcoma, as well as with clinical stage and lymph node metastasis in gastric cancer [2–5]. We hitherto reported a strong association of hyperfibrinogenemia with lymph node metastasis, liver metastasis, and consequently poor prognosis in gastric cancer [6, 7]. Elevated plasma fibrinogen was also a relevant factor associated with depth of tumor invasion and poor prognosis in colorectal cancer [8]. On the other hand, we recently demonstrated that pre-operative thrombocytosis in colorectal cancer patients significantly correlates with tumor size, depth of invasion, and poor prognosis [9]. Thus, the abnormal activation of the hemostatic system may be closely associated with cancer progression [10].
Pre-operative chemoradiotherapy (CRT) is currently recognized as one of the standard therapeutic strategies for advanced rectal cancer. Many clinical studies have demonstrated that CRT contributes to reduce the rate of postoperative local recurrence and increase the rate of sphincter-preserving surgery [11–13]. Since the response to CRT differs among patients, and poor response usually impairs prognosis, the prediction of the response to therapy preoperatively would help the decision of the indication of CRT, avoiding the time loss and the high expenses of a therapy for those who will not benefit of it. If the sensitivity of radio- or chemoradiotherapy could be accurately predicted, and those cases with low or without sensitivity to radiotherapy could be withdrawn, such cases would avoid receiving an unnecessary burden prior to receiving a surgical resection. Several markers for the pre-operative prediction of the sensitivity of rectal cancer to radio- or chemoradiotherapy have been reported, such as p53 [14], endothelial growth factor receptor [15, 16], Ki-67 [17], and p21 [15]. We have also reported Ku70, Ku86, P16, and telomerase reverse transcriptase as promising biomarkers in predicting the radiosensitivity of rectal cancer [18–20]. Furthermore, we recently reported that the gene expression profiles of rectal cancer determined by DNA microarray analysis were correlated with histological regression [21]. However, complex procedures, such as immunohistochemistry and mRNA analysis using biopsy specimens from tumors, are necessary for the identification of these markers, and very often the small biopsy specimens do not adequately reflect the total tumor, the different results being obtained from biopsy specimens and the surgically resected ones. Therefore, predictive markers, which can be more easily and conveniently analyzed prior to radio- or chemoradiotherapy, are desired.
We recently reported that the decrease in plasma fibrinogen levels during CRT significantly correlates with the tumor regression induced by CRT [22]. Post-CRT, but not pre-CRT, fibrinogen level significantly correlated with the post-CRT local extent of rectal tumor such as depth of invasion, tumor size, and poor prognosis. These results backed our hypothesis that plasma fibrinogen level reflects local tumor volume; however, the use of post-CRT fibrinogen level as a predictive factor of the response rate to pre-operative CRT would not be feasible. In the present study, we focused on thrombocytosis, another coagulation disorder which we recently reported to be significantly associated with the progression of colorectal cancer [9]. We assessed the peripheral blood platelet count in patients receiving pre-operative CRT and evaluated its correlation with the clinicopathological features, response to CRT, and prognosis.
Methods
The medical records of 108 consecutive patients with rectal adenocarcinoma diagnosed between November 2003 and June 2012 and who received CRT at the Department of Surgical Oncology, the University of Tokyo Hospital, were retrospectively reviewed. Patients with concomitant unresectable metastases and uncontrollable neoplasm of extracolonic origin diagnosed before CRT and those who developed unresectable metastasis during CRT were excluded from this study. All patients enrolled in the study received a total dose of 50.4 Gy of radiation and concomitant 5-FU-based chemotherapy and underwent standardized curative resection, following an interval of 6–8 weeks after CRT. The following three regimens were used in this study: tegafur–uracil and leucovorin by oral administration, tegafur–gimeracil–oteracil potassium by oral administration, and continuous intravenous infusion of 5FU.
Pre-CRT blood samples were obtained from patients within 2 weeks prior to CRT and post-CRT samples between 3 and 5 weeks after CRT. In the present study, platelet count >36.5 × 10,000 /μl was defined as thrombocytosis according to the normal range of platelet count in our institution. Computed tomography (CT) was also performed before and after CRT, and tumor size reduction was classified into partial response and stable disease according to the Response Evaluation Criteria in Solid Tumors (RECIST) [23]. Presence of distant metastasis was also evaluated by these CTs. Also, double-contrast barium enema, performed prior to and after the CRT, was used to calculate the tumor shrinkage rate using the longitudinal dimension of the tumor, as described by Suzuki et al. [24, 25], which gave the same result as the CT. After resection of the tumor, all specimens were histopathologically analyzed, and the pathological TNM classification and staging were determined according to the classification established by the American Joint Committee on Cancer. Tumor regression grading was also determined as proposed by Dworak et al. [26]. Briefly, tumor samples without any fibrosis/regression were considered as grade 0, whereas complete regression (grade 4) was defined as the absence of viable tumor cells in the primary tumor and the lymph nodes. Tumor samples with > 50 % viable tumor cells (< 50 % fibrosis) were considered as grade 1. A regression within 50–80 % was classified as grade 2, and if regression exceeded 80 %, they were classified as Grade 3. This study was conducted with the approval of the Ethics Committee of the University of Tokyo.
The univariate analyses of clinicopathological variables were carried out as follows: the continuous variables, i.e., age and tumor size, were analyzed using correlation coefficients; variables were categorized into two groups, i.e., gender, histological type, and presence/absence of metastasis, using unpaired t-test; and ordinal-scale variables were categorized into three or more groups, i.e., depth of invasion, node metastasis, and pathological tumor regression grading, using Spearman rank correlation coefficients. Kaplan–Meier estimator, log-rank test, and Cox proportional hazard model were used for survival analysis, and the percentage of patients without recurrence 5 years after surgery, as estimated by the Kaplan–Meier estimator, was defined as 5-year survival rate. Similarly, the percentage of alive patients at 5 years was defined as 5-year overall survival, that of patients without local recurrence as 5-year local recurrence-free survival, and that of patients without distant metastasis as 5-year distant metastasis-free survival. Parameters with P < 0.2 in log-rank test were used in Cox proportional hazard model. All analyses were performed with JMP8.0 software, and differences at P < 0.05 were considered as statistically significant.
Results
The general characteristics of the patients are shown in Table 1. Of the 108 rectal cancer patients who received CRT, 62 (57.4 %) were male and 46 (42.6 %) were female. The mean platelet count before CRT was 29.2 ± 12.0×10,000/μl, and thrombocytosis was observed in 20.4 % of patients. As shown in Fig. 1, the majority of plotted dots located below the slant line, which means that the platelet counts decreased during CRT in most cases. There were no cases of post-CRT thrombocytosis among those without pre-CRT thrombocytosis.
Table 2 shows the univariate analysis of the association between pre- and post-CRT platelet counts and the clinicopathological features. Pre-CRT platelet counts significantly correlated with the response rate evaluated by barium enema and RECIST, venous invasion, tumor size, depth of invasion, and pathological tumor regression grading, whereas post-CRT platelet counts correlated with the response rate evaluated by barium enema and RECIST, venous invasion, tumor size, and depth of invasion, but not with pathological tumor regression grading. Post-CRT platelet counts elevated along with the extent of tumors, which means that the deeper the tumor invasion and the larger the tumor size, the higher platelet counts were found. On the other hand, pre- and post-CRT platelet counts showed no correlation with metastasis of cancer, either lymph nodal or hematogenic. Figure 2a, b shows the correlation between the pre-CRT platelet counts and the tumor regression evaluated by barium enema, RECIST, and the pathological findings. Elevated platelet count before CRT strongly correlated with poor response, i.e., lower tumor regression as analyzed by both methods. It is known that anemia can induce thrombocytosis, and the pre-CRT anemia is correlated with the response to radiation therapy [27]. Indeed pre-CRT hemoglobin levels and platelet counts, in our series, were significantly correlated (Fig. 2c). Therefore, we also evaluated other factors which could be affecting the response to CRT, i.e., hemoglobin level before CRT, interval between CRT and surgery, dose reduction of chemotherapy, and variation of the chemotherapy regimen (Table 3). Among these factors, platelet count before CRT was the only factor affecting pathological tumor regression (P = 0.027). Table 4 shows the correlation of thrombocytosis and pathological grade 1–3, which is defined as “cases which could not achieve pathological complete response”. Because there were many cases of pathological grade 1–3 without thrombocytosis, the sensitivity and its negative predictive value remained very low (21.2 and 9.3 %, respectively). In contrast, the specificity and its positive predictive value were markedly high (88.9 and 95.5 %, respectively).
Correlations between pre-CRT platelet counts and the tumor regression rate or pre-CRT hemoglobin level. a, b The correlation between the pre-CRT platelet counts and the tumor regression rate as evaluated by pathological tumor regression grading (a) and barium enema (b) is shown. c The correlation between platelet counts and hemoglobin level of pre-CRT is shown
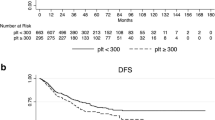
Because the response to CRT had a better correlation with pre-CRT platelet counts rather than the post-CRT ones and post-CRT thrombocytosis was observed in only a few cases (4.0 %), we then evaluated the association between pre-CRT platelet count and prognosis. Since the prognosis of cases with distant metastasis, i.e., UICC stage IV, had a quite worse prognosis than those without distant metastasis, two cases with pre-CRT distant metastasis and six cases which developed distant metastasis during CRT were excluded from further prognostic analysis (Fig. 3). Figure 4 shows the survival rate of the cases with and without thrombocytosis by Kaplan–Meier analysis. Pre-CRT thrombocytosis was associated with a relatively worse disease-free survival rate, but the difference was marginally significant (P = 0.0513). Interestingly, thrombocytosis was not associated with distant metastasis-free survival (P = 0.6360), whereas it significantly shortened the local recurrence-free survival (P < 0.0001). However, pre-CRT thrombocytosis did not affect the patients’ overall survival (data not shown). It can be attributed to the relatively short median follow-up time (22.5 months) of the present study; thus, most of the patients with cancer recurrence are still alive and receiving chemotherapy. By the multivariate analysis, as shown in Table 5, both depth of invasion and presence of lymph node metastasis, but not the presence of distant metastasis, were independent factors associated with local recurrence. Pre-CRT thrombocytosis seemed to be the factor more strongly associated with local recurrence after CRT, followed by surgical resection than the previously known ypT and ypN factors. In contrast, pre-CRT thrombocytosis was not an independent prognostic factor for distant metastasis.
Correlations between the pre-CRT platelet counts and disease-free survival (a), distant metastasis-free survival (b), and local recurrence-free survival (c). The survival curves of cases with or without pre-CRT thrombocytosis are presented using Kaplan–Meier estimator, and the P values were calculated using log-rank test
Discussion
During the last decades, the close association of the hemostatic and coagulation systems with the progression of different types of cancer has been focused. The various distinct factors involved in the complex process of hemostasis are now recognized as important factors also involved in different steps of cancer progression. Recently, platelets have been suggested to play a central role in the complex interaction between the hemostatic system and cancer progression [28]. Clinically, thrombocytosis has been observed to correlate with poor prognosis in various types of malignancies, such as gastric, lung, endometrial, and esophageal cancer [29–32]. Recently, we reported that pre-operative thrombocytosis is a significant prognostic factor associated with poor cancer survival also in colorectal cancer patients [9].
Based on our previous finding that platelet counts significantly correlated with local cancer progression in colorectal cancer patients [9], in the present study, we aimed to investigate the possible role of thrombocytosis as a marker for the prediction of response to CRT, and for this purpose, we evaluated the correlation between thrombocytosis and the response to CRT in rectal cancer patients. Although in the majority of cases platelet count was reduced by CRT, the reduction range did not correlate with the tumor regression rate as evaluated by double-contrast barium enema, RECIST, and pathological examination (data not shown). Therefore, the thrombocytopenia induced by CRT might be dependent on the myelosuppression induced by CRT rather than on the tumor volume reduction. Although both platelet counts before and after CRT correlated with local cancer progression, the post-CRT platelet count showed a higher correlation with response to CRT in terms of tumor size, depth of invasion, and lymphatic invasion. In contrast, neither the pre- nor post-CRT platelet counts showed correlation with the metastatic state of cancer, either nodal or hematogenic. In a previous report, we have demonstrated that pre-operative thrombocytosis is not only an independent indicator of poor cancer-specific survival in patients with colorectal cancer but also an independent predictor of poor disease-free survival in patients with stage II colorectal cancer [9]. In the present study, we found that pre-CRT thrombocytosis also strongly correlated with poor response to CRT, as confirmed by barium enema, RECIST, and pathological examination, and consequently, a significantly higher risk of local recurrence, but not distant metastasis. ypN was also found to be an independent prognostic factor for local recurrence, but not for distant metastasis. Whereas ypN status is affected by the CRT effect, the incidence of distant metastasis is not; therefore, the absence of correlation between ypN and distant metastasis may be reasonable. No other factors evaluated, including pre-CRT hemoglobin level and the variation of chemotherapy regimen, correlated with response to CRT. Since only one case out of 22 with pre-CRT thrombocytosis resulted in pathological CR, it can be hypothesized that achieving pathological CR by CRT is difficult in rectal cancer patients with thrombocytosis. Thrombocytosis may be a factor negatively affecting the achievement of pathological CR by CRT in rectal cancer patients. It still remains inconclusive whether rectal cancer patients with thrombosis should receive immediate surgery without pre-operative CRT because there is no randomized trial comparing the effect of pre-operative CRT on the prognosis of those patients with thrombocytosis. However, at least postoperative adjuvant chemotherapy should be considered for those cases with pre-CRT thrombocytosis, regardless of ypN or ypT, because pre-CRT thrombocytosis is a strong independent predictive factor for local recurrence.
Various mechanisms of platelet-induced cancer progression, through platelet-cancer interaction, have been reported. Upon activation, platelets release microparticles which contain platelet membrane- and cytoplasm-derived proteins. These microparticles have been shown to increase adhesion, proliferation, chemotaxis, and survival of cancer cells [33, 34]. Platelets are also able to bind directly to cancer cells through various receptors expressed on their surface, such as P-selectin, GPIIb-IIIa, and β1- and β3-integrins [35–37], and this aggregation of platelets and the fibrin network formation around tumor cells increase their metastatic potential. Platelets protect cancer cells from the cytolytic activity of natural killer cells, the important effectors of the immune machinery that eliminate circulating cancer cells [38]. In addition, platelets store various angiogenesis-inducing factors such as vascular endothelial growth factor, platelet-derived growth factor, fibroblast growth factor, and epidermal growth factor (EGF), and promote angiogenesis by releasing these factors [39]. Furthermore, in recent years, lysophospholipids, such as sphingosine 1-phospate (S1P) and lysophosphatidic acid (LPA), which are abundantly stored in platelets, and released upon activation, have been focused as important chemical mediators, exerting various effects on surrounding cells by binding specific receptors [40, 41]. They also play an important role in transactivating the EGF receptor and increasing cyclooxygenase-2 expression [42–45], consequently promoting the growth and migratory properties of cancer cells, such as gastric and colorectal types. Therefore, there is a possibility that S1P and LPA are the key mediators of platelet-mediated cancer progression.
Presently, no reports have shown the role or the involvement of platelets in radio-resistance of cancer cells. Therefore, we believe that our present findings have important implications in the context of platelet-induced cancer progression and platelet-regulated chemo/radio-resistance. For the first time, we demonstrated that platelets may play a pivotal role in the regulation of radio-resistance in colorectal cancer. However, the mechanisms underlying the radio-resistance conferred by platelets remain to be completely elucidated. As discussed above, S1P and LPA may be abundantly released by platelets once activated, and cancer cells may activate them. The released S1P and LPA, acting through the S1P or LPA receptors, which are expressed on the different cells types, may have modulatory effects on the immune system [46, 47]. Another interesting finding here was that thrombocytosis may be a promising marker for the prediction of sensitivity to radiotherapy. We observed that thrombocytosis diagnosed pre-CRT was significantly associated with local recurrence of rectal cancer after CRT. Predicting one’s response to CRT is an important matter when deciding on the treatment strategy for rectal cancer patients, especially for the selection of those who will benefit of a pre-operative CRT, which would allow conservative approaches such as local excision or sphincter-sparing surgery or the indication of adjuvant chemotherapy immediately after surgical procedure for the group of patients who had a predicted poor prognosis. Although we have previously shown that low post-CRT plasma fibrinogen level significantly correlated with response to CRT in rectal cancer patients [22], its application as a predictive factor of the treatment response is not feasible, such being limited to the evaluation of the response rate after the treatment.
In conclusion, platelet count is a simple test, available in every hospital, which does not need special techniques or expertise and can be tested prior to CRT as a potential predictive biomarker of the response to treatment and of the risk of local recurrence after treatment in rectal cancer patients receiving CRT.
References
Piccioli A, Lensing AW, Prins MH, Falanga A, Scannapieco GL, Ieran M et al (2004) Extensive screening for occult malignant disease in idiopathic venous thromboembolism: a prospective randomized clinical trial. J Thromb Haemost 2(6):884–889
Komurcuoglu B, Ulusoy S, Gayaf M, Guler A, Ozden E (2011) Prognostic value of plasma D-dimer levels in lung carcinoma. Tumori 97(6):743–748
Kilic M, Yoldas O, Keskek M, Ertan T, Tez M, Gocmen E et al (2008) Prognostic value of plasma D-dimer levels in patients with colorectal cancer. Colorectal Dis Off J Assoc Coloproctology G B Irel 10(3):238–241
Morii T, Mochizuki K, Tajima T, Ichimura S, Satomi K (2011) D-dimer levels as a prognostic factor for determining oncological outcomes in musculoskeletal sarcoma. BMC Musculoskelet Disord 12:250
Kwon HC, Oh SY, Lee S, Kim SH, Han JY, Koh RY et al (2008) Plasma levels of prothrombin fragment F1+2, D-dimer and prothrombin time correlate with clinical stage and lymph node metastasis in operable gastric cancer patients. Jpn J Clin Oncol 38(1):2–7
Yamashita H, Kitayama J, Kanno N, Yatomi Y, Nagawa H (2006) Hyperfibrinogenemia is associated with lymphatic as well as hematogenous metastasis and worse clinical outcome in T2 gastric cancer. BMC Cancer 6:147
Yamashita H, Kitayama J, Nagawa H (2005) Hyperfibrinogenemia is a useful predictor for lymphatic metastasis in human gastric cancer. Jpn J Clin Oncol 35(10):595–600
Yamashita H, Kitayama J, Taguri M, Nagawa H (2009) Effect of preoperative hyperfibrinogenemia on recurrence of colorectal cancer without a systemic inflammatory response. World J Surg 33(6):1298–1305
Sasaki K, Kawai K, Tsuno NH, Sunami E, Kitayama J (2012) Impact of preoperative thrombocytosis on the survival of patients with primary colorectal cancer. World J Surg 36(1):192–200
Kvolik S, Jukic M, Matijevic M, Marjanovic K, Glavas-Obrovac L (2010) An overview of coagulation disorders in cancer patients. Surg Oncol 19(1):e33–e46
Bosset JF, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L et al (2006) Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med 355(11):1114–1123
Sauer R, Becker H, Hohenberger W, Rodel C, Wittekind C, Fietkau R et al (2004) Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 351(17):1731–1740
Watanabe T, Nagawa H (2004) Sphincter preservation in locally advanced rectal cancer due to the addition of continuous infusion 5-FU to preoperative radiation therapy or advances in surgical techniques? Int J Radiat Oncol Biol Phys 59(2):618, author reply
Fu CG, Tominaga O, Nagawa H, Nita ME, Masaki T, Ishimaru G et al (1998) Role of p53 and p21/WAF1 detection in patient selection for preoperative radiotherapy in rectal cancer patients. Dis Colon Rectum 41(1):68–74
Kuremsky JG, Tepper JE, McLeod HL (2009) Biomarkers for response to neoadjuvant chemoradiation for rectal cancer. Int J Radiat Oncol Biol Phys 74(3):673–688
Marquardt F, Rodel F, Capalbo G, Weiss C, Rodel C (2009) Molecular targeted treatment and radiation therapy for rectal cancer. Strahlenther Onkol 185(6):371–378
Kikuchi M, Mikami T, Sato T, Tokuyama W, Araki K, Watanabe M et al (2009) High Ki67, Bax, and thymidylate synthase expression well correlates with response to chemoradiation therapy in locally advanced rectal cancers: proposal of a logistic model for prediction. Br J Cancer 101(1):116–123
Komuro Y, Watanabe T, Hosoi Y, Matsumoto Y, Nakagawa K, Tsuno N et al (2002) The expression pattern of Ku correlates with tumor radiosensitivity and disease free survival in patients with rectal carcinoma. Cancer 95(6):1199–1205
Komuro Y, Watanabe T, Tsurita G, Muto T, Nagawa H (2005) Evaluating the combination of molecular prognostic factors in tumor radiosensitivity in rectal cancer. Hepatogastroenterology 52(63):666–671
Komuro Y, Watanabe T, Tsurita G, Muto T, Nagawa H (2005) Expression pattern of telomerase reverse transcriptase in rectal carcinoma predicts tumor radiosensitivity, local recurrence and disease-free survival. Hepatogastroenterology 52(64):985–989
Watanabe T, Komuro Y, Kiyomatsu T, Kanazawa T, Kazama Y, Tanaka J et al (2006) Prediction of sensitivity of rectal cancer cells in response to preoperative radiotherapy by DNA microarray analysis of gene expression profiles. Cancer Res 66(7):3370–3374
Kawai K, Kitayama J, Tsuno NH, Sunami E, Nagawa H (2011) Hyperfibrinogenemia after preoperative chemoradiotherapy predicts poor response and poor prognosis in rectal cancer. Int J Color Dis 26(1):45–51
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45(2):228–247
Suzuki T, Sadahiro S, Fukasawa M, Ishikawa K, Kamijo A, Yasuda S et al (2004) Predictive factors of tumor shrinkage and histological regression in patients who received preoperative radiotherapy for rectal cancer. Jpn J Clin Oncol 34(12):740–746
Sadahiro S, Suzuki T, Maeda Y, Tanaka Y, Nakamura T, Saguchi T et al (2007) Predictors of tumor downsizing and regression with preoperative radiotherapy alone and with concomitant tegafur/uracil (UFT) for resectable advanced rectal adenocarcinoma. Hepato-Gastroenterology 54(76):1107–1112
Dworak O, Keilholz L, Hoffmann A (1997) Pathological features of rectal cancer after preoperative radiochemotherapy. Int J Colorectal Dis 12(1):19–23
Hoff CM (2012) Importance of hemoglobin concentration and its modification for the outcome of head and neck cancer patients treated with radiotherapy. Acta Oncol 51(4):419–432
Jain S, Harris J, Ware J (2010) Platelets: linking hemostasis and cancer. Arterioscler Thromb Vasc Biol 30(12):2362–2367
Hwang SG, Kim KM, Cheong JH, Kim HI, An JY, Hyung WJ, et al (2012) Impact of pretreatment thrombocytosis on blood-borne metastasis and prognosis of gastric cancer. Eur J Surg Oncol 38(7):562–7
Cakar B, Karaoglanoglu M, Sayici Y, Gonullu Demirag G, Yucel I (2011) The prognostic value of thrombocytosis in newly diagnosed lung cancer patients: a retrospective analysis. J Buon 16(4):677–81
Metindir J, Bilir Dilek G (2009) Preoperative hemoglobin and platelet count and poor prognostic factors in patients with endometrial carcinoma. J Cancer Res Clin Oncol 135(1):125–129
Aminian A, Karimian F, Mirsharifi R, Alibakhshi A, Dashti H, Jahangiri Y et al (2011) Significance of platelet count in esophageal carcinomas. Saudi J Gastroenterol 17(2):134–137
Baj-Krzyworzeka M, Majka M, Pratico D, Ratajczak J, Vilaire G, Kijowski J et al (2002) Platelet-derived microparticles stimulate proliferation, survival, adhesion, and chemotaxis of hematopoietic cells. Exp Hematol 30(5):450–459
Dashevsky O, Varon D, Brill A (2009) Platelet-derived microparticles promote invasiveness of prostate cancer cells via upregulation of MMP-2 production. Int J Cancer 124(8):1773–1777
Borsig L, Wong R, Feramisco J, Nadeau DR, Varki NM, Varki A (2001) Heparin and cancer revisited: mechanistic connections involving platelets, P-selectin, carcinoma mucins, and tumor metastasis. Proc Natl Acad Sci U S A 98(6):3352–3357
Trikha M, Zhou Z, Timar J, Raso E, Kennel M, Emmell E et al (2002) Multiple roles for platelet GPIIb/IIIa and alphavbeta3 integrins in tumor growth, angiogenesis, and metastasis. Cancer Res 62(10):2824–2833
Karpatkin S, Pearlstein E, Ambrogio C, Coller BS (1988) Role of adhesive proteins in platelet tumor interaction in vitro and metastasis formation in vivo. J Clin Invest 81(4):1012–1019
Nieswandt B, Hafner M, Echtenacher B, Mannel DN (1999) Lysis of tumor cells by natural killer cells in mice is impeded by platelets. Cancer Res 59(6):1295–1300
Italiano JE Jr, Richardson JL, Patel-Hett S, Battinelli E, Zaslavsky A, Short S et al (2008) Angiogenesis is regulated by a novel mechanism: pro- and antiangiogenic proteins are organized into separate platelet alpha granules and differentially released. Blood 111(3):1227–1233
Pyne S, Pyne NJ (2000) Sphingosine 1-phosphate signalling in mammalian cells. Biochem J 349(Pt 2):385–402
Tanikawa T, Kurohane K, Imai Y (2010) Regulatory effect of lysophosphatidic acid on lymphocyte migration. Biol Pharm Bull 33(2):204–208
Mori K, Kitayama J, Shida D, Yamashita H, Watanabe T, Nagawa H (2006) Lysophosphatidic acid-induced effects in human colon carcinoma DLD1 cells are partially dependent on transactivation of epidermal growth factor receptor. J Surg Res 132(1):56–61
Shida D, Fang X, Kordula T, Takabe K, Lepine S, Alvarez SE et al (2008) Cross-talk between LPA1 and epidermal growth factor receptors mediates up-regulation of sphingosine kinase 1 to promote gastric cancer cell motility and invasion. Cancer Res 68(16):6569–6577
Shida D, Kitayama J, Yamaguchi H, Okaji Y, Tsuno NH, Watanabe T et al (2003) Lysophosphatidic acid (LPA) enhances the metastatic potential of human colon carcinoma DLD1 cells through LPA1. Cancer Res 63(7):1706–1711
Shida D, Kitayama J, Yamaguchi H, Yamashita H, Mori K, Watanabe T et al (2005) Lysophosphatidic acid transactivates both c-Met and epidermal growth factor receptor, and induces cyclooxygenase-2 expression in human colon cancer LoVo cells. World J Gastroenterol: WJG 11(36):5638–5643
Chi H (2011) Sphingosine-1-phosphate and immune regulation: trafficking and beyond. Trends Pharmacol Sci 32(1):16–24
Weigert A, Weis N, Brune B (2009) Regulation of macrophage function by sphingosine-1-phosphate. Immunobiology 214(9–10):748–760
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kawai, K., Kitayama, J., Tsuno, N.H. et al. Thrombocytosis before pre-operative chemoradiotherapy predicts poor response and shorter local recurrence-free survival in rectal cancer. Int J Colorectal Dis 28, 527–535 (2013). https://doi.org/10.1007/s00384-012-1594-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-012-1594-4