Abstract
Purpose
Although small rectal carcinoid tumors can be treated using local excision, complete resection can be difficult because tumors are located in the submucosal layer. We evaluate the factors associated with pathologically complete local resection of rectal carcinoid tumors.
Methods
Data were analyzed of 161 patients with 166 rectal carcinoid tumors who underwent local excision with curative intent from January 2001 to December 2010. A pathologically complete resection (P-CR) was defined as an en bloc resection with tumor-free lateral and deep margins. The study classified treatments into three categories for analysis: conventional polypectomy (including strip biopsy, snare polypectomy, and hot biopsy), advanced endoscopic techniques (including endoscopic mucosal resection with cap and endoscopic submucosal dissection), and surgical local excision (including transanal excision and transanal endoscopic microsurgery). We evaluated the P-CR rate according to treatment method, tumor size, initial endoscopic impression and the use of endoscopic ultrasound (EUS) or transrectal ultrasound (TRUS).
Results
The mean tumor size was 5.51 ± 2.43 mm (range 2–18 mm) and all lesions were confined to the submucosal layer. The P-CR rates were 30.9, 72.0, and 81.8 % for conventional polypectomy, advanced endoscopic techniques, and surgical local excision, respectively. Univariate analysis showed that P-CR was associated with treatment method, use of EUS or TRUS, and initial endoscopic impression. Multivariate analysis showed that only treatment method was associated with P-CR.
Conclusion
Pathologically complete resection of small rectal carcinoid tumors was more likely to be achieved when using advanced endoscopic techniques or surgical local excision rather than conventional polypectomy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The number of cases of neuroendocrine tumors has increased over time, most likely due to increased awareness among physicians and greater use of endoscopy [1, 2]. Endoscopic screening has not only increased the number of rectal carcinoid tumor cases, but has also led to earlier detection [2].
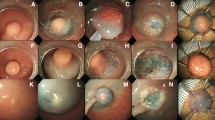
A standardized treatment for small rectal carcinoid remains to be established. Small rectal carcinoid tumors without muscularis propria invasion can be treated using local excision since they rarely metastasize [3–6]. Following local excision, patients may need to undergo further treatment according to margin status, the size of the primary tumor, the depth of invasion, the presence of angiolymphatic invasion, and the mitotic rate. Achieving complete excision has a major bearing on the type of post-excision treatment. However, carcinoid tumors arise from the deep portion of the epithelial glands and then penetrate the muscularis mucosa into the submucosal layer where they form a nodular lesion. Those characteristics have made it difficult to achieve a tumor-free resection margin using conventional polypectomy and have led to the development of new endoscopic resection methods, including endoscopic mucosal resection with cap (EMR-C) and endoscopic submucosal dissection (ESD) [7–12].
The present study analyzed the treatment and outcomes of rectal carcinoid tumor patients who underwent local excision in our hospital. The study identified factors that were associated with a pathologically compete resection (P-CR).
Patients and methods
Patients
From January 2001 to December 2010, a total of 299 patients with colorectal carcinoid tumors were treated at the National Cancer Center in South Korea. We reviewed patient medical records and analyzed the endoscopic findings, procedures, and pathology reports. The study was approved by our institutional review board (NCCNCS-11-530). The study excluded patients with metastatic disease (n = 10), who underwent radical surgery for primary therapy (n = 33) and concurrent chemoradiotherapy for synchronous anal cancer (n = 1), who were diagnosed pathologically in other hospitals (n = 91), and who had colon carcinoid tumors located more than 15 cm above the anal verge (n = 3). Following exclusions, there were 161 patients who had 166 rectal carcinoid tumors which were diagnosed as well-differentiated neuroendocrine tumors (Grade I) according to the WHO classification.
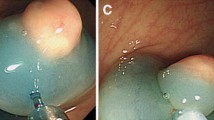
Patients underwent local excision using endoscopic or surgical resection. Resection was performed based on a colonoscopy diagnosis of a rectal polyp or a submucosal tumor regardless of histological confirmation. All resected lesions were ultimately diagnosed as being rectal carcinoid tumors upon histological examination. All patients were evaluated for metastasis to a distant organ or a regional lymph node using abdominopelvic computed tomography (CT). All specimens were referred to pathologists and examined microscopically for tumor size, depth of invasion, angiolymphatic invasion, and resection margin status. P-CR was defined as an en bloc resection with tumor-free lateral and deep margins.
Endoscopic procedures
Before October 2003, local excision of small rectal carcinoid tumors was achieved using conventional polypectomy (including strip biopsy, snare polypectomy, and hot biopsy), or surgical local excision (including TAE and TEM). The advanced endoscopic procedures of EMR-C and ESD were used from October 2003 and March 2007, respectively. Endoscopists have preferred ESD to EMR-C for resecting the carcinoid tumor since March 2007. EMR-C and ESD were performed by five and four expert endoscopists, respectively. Endoscopic ultrasound (EUS) was used to assess rectal carcinoid tumor depth of invasion from 2007. The choice of resection method for rectal carcinoid tumors and the use of EUS were left to the respective operators. The details of all procedures have been presented in previous reports [13, 14].
Data analysis
We evaluated the P-CR rate according to treatment method, tumor size, initial impression at colonoscopy and the use of EUS or transanal ultrasound (TRUS). Tumor size (i.e., the longest diameter) was determined using colonoscopic findings. For analysis, tumors were categorized into three sizes: (a) <5 mm, (b) ≥5 mm but <10 mm, and (c) ≥10 mm.
Categorical data were compared using chi-squared or Fisher’s exact tests. Multivariate logistic regression analysis was performed to identify factors associated with P-CR. P < 0.05 was considered to indicate significance. Statistical analyses were performed using the SPSS 14.0 statistical software package for Windows (SPSS Inc, Chicago, IL, USA).
Follow-up
Follow-up treatment differed according to whether P-CR was achieved. Patients with a tumor-free resection margin were recommended for repeat sigmoidoscopy every 6 months for the first 2 years. Patients with tumor removal that was pathologically incomplete but clinically complete were recommended to undergo a sigmoidoscopy every 3 months with a biopsy on scar tissue for the first 1 year. Rectal carcinoid tumors that were ≥10 mm in size, were positive for angiolymphatic invasion, or had invaded deeper than the submucosal layer were recommended to be treated with radical resection. Endoscopic resection or surgical local excision was again attempted in cases of clinically incomplete removal but in the absence of the aforementioned features (i.e., ≥10 mm in size, positive for angiolymphatic invasion, and had invaded deeper than the submucosal layer). All patients underwent an annual follow-up involving chest radiography and abdominopelvic CT, in addition to endoscopy.
Results
The mean age of the 161 patients was 52.65 ± 10.15 years (range 32–77 years, median 52.0 years), and the mean tumor diameter for the 166 lesions was 5.51 ± 2.43 mm (range 2–18 mm, median 5.0 mm) (Table 1). All lesions were confined to the submucosal layer without invading the proper muscle layer.
The overall P-CR rate was 59.0 % (98 of 166 lesions). The P-CR rate was 66.2 % (90 of 136 lesions) when the initial endoscopic impression was a submucosal tumor. The P-CR rates were 30.9, 72.0, and 81.8 % for conventional polypectomy, advanced endoscopic techniques, and surgical local excision, respectively. Univariate analysis showed that P-CR was associated with treatment method, use of EUS or TRUS, and initial endoscopic impression (Table 2). We performed a multivariate analysis to determine whether treatment method, use of EUS, or initial endoscopic impression had an effect on P-CR. We found that P-CR was only associated with treatment method (Table 3).
Salvage treatment and follow-up
A total of 153 lesions were followed-up, and the median follow-up period was 31.0 months (mean 33.26 ± 24.02, range 1–105 months). After histopathologic assessment, additional treatment was undertaken for 25 lesions; those treatments comprised 15 endoscopic excisions, five surgical local excisions, and five radical operations with regional lymph node dissection.
Although 14 lesions met the criteria for additional radical surgery, only five lesions underwent radical operations due to patient refusal, old age, or another coexisting malignancy. Four cases showed metastasis to a regional lymph node according to the final pathology report after radical surgery. Of those, two cases had tumors less than 10 mm in size with positive angiolymphatic invasion. Neither local recurrence nor metastasis to regional lymph node or distal organs was found in any of the 153 lesions during the follow-up period.
Discussion
Small rectal carcinoid tumors located within the submucosal layer are considered candidates for local treatment even though a consensus has yet to be reached regarding a threshold size for local excision. Achieving complete tumor removal has a major impact on the type of further treatment, and various treatment modalities have been reported to achieve clear margins using deeper resection [7–12].
The present study found that treatment method was the only factor that independently affected the P-CR rate. Advanced endoscopic resection techniques including EMR-C and ESD or surgical local excision were found to be superior to conventional polypectomy to achieve the P-CR. However, the P-CR rates for advanced endoscopic technique and surgical local excision were found to be similar. The present ESD P-CR rate was lower than rates reported elsewhere [15–17]. This may have been because our study included our early experience with ESD, which may have lowered the P-CR rate for that technique.
The current study was mainly focused on P-CR rather than clinically complete resection. Some endoscopists regard successful en bloc resection as clinically complete resection and put more weight on it than P-CR based on endoscopic cauterization eradicating tumor cells close to the resection margin. However, only lesions considered completely clinically removed but pathologically incompletely removed are considered to need more frequent surveillance, albeit that a consensus protocol has yet to be established. An incompletely removed conventional polypectomy resection specimen can sometimes be difficult to histopathologically evaluate in order to determine the size of the primary tumor and angiolymphatic invasion. In our study, four of the five cases that underwent radical operations were found to involve lymph node metastasis. All of those cases involved tumors ≥10 mm with a tumor-involved resection margin or a positive angiolymphatic invasion. Those factors (i.e., tumor size and angiolymphatic invasion) have been shown to be risk factors for lymph node metastasis [5, 6, 18]. Tumor size and angiolymphatic invasion are important factors in terms of treatment following local excision, and a completely removed specimen would be helpful to determine the precise size and the occurrence of angiolymphatic invasion.
We usually applied local treatment for rectal carcinoid tumors lesser than 10 mm in diameter and confined to the submucosal layer without definite evidence of lymph node metastasis in imaging test including abdominal and pelvic CT scan. Patients with carcinoid tumors more than 10 mm in size or positive angiolymphatic invasion were recommended with radical operation. In our study, 14 patients were recommended with radical operation after local treatment but only five patients underwent the additional operation (Table 4). Although recurrences or metastases were not occurred yet during follow-up period in our patients, further longer follow-up should be needed because carcinoid tumors are known to be slow-growing tumors.
We anticipated that using EUS to determine the degree of submucosal invasion would result in better treatment selection and hence a better P-CR rate. For example, surgical local excision or deeper endoscopic submucosal dissection could be chosen for carcinoid tumors located deep in the submucosal layer. However, we found that use of EUS did not improve the P-CR rate (data not shown).
This study found the increase of P-CR rate when the submucosal tumor was suspected on colonoscopy. This increase was thought to be because advanced endoscopic techniques or surgical local excision was usually performed in those cases. Rectal carcinoid tumors typically appear as sessile, submucosal tumors covered with yellow-discolored mucosa on colonoscopy [19], and endoscopically, it is not very difficult to distinguish them from polyps. Therefore, when the gross endoscopic impression is a submucosal tumor including a rectal carcinoid, it would be expected that complete resection would be achieved using one of the advanced endoscopic resection methods or surgical local excision rather than conventional polypectomy.
In conclusion, advanced endoscopic techniques and surgical local excision were found to more likely result in pathologically complete resection compared to conventional polypectomy in local treatment of small rectal carcinoid tumors.
References
Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, Abdalla EK, Fleming JB, Vauthey JN, Rashid A, Evans DB (2008) One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol 26:3063–3072
Scherübl H (2009) Rectal carcinoids are on the rise: early detection by screening endoscopy. Endoscopy 41:162–165
Higaki S, Nishiaki M, Mitani N, Yanai H, Tada M, Okita K (1997) Effectiveness of local endoscopic resection of rectal carcinoid tumors. Endoscopy 29:171–175
Ishikawa H, Imanishi K, Otani T, Okuda S, Tatsuta M, Ishiguro S (1989) Effectiveness of endoscopic treatment of carcinoid tumors of the rectum. Endoscopy 21:133–135
Soga J (2005) Early-stage carcinoids of the gastrointestinal tract: an analysis of 1914 reported cases. Cancer 103:1587–1595
Modlin IM, Kidd M, Latich I, Zikusoka MN, Shapiro MD (2005) Current status of gastrointestinal carcinoids. Gastroenterology 128:1717–1751
Ono A, Fujii T, Saito Y, Matsuda T, Lee DT, Gatoda T, Saito D (2003) Endoscopic submucosal resection of rectal carcinoid tumors with a ligation device. Gastrointest Endosc 57:583–587
Nagai T, Torishima R, Nakashima H, Ookawara H, Uchida A, Kai S, Sato R, Murakami K, Fujioka T (2004) Saline-assisted endoscopic resection or rectal carcinoids: cap aspiration method versus simple snare resection. Endoscopy 36:202–205
Kajiyama T, Hajiro K, Sakai M, Inoue K, Konishi Y, Takakuwa H, Ueda S, Okuma M (1996) Endoscopic resection of gastrointestinal submucosal lesions: a comparison between strip biopsy and aspiration lympectomy. Gastrointest Endosc 44:404–410
Imada-Shirakata Y, Sakai M, Kajiyama T, Kin G, Inoue K, Torii A, Kishimoto H, Ueda S, Okuma M (1996) Endoscopic resection of rectal carcinoid tumors using aspiration lumpectomy. Endoscopy 29:34–38
Oshitani N, Hamasaki N, Sawa Y, Hara J, Nakamura S, Matsumoto T, Kitano A, Arakawa T (2000) Endoscopic resection of small rectal carcinoid tumours using aspiration method with a transparent overcap. J Int Med Res 28:241–246
Onozato Y, Kakizaki S, Ishihara H, Iizuka H, Sohara N, Okamura S, Mori M, Itoh H (2007) Endoscopic submucosal dissection for rectal tumors. Endoscopy 39:423–427
Sohn DK, Han KS, Hong CW, Chang HJ, Jeong SY, Park JG (2008) Selection of cap size in endoscopic submucosal resection with cap aspiration for rectal carcinoid tumors. J Laparoendosc Adv Surg Tech A 18:815–818
Moon SH, Hwang JH, Sohn DK, Park JW, Hong CW, Han KS, Chang HJ, Oh JH (2011) Endoscopic submucosal dissection for rectal neuroendocrine (carcinoid) tumors. J Laparoendosc Adv Surg Tech A 21:695–699
Lee DS, Jeon SW, Park SY, Jung MK, Cho CM, Tak WY, Kweon YO, Kim SK (2010) The feasibility of endoscopic submucosal dissection for rectal carcinoid tumors: comparison with endoscopic mucosal resection. Endoscopy 42:647–651
Park HW, Byeon JS, Park YS, Yang DH, Yoon SM, Kim KJ, Ye BD, Myung SJ, Yang SK, Kim JH (2010) Endoscopic submucosal dissection for treatment of rectal carcinoid tumors. Gastrointest Endosc 72:143–149
Zhou PH, Yao LQ, Qin XY, Xu MD, Zhong YS, Chen WF, Ma LL, Zhang YQ, Qin WZ, Cai MY, Ji Y (2010) Advantages of endoscopic submucosal dissection with needle-knife over endoscopic mucosal resection for small rectal carcinoid tumors: a retrospective study. Surg Endosc 24:2607–2612
Fahy BN, Tang LH, Klimstra D, Wong WD, Guillem JG, Paty PB, Temple LK, Shia J, Weiser MR (2007) Carcinoid of the rectum risk stratification(CaRRs): a strategy for preoperative outcome assessment. Ann Surg Oncol 14:1735–1743
Jetmore AB, Ray JE, Gathright JB Jr, McMullen KM, Hicks TC, Timmcke AE (1992) Rectal carcinoids: the most frequent carcinoid tumor. Dis Colon Rectum 35:717–725
Acknowledgments
This study was supported in part by the National Cancer Center Grant (grant No. 0910520).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Son, HJ., Sohn, D.K., Hong, C.W. et al. Factors associated with complete local excision of small rectal carcinoid tumor. Int J Colorectal Dis 28, 57–61 (2013). https://doi.org/10.1007/s00384-012-1538-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-012-1538-z