Abstract
Purpose
With a theoretical link between stent insertion and increased risk of tumour seeding, there is concern about long-term survival after the use of self-expanding metallic stents (SEMS) as a “bridge to surgery” in the treatment of left-sided obstructing colorectal cancer. This cohort study aims to determine if preoperative stenting adversely affects long-term survival by comparing a group of patients having preoperative stenting (group A) with a group of patients having elective surgery (group B) in a single centre.
Methods
The study is retrospective. Survival was calculated with Kaplan–Meier analysis and compared using the log-rank test. Other group characteristics were compared with Fisher's exact test.
Results
From November 1998 to November 2008, 15 patients had preoperative SEMS and were entered in group A. This represented 11.5 % of a total of 130 patients undergoing SEMS insertion in the same period. Group B included 88 consecutive patients undergoing elective left-sided colonic resection for Dukes' B and C cancer excluding mid and low rectal tumours between January 2003 and December 2007. The 30-day mortality rate for groups A and B was 6.7 % (one patient) and 5.7 % (five patients), respectively. The 5-year survival rate was 60 % and 58 %, respectively, with a p value of 0.96.
Conclusions
In our own practice, patients undergoing SEMS as a “bridge to surgery” have the same long-term survival with those undergoing elective surgery. This finding needs to be confirmed in larger scale studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
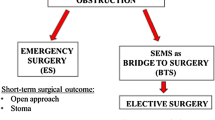
Traditional management of acute malignant bowel obstruction has focused on emergency resection, often with formation of a defunctioning stoma. Currently approximately 21 % of major colorectal resections are carried out as urgent or emergency cases. The 30-day mortality rates are considerably higher in this group at 9.3 % with colon cancer, 11.7 % with rectosigmoid cancer, and 12.3 % with rectal cancer [1]. In addition, stoma formation is known to impact negatively on many aspects of patient quality of life—Nugent et al. [2] reported that 80 % of patients with a stoma experience significant change in lifestyle and more than 40 % have difficulties with sexual function. Stoma closure is frequently not performed for a variety of reasons including advanced patient age and the presence of significant comorbidities. In those who do undergo reversal, this further surgery confers additional risks—Pearce et al. [3] reported that of 145 patients having had a Hartmann's procedure, just over half (80 patients) underwent reversal and of these, only 28 (19.3 % of the whole group) had a completely uneventful recovery.
In 1991, Dohmoto reported the first successful palliative stenting of an obstructing rectal carcinoma [4]. Since then, the technique of self-expanding metallic stent (SEMS) insertion has been widely utilised in a variety of settings. Early studies demonstrated good rates of technical and clinical success [5, 6]. Subsequent systematic reviews have confirmed this—studies with large numbers of patients have reported technical success of 92 % to 94 % and clinical success in 88 % to 91 % [7, 8]. Authors have concluded that colorectal stents offer good palliation in inoperable tumours as well as being safe and effective as a “bridge to surgery”.
Several non-randomised studies have compared the use of SEMS prior to elective resection with emergency surgery in the management of patients with malignant colonic obstruction. Results have demonstrated that the use of SEMS is associated with higher rate of primary anastomosis [9, 10], lower rate of post-operative complications [11] and shorter stay in intensive care unit and hospital [12]. A systematic review of available literature published in 2007 concluded that combining SEMS insertion with elective surgery appeared safer and more effective than emergency surgery [13]. In 2009, a randomised controlled trial comparing patients undergoing emergency open surgery for obstructing left-sided colon cancer with those undergoing endoluminal stenting followed by laparoscopic resection demonstrated that the endolaparoscopic approach was associated with fewer complications and a higher rate of successful one-stage operation [14].
There are, however, concerns that the use of SEMS may adversely affect oncological outcomes. The principles of haematogenous spread of tumour cells by mechanical handling of cancers have been known for some time, leading to the development of a variety of minimal-handling techniques. Studies have detected tumour cells in the peripheral blood of patients with colorectal malignancies undergoing colonoscopy, endorectal ultrasound scan and stenting [15–17]. In some cases, these cells were not present prior to the investigation or intervention. The oncological consequences of this have not yet been examined. Clearly, however, there is concern that SEMS insertion may lead to clinically significant tumour seeding. There is also concern that subclinical perforations caused by the stent may cause dissemination.
Long-term results of studies comparing the use of stents as a bridge to surgery with emergency surgery are reassuring, with most demonstrating no significant differences in 3- and 5-year mortality rate [11, 18]. Such comparisons, however, may be confounded by the fact that patients operated on immediately without prior bowel decompression have worse outcomes as they are less well at the time of surgery. This makes outcomes in this group poorer, possibly obscuring any adverse effects of stenting when attempting a direct comparison. To overcome this, a recent study compared the long-term outcomes after stenting as a bridge to surgery and after elective surgery for non-obstructing left-sided colorectal cancer. Results demonstrated a possible adverse effect of stenting on overall 5-year survival as well as disease-free 5-year survival [19]. In our study, the aim is to determine if the long-term outcomes of our cohort of patients undergoing stent insertion as a bridge to surgery are significantly different to those undergoing elective resections.
Methods
This is a retrospective study comparing a cohort of patients having SEMS insertion prior to surgical resection of obstructing left-sided colorectal cancer (group A) and a cohort of patients having elective surgery for non-obstructing left-sided colorectal cancer (group B). Group A patients were identified from an electronic registry of colonic stenting procedures performed from November 1998 to November 2008 at Eastbourne District General Hospital (EDGH). Preoperative or bridge to surgery stenting was defined as urgent attempted stenting for acute obstruction followed by urgent or elective therapeutic resection in the absence of metastatic disease. Fluoroscopic technique was used in all cases as described previously [20], all procedures being carried out in the interventional radiology suite. All patients in group A had clinical features of left-sided colonic obstruction, confirmed by plain abdominal X-ray and urgent computed tomography scanning.
Group B patients were identified using theatre log records and the local cancer registry database. A consecutive series of those undergoing elective left-sided colonic resections (left hemicolectomy, sigmoid colectomy, high anterior resection and Hartmann's procedure) for Dukes B, C1, and C2 tumours from January 2003 to December 2007 were included. Those with mid- and low rectal tumours were excluded. All patients in group B were operated on by consultant colorectal surgeons. Patients in group A were operated on by consultant colorectal surgeons if their surgery was elective. If the surgery was performed as an emergency (failed stenting or perforation after stenting) or if further surgery was performed following an anastomotic leak in either group their surgery was performed under the care of the on-call surgeon. At the time of the study, there were five consultant surgeons participating in the on-call rota (four colorectal surgeons and one general surgeon).
Further information was collected from E-searcher (an electronic hospital database), Picture Archiving and Communication Systems (an imaging system) and individual case notes. Data was gathered on patient demographics, tumour site, Dukes stage, type of surgery, colostomy formation rate, complications, 30-day mortality rate, and long-term survival. Collected data was stored in an Excel spreadsheet (Microsoft Office Excel 2003). The GraphPad Prism statistical package was used for statistical analysis (GraphPad Prism version 5.02 for Windows, GraphPad Software, San Diego California USA, http://www.graphpad.com). Survival curves were plotted using the Kaplan–Meier analysis and compared using the log–rank (Mantel–Cox) test. The Fisher's exact test was used to compare other clinicopathologic parameters. A two-sided p value of less than 0.05 was considered significant.
Results
Group A included 15 patients who had attempted colonic stenting for colorectal cancer as a bridge to surgery. They represented 22 % of a total of 68 patients who had urgent attempted stenting for acute colonic obstruction from colorectal cancer. In total 130 patients had attempted stenting for colonic obstruction in the same centre and during the same period, irrespective of underlying pathology and urgency of procedure. Group B consisted of 88 consecutive patients who had elective surgery and met the inclusion criteria described above. Patient demographics, tumour site and Dukes stage are summarised in Table 1. The median age for group A was 71 years with a range from 59 to 83 years, and for group B 74 years with a range from 35 to 94 years (p = 0.41). In group A, there were five (33.3 %) female and ten (66.7 %) male patients and in group B 41 (46.6 %) and 47 (53.4 %), respectively (p = 0.58). The site of tumour in group A was rectosigmoid junction in one (6.7 %) patient, sigmoid colon in nine (60 %), descending colon/splenic flexure in five (33.3 %) and in group B it was upper rectum in five (5.7 %), rectosigmoid junction in 19 (21.6 %), sigmoid colon in 50 (56.8 %), descending colon in 14 (15.9 %). The differences between groups A and B were not statistically significant (p = 0.22). The Dukes' stage in group A was B in five (33.3 %) patients, C1 in eight (53.3 %), C2 in two (13.3 %), and in group B, it was B in 46 (52.3 %), C1 in 39 (44.3 %), C2 in 3 (3.4 %)—the p value was 0.15.
Of the 15 patients undergoing preoperative stenting, technical and clinical success was achieved in 11 (73 %). There was one incidence of perforation necessitating urgent Hartmann's procedure. In one patient, the stent migrated 3 days after placement, but the patient was successfully restented. There were no other complications relating to SEMS insertion. The median time duration between successful stenting and elective surgery was 18.5 days (range 5 to 95 days).
The operations performed in both groups are shown in Table 2. In group A, three patients had Hartmann's operation, five had high anterior resection, one underwent sigmoid colectomy, five had left hemicolectomy, and one an extended right hemicolectomy. In group B, 43 patients had high anterior resection, 20 sigmoid colectomy, and 25 left hemicolectomy. In group A, 3 (27.3 %) of the 11patients who had successful stent insertion required a stoma. Of the four in whom stent insertion was not successful, one underwent urgent Hartmann's procedure, one an urgent left hemicolectomy with defunctioning ileostomy, one an urgent high anterior resection with defunctioning ileostomy and one an urgent extended right hemicolectomy. Overall, six patients in group A required a stoma (40 %)—four end colostomies and two loop ileostomies. In group B, 6 of the 88 patients required a stoma (five loop ileostomies and one loop colostomy).
In group A, one patient who had undergone an anterior resection after successful stenting developed an anastomotic leak and returned to theatre for a Hartmann's procedure. A second patient in this group in whom stenting had not been successful also developed an anastomotic leak after anterior resection. In this instance, a defunctioning ileostomy was performed. There were no other post-operative complications. In group B, post-operative complication details were only available for 64 patients. Of these, three developed anastomotic leaks, three had intra-operative splenic injuries, three developed wound infections, three experienced medical complications (chest infection, renal impairment, tachyarrhythmia) and a further five patients developed incisional hernias.
The 30-day mortality rate for groups A and B was 6.7 % (one patient) and 5.7 % (five patients), respectively. The 5-year survival rate was 60 % and 58 %, respectively. The survival curves were calculated with Kaplan–Meier analysis (Fig. 1). Statistical analysis using the log-rank (Mantel–Cox) test gave a p value of 0.96, demonstrating no significant difference between the two groups.
Discussion
SEMS have proven a very useful tool in the management of colorectal malignancies. Use of stenting as a bridge to surgery confers many theoretical benefits. Patients with acute large bowel obstruction often suffer fluid, electrolyte, and nutritional imbalances. A period of decompression and resuscitation prior to undertaking major colonic resection may allow normalisation of some of these parameters, reducing the inherent risks of surgery and the post-operative period. ‘Buying time’ may also allow formal staging, perhaps preventing unnecessary surgery in those with advanced inoperable cancers. In addition, older frail patients gain the opportunity to make an informed decision about having surgery without being pressurised by the urgency of acute obstruction. For those with resectable disease, pre-operative relief of bowel obstruction could result in lower rates of incomplete, ‘non-oncological’ resections and lower rates of stoma formation.
There has, however, been some concern that manipulation of a tumour in this way may precipitate distant spread and so compromise the long-term outcomes for this patient group. Koch et al. [15] detected tumour cells in the peripheral blood of patients with colorectal malignancies undergoing diagnostic colonoscopies. In 6 of 44 patients, these cells were not present prior to the procedure. Further work by the team identified similar findings in patients undergoing endorectal ultrasound scan, with a trend towards worse prognosis in these patients which although not statistically significant, raised concerns that mechanical manipulation of tumours may be contributing to haematogenous spread [16]. Maruthachalam et al. [17] repeated some of this work finding evidence of peripheral occult tumour cells after SEMS insertion, but not after colonoscopy. There is also a possibility of tumour dissemination caused by stent perforations. Such injuries to the bowel may not be clinically apparent—Pirlet et al. [21] reported two stent perforations and eight silent perforations (detected at the time of histological examination of the resection specimen) in a series of 30 patients undergoing colonic stenting as a bridge to surgery. The clinical consequences of such occult perforations are not known, but clearly, the potential for dissemination is a concern.
Investigating the theoretical link between stent insertion and increased risk of metastasis, invasion and advancement of cancer, Saida et al. [11] compared the long-term outcomes of 44 patients stented as a bridge to surgery with 40 patients undergoing emergency operation. Long-term prognosis did not significantly differ: 3-year overall survival rate was 50 % vs. 48 %; 5 year survival was 44 % vs. 40 % in the emergency operation and SEMS groups, respectively. Their results were confounded by including Dukes D patients in their study. Li et al. [18] reported outcomes of 52 patients undergoing resection with a mean of 8 days ±2 days after stent insertion. All underwent elective one-stage procedures. Mean follow-up was 36 ± 12 months, at which time all the patients were alive. Another study randomised patients with left-sided colonic obstruction to either stenting (n = 47) or emergency resection (n = 51), and interim analysis showed increased 30-day morbidity in the stent group which led to suspension of the trial [22]. Final analysis did not however show any differences in 30-day mortality, overall mortality, morbidity or stoma rates.
The results of our study do not demonstrate that stent insertion as a bridge to surgery has any adverse effect on long-term survival. A similar study by Kim et al. [19] compared the outcomes of bridge to surgery after SEMS insertion and non-obstructing elective surgery, identifying 35 patients with left-sided malignancies who underwent resection after SEMS insertion, matching these to 350 patients with non-obstructing but similar stage disease who underwent elective surgery. In the subgroup of stage II and III patients (24 and 240, respectively) SEMS insertion had an adverse effect on the overall 5-year survival (43.6 % in the stent group vs. 86.9 % in the control group) and the 5-year disease-free survival rate (43.1 % in the stent group vs. 80.5 % in the control group). The authors concluded that SEMS insertion as a bridge to surgery in the management of left-sided colon cancer obstruction is possibly associated with adverse oncologic outcomes. We have not confirmed these results in our series.
There is a bias inherent in a comparative study of this nature as we attempt to compare a group of patients with obstructing colonic lesions to those whose lesions are not obstructing. Although the control group patients were matched according to Dukes' stage, colonic obstruction secondary to tumour is known to adversely impact outcome regardless of pathological staging [23–25], and we would therefore expect that the stent group outcomes would be poorer. The results of this study which show no such adverse effect can reassure clinicians that the possible tumour seeding caused by mechanical trauma during stent insertion is unlikely to have any significant effect on overall outcomes. In addition, our study suggests that preoperative stenting may diminish the adverse impact of obstruction resulting to outcomes similar to those undergoing elective surgery. We acknowledge that our numbers are small and that there is a possible selection bias as stented patients who were elderly and had significant comorbidities may have been less likely to proceed to surgery than similar patients who presented as elective cases.
During 1998 to 2008 at EDGH, a total of 68 patients underwent attempted urgent colonic stenting for acute large bowel obstruction from colorectal cancer. The relatively small number proceeding to surgery is a reflection of the fact that our hospital serves one of the most elderly communities in the United Kingdom and many of the patients were not fit for surgery even in the absence of metastatic disease. The success rate of stenting presented (73 %) is lower than in some similar studies [5, 6]. This may be attributable to the use of fluoroscopy alone rather than a combination approach utilising endoscopic techniques. Patient characteristics may also play a role—elderly patients often have extensive diverticulosis which can make stent insertion technically very challenging. It is also possible that published figures are subject to publication bias, with only studies which demonstrate high success rates appearing in print.
Complication rates from stenting in this series are comparable with published results—Watt et al. [13] reported a perforation rate of 4.5 % in a systematic review. This study confirms that colonic stenting as a bridge to surgery is safe. Our median time from successful stenting to surgery (18.5 days) is longer than that reported in other series [6, 18]. This may reflect the additional burdens imposed on the service by the various United Kingdom government waiting time pathways regulations resulting to delays on those presenting outside these pathways.
Stoma formation rates were higher in the stent group. Where stent insertion successfully relieved bowel obstruction, a stoma was required in 27.3 % of patients at the time of subsequent surgery. When we include those in whom stent deployment was not successful, the overall stoma formation rate for the stent group was 40 %. This compares to a stoma formation rate of 6.8 % in the elective control group. These findings echo those of Kim et al. [19] who also found that stoma formation rates were higher in the bridge to surgery group when compared to the elective controls. The authors speculated that this may be ascribed to a surgeons' individual decision to defunction an anastomosis in cases where the colon has previously been obstructed and where healing may be compromised.
Conclusions
Colonic stenting as a bridge to surgery is safe and can ‘buy time’ to fully assess and optimise patients with obstructing colorectal malignancies. Fears about adversely affecting long-term survival have not been confirmed in this study but there is clearly a need for more information. Ongoing trials comparing outcomes of colonic stenting followed by elective surgery with emergency surgery for the management of acute left-sided malignant colonic obstruction may provide some answers when long-term follow-up results become available. In the meantime, centres that have accumulated experience of stenting as a bridge to surgery need to publish their long-term results in order to address the concerns that colonic stenting may have a negative impact on long-term survival of cancer patients.
References
National Bowel Cancer Audit. NHS Information Centre (2010) http://www.ic.nhs.uk/webfiles/Services/NCASP/audits%20and%20reports/NHS_Bowel_Cancer_2010_INTERACTIVE.pdf Accessed 06 December 2011
Nugent KP, Daniels P, Stewart B, Patankar R, Johnson CD (1999) Quality of life in stoma patients. Dis Colon Rectum 42:1569–1574
Pearce NW, Scott SD, Karran SJ (1992) Timing and method of reversal of Hartmann's procedure. Br J Surg 79:839–841
Dohmoto M (1991) New method—endoscopic implantation of rectal stent in palliative treatment of malignant stenosis. Endoscopia Digestiva 3:1507–1512
Mainar A, De Gregorio MA, Tejero E, Tobio R, Alfonso E, Pinto I, Herrera M, Fernandez JA (1999) Acute colorectal obstruction: treatment with self-expandable metallic stents before scheduled surgery—results of a multicenter study. Radiology 210:65–69
Camunez F, Echenagusia A, Simo G, Turegano F, Vazquez J, Barreiro-Meiro I (2000) Malignant colorectal obstruction treated by means of self-expanding metallic stents: effectiveness before surgery and in palliation. Radiology 216:492–497
Khot UP, Lang AW, Murali K, Parker MC (2002) Systematic review of the efficacy and safety of colorectal stents. Br J Surg 89:1096–1102
Sebastian S, Johnston S, Geoghegan T, Torregiani W, Buckley M (2004) Pooled analysis of the efficacy and safety of self-expanding metal stenting in malignant colorectal obstruction. Am J Gastroenterol 99:2051–2057
Ng KC, Law WL, Lee YM, Choi HK, Seto CL, Ho JW (2006) Self-expanding metallic stent as a bridge to surgery versus emergency resection for obstructing left-sided colorectal cancer: a case-matched study. J Gastrointest Surg 10:798–803
Martinez-Santos C, Lobato RF, Fradejas JM, Pinto I, Ortega-Deballon P, Moreno-Azcoita M (2002) Self-expandable stent before elective surgery vs. emergency surgery for the treatment of malignant colorectal obstructions: comparison of primary anastomosis and morbidity rates. Dis Colon Rectum 45:401–406
Saida Y, Sumiyama Y, Nagao J, Uramatsu M (2003) Long-term prognosis of pre-operative “bridge to surgery” expandable metallic stent insertion for obstructive colorectal cancer: comparison with emergency operation. Dis Colon Rectum 46:S44–S49
Law WL, Choi HK, Chu KW (2003) Comparison of stenting with emergency surgery as palliative treatment for obstructing primary left-sided colorectal cancer. Br J Surg 90:1429–1433
Watt AM, Faragher IG, Griffin TT, Reiger NA, Maddern GJ (2007) Self-expanding metallic stents for relieving malignant colorectal obstruction: a systematic review. Ann Surg 246:24–30
Cheung HYS, Chung CC, Tsang WWC, Wong JCH, Yau KKK, Li MKW (2009) Endolaparoscopic approach vs conventional open surgery in the treatment of obstructing left-sided colon cancer. Arch Surg 144(12):1127–1132
Koch M, Kienle P, Sauer P, Willeke F, Buhl K, Benner A, Lehnert T, Herfarth C, Weitz J, von Knebel Doeberitz M (2004) Hematogenous tumor cell dissemination during colonscopy for colorectal cancer. Surg Endosc 18:587–591
Koch M, Antolovic D, Kienle P, Horstmann J, Herfarth C, von Knebel Doeberitz M, Weitz J (2007) Increased detection rate and potential prognostic impact of disseminated tumor cells in patients undergoing endorectal ultrasound for rectal cancer. Int J Colorectal Dis 22:359–365
Maruthachalam K, Lash GE, Shenton BK, Horgan AD (2009) Tumour cell dissemination following endoscopic stent insertion. Br J Surg 94:1151–1154
Li YD, Cheng YS, Li MH, Fan YB, Chen NW, Wang Y, Zhao JG (2010) Management of acute malignant colorectal obstruction with a novel self-expanding metallic stent as a bridge to surgery. Eur J Radiol 73:566–571
Kim JS, Hur H, Min SB, Sohn SK, Cho CH, Kim NK (2009) Oncologic outcomes of self-expanding metallic stent insertion as a bridge to surgery in the management of left-sided colon cancer obstruction: comparison with non-obstructing elective surgery. World J Surg 33:1281–1286
Clark JS, Buchanan GN, Khawaja AR, Rowe PH, Saunders MP et al (2003) Use of the Bard Memotherm self-expanding metal stent in the palliation of colonic obstruction. Abdom Imaging 28:518–524
Pirlet IA, Slim K, Kwiatkowski F, Michot F, Millat BL (2011) Emergency preoperative stenting versus surgery for acute left-sided malignant colonic obstruction: a multicenter randomized controlled trial. Surg Endosc 25:1814–1821
Van Hooft JE, Bemelman WA, Oldenburg B, Marinelli AW, Lutke Holzik MJ, Grubben MJ, Sprangers MA, Dijkgraaf MG, Fockens P (2011) Colonic stenting versus emergency surgery for acute left-sided malignant colonic obstruction: a multicentre randomised trial. Lancet Oncol 12:344–352
Chen HS, Sheen-Chen SM (2000) Obstruction and perforation in colorectal adenocarcinoma: an analysis of prognosis and current trends. Surgery 127:370–376
McArdle CS, Hole DJ (2004) Emergency presentation of colorectal cancer is associated with poor 5-year survival. Br J Surg 91:605–609
McArdle CS, McMillan DC, Hole DJ (2006) The impact of blood loss, obstruction and perforation on survival in patients undergoing curative resection for colon cancer. Br J Surg 93:483–488
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Knight, A.L., Trompetas, V., Saunders, M.P. et al. Does stenting of left-sided colorectal cancer as a “bridge to surgery” adversely affect oncological outcomes? A comparison with non-obstructing elective left-sided colonic resections. Int J Colorectal Dis 27, 1509–1514 (2012). https://doi.org/10.1007/s00384-012-1513-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-012-1513-8