Abstract
Introduction
Probiotics are defined as live microorganisms that, when administered in adequate amounts, confer a health benefit on the host. These probiotic effects are considered to be displayed through the mediation of effective molecules derived from these bacteria because live bacteria as well as their conditioned media exhibit beneficial effects in many cases. However, many of the probiotic-derived molecules which mediate such benefits have so far been poorly characterized. We previously found that competence and sporulation factor (CSF) activates the Akt and p38 MAPK pathways and protects epithelial cells from oxidant stress in the mammalian intestine. The purpose of this study is to determine the CSF effect on reducing intestinal inflammation.
Methods and results
A protein array demonstrated that CSF induced the anti-inflammatory cytokine, IL-10, and decreased the release of pro-inflammatory mediators, IL-4, IL-6 and CXCL-1, induced by TNF-α in Caco2/bbe cells. CSF also induced the cytoprotective protein Hsp 27 in Caco2/bbe cells. The histological score of intestinal inflammation in 2% dextran sodium sulfate (DSS)-treated mice with the administration of 10 nM CSF was significantly lower than that of control mice. CSF also improved the survival rate of mice treated with a lethal concentration of DSS.
Conclusion
Therefore, CSF is a potentially effective treatment for intestinal inflammation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The mammalian intestine contains a diverse population of commensal bacteria that are involved in the metabolism of nutrients, fortification of the mucosal barrier, xenobiotic metabolism, angiogenesis [1], and development of intestinal lymphoid tissue [2]. These bacteria are present in the intestinal microflora and play an indispensable role in maintaining intestinal homeostasis [3]. Some of the commensal bacteria are called probiotics which are living organisms, mostly found in food supplements, which provide health benefits beyond their mere nutritive value. Indeed, the administration of certain probiotics is known to improve abdominal symptoms [4] and intestinal damage in patients with inflammatory bowel disease (IBD) [5–9], antibiotics-induced colitis [10, 11], and necrotizing enterocolitis [12, 13]. Some mechanisms of these probiotic functions were identified to be the enhancement of innate immunity through the induction of anti-microbial peptide defensins [14–16], the enhancement of the barrier functions of intestinal epithelia through the activation of mitogen-activated protein kinases (MAPKs) [17, 18] and the regulation of the inflammatory status through the modulation of inflammation-related cytokines [18–31]. These therapeutic effects are thought to occur through the mediation of effective molecules derived from these bacteria because live bacteria as well as their conditioned media appear to exhibit beneficial effects in many cases [17, 18]. However, the specific molecules which mediated their benefits for host health are poorly characterized [32–34].
Bacillus subtilis, which is a soil and water saprophyte found in the enteric flora of many species, is regarded as a beneficial bacteria and has probiotic activities on the health status of grower pigs [35] and on the cytoprotection of mice [33, 36] and human epithelial cells [33]. B. subtilis secretes many small peptides including competence and sporulation factor (CSF) which is a quorum-sensing molecule. CSF, whose amino acid sequence is ERGMT, is a key molecule involved in communication among bacteria and is associated with bacterial proliferation and sporulation [37–40]. We have previously ascertained that CSF induces the expression of the cytoprotective proteins and heat-shock protein (Hsp) 25/27 and 72 [41–43], activates the Akt and p38 MAPK pathway in mammalian intestinal epithelia, and protects intestinal tissues from oxidant stress. Furthermore, this beneficial activity is mediated by the transport of CSF through the transport of novel organic cation transporter isotype 2 [33], thus suggesting a novel system of host–microbial interaction in the gut.
The current study demonstrates that CSF improves epithelial cell injury caused by intestinal inflammation through the modulation of pro-inflammatory mediators and inductions of cytoprotective Hsps.
Methods
Cell culture
Human colonic epithelial Caco2/bbe cells purchased from ATCC were grown in high-glucose Dulbecco's modified eagle's medium (DMEM) supplemented with 10% (vol/vol) fetal bovine serum (FBS), 2 mM l-glutamine, 50 U/ml penicillin, 50 μg/ml streptomycin, and 10 μg/ml transferrin (all from Invitrogen/GIBCO, Grand Island, NY) in a humidified atmosphere of 5% CO2. The cells were plated on 6- or 12-well plates at a density of 105 cells/cm2 and then were allowed to differentiate for 10–14 days before the experiments.
Protein array
Caco2/bbe cells were treated with or without 100 ng/ml TNF-α and/or 10 nM CSF, which were purchased from Hokkaido System Science (Hokkaido, Japan), in FBS-free DMEM for 48 h, and then, the conditioned media were collected from each well. The expression of inflammatory mediators in the conditioned media was examined using the Human Cytokine Antibody Array V (RayBiotech, GA) (Table 1) according to the manufacturer's instructions.
Western blots
Proteins of Caco2/bbe cells were washed with phosphate-buffered saline (PBS), extracted with 200 μl lysis buffer (1% vol/vol Triton X 100; 20 mM Tris, pH 8; 50 mM NaCl; 5 mM EDTA; 0.2% wt/vol BSA; and complete protease cocktail (Roche Molecular Biochemicals, Indianapolis, IN)), and analyzed by Western blotting. Five to twenty micrograms of each sample was resolved by SDS-PAGE (10–12%) and immediately transferred to a polyvinylidene difluoride (PVDF) membrane using 1× transfer buffer (25 mM Tris, pH 8.8; 192 mM glycine with 15% (vol/vol) methanol). PVDF membranes were incubated in PBS with 0.05% (vol/vol) Tween 20 (T-PBS) containing 5% (wt/vol) milk for 1 h at room temperature to block nonspecific binding. The blots were incubated overnight at 4°C with anti-human Hsp27 and Hsc70 antibody (Stressgen, Victoria, British Columbia, Canada) as the primary antibody. Blots were washed five times for 10 min each in T-PBS at room temperature, incubated for 60 min in species-appropriate horseradish peroxidase-conjugated secondary antibody (Jackson Immunoresearch, West Grove, PA) in T-PBS, washed four times in T-PBS and once in PBS, and developed using the Super-Signal West Pico enhanced chemiluminescence system (Pierce Chemical, Rockford, IL).
Dextran sodium sulfate-induced colitis in vivo
These studies were approved by the Institutional Animal Care and Use Committee of Asahikawa Medical College. DSS with a molecular weight of 25,000 (Tokyo Chemical Industry Co., Ltd.) was dissolved in tap water to obtain a 2% DSS solution. C57/Bl6 mice (18–25 g) were purchased from Sankyo Labo Service Co., Inc. (Tokyo, Japan) and allowed free access to this solution as drinking water for 6 days to prepare a mouse model of DSS-induced colitis. The mice were transanally administered various concentrations of CSF at day 6. Twenty-four hours after the administration of CSF, the mice were sacrificed, and a 3-mm piece of their colon was fixed in 10% buffered formalin, sectioned at 4 μm, and stained with hematoxylin and eosin for light microscopic examination. Protein samples were prepared as described above. In another set of experiments, the cumulative survival rate of the mice which were orally treated with 4% DSS and were transanally administered either 10 nM CSF or PBS for every 2 days was investigated.
Histological analysis
The histological activity was assessed according to Berg's score described below [44]. Because intestinal lesions showed multifocal and variable severity, the grades were assessed in three representative parts of the colon in each mouse. A score from 0 to 4 was based on the following criteria: (grade 0) no change from normal tissue; (grade 1) one or a few multifocal mononuclear cell infiltrates in the lamina propria accompanied by minimal epithelial hyperplasia and slight to no depletion of mucus from goblet cells; (grade 2) the lesions tended to involve more of the intestine than grade 1 lesions or were more frequent. Typical changes included mild inflammatory cell infiltrates in the lamina propria composed primarily of mononuclear cells with a few neutrophils. Small epithelial erosions were occasionally present, and inflammation rarely involved the submucosa; grade 3 lesions involved a large area of the mucosa or were more frequent than grade 2 lesions. Inflammation was moderate and often involved the submucosa but was rarely transmural. Inflammatory cells were a mixture of mononuclear cells as well as neutrophils, and crypt abscesses were sometimes observed. Ulcers were occasionally observed; grade 4 lesions usually involved most of the intestinal section and were more severe than grade 3 lesions. Inflammation was severe, including mononuclear cells and neutrophils, and was sometimes transmural. Crypt abscesses and ulcers were present.
Statistical analysis
The histological scores were analyzed with the Mann–Whitney U test. The survival curves were constructed with the Kaplan–Meyer method, and univariate survival distributions were compared using the Log rank test. A p value of <0.05 was considered to be statistically significant.
Results
CSF modulated the secretion of inflammatory mediators under the control of TNF-α and induced Hsp 27 in Caco2/bbe cells
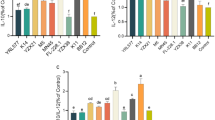
IL-10, which is an essential cytokine with immunosuppressive properties [45], was secreted from Caco2/bbe cells with no influence on the release of pro-inflammatory mediators 48 h after the incubation with 10 nM of CSF (Fig. 1a and b). Furthermore, CSF inhibits TNF-α-induced secretion of pro-inflammatory mediators including IL-4, IL-6, and IL-7, and CXCL-1 (Fig. 1c and d) but not IL-10. This suggests that CSF exerts an anti-inflammatory effect through inducing IL-10 and inhibiting the release of pro-inflammatory mediators derived from intestinal epithelia under inflammatory conditions. Various concentrations of CSF (0.1 to 100 nM) also induced Hsp27 in Caco2/bbe cells, thus suggesting that CSF has a protective effect on the intestinal epithelia (Fig. 1e).
Protein array for inflammatory mediators and Hsp 27 induction in Caco/2bbe cells treated with CSF. Caco/2bbe cells were treated with PBS (a), 10 nM CSF (b), 100 ng/ml TNF-α (c), or 100 ng/ml TNF-α with 10 nM CSF (d). The expression of inflammatory mediators in conditioned media of each well was analyzed with the Human Cytokine Antibody Array V (RayBiotech, GA). CSF induced the release of IL-10 from Caco2/bbe cells. TNF-α induced secretion of IL-4, IL-46, and IL-47 and CXCL-1 in the conditioned media of Caco2/bbe cells were negated by CSF. Treatment with either 1 nM or 100 nM CSF showed similar results as that of 10 nM CSF (data not shown). The Caco2/bbe cells were incubated with CSF for 16 h and examined Hsps expression in the cells (e). Hsp 27 was induced by 0.1 to 100 nM, particularly by 10 nM CSF, whereas neither experimental condition altered the expression of the constitutively expressed heat-shock cognate Hsc70
CSF improves intestinal injury in mice treated with DSS
The length of the large intestine was significantly longer in the mice orally treated with 2% DSS and a 10 nM CSF enema, than that in the mice without the CSF enema, while the weight of the mice showed no difference between the two groups (Fig. 2).
Weight and intestinal length of mice treated with 2% DSS. While the weight of DSS-colitis mice treated with 10 nM CSF solution was similar to the control mice, the length of mice colon treated with CSF solution was significantly longer than that of the control (a weight of mice, b length of mice colon, N.S. not significant) (N = 4). *p < 0.05 in comparison to the control based on an analysis of variance
The histological activity of the large intestine of DSS-colitis mice treated with CSF was assessed by Berg's score. Whereas DSS treatment caused moderate to severe colitis corresponding to grade 3 or more, 10 nM CSF significantly reduced the histological severity (2.0 ± 0.4) in comparison to the control (3.3 ± 0.2), thus indicating the anti-inflammatory effect of CSF treatment an in vivo colitis model (Fig. 3).
Histological findings and scores of the large intestine in the mice treated with 2% DSS and/or 10 nM CSF. The mice were treated with PBS (a), DSS (b), or DSS with 10 nM CSF (c). Whereas DSS treatment caused severe colitis corresponding to grade 3 or more, 10 nM CSF diminished inflammatory cell infiltration and improved epithelial defects. The histological score of the mice large intestine treated with 10 nM CSF (2.0 ± 0.4) was significantly lower than that of the mice treated with 2% DSS only (control) (3.3 ± 0.2) (d)
CSF improved survival rate of mice treated with a lethal dose of DSS
The 50% survival period of the control mice treated with 4% DSS was 9 days while 10 nM CSF prolonged the 50% survival period to 11 days. The cumulative survival rate of CSF-treated mice was significantly higher than that of control mice (p ≤ 0.05). This demonstrated the CSF effect to improve the survival ability of mice with severe colitis (Fig. 4).
The cumulative survival rates of the DSS-treated mice with or without the administration of CSF. Four-percent DSS-treated mice were anally administered either PBS or 10 nM CSF every 2 days and observed until their death. Whereas the control mice showed 50% survival periods of 9 days (dotted line), CSF prolonged the 50% survival periods to 11 days (solid line). The cumulative survival rate of CSF-treated mice was significantly higher than that of the control mice
Discussion
The current study demonstrated that the B. subtilis quorum-sensing molecule, CSF, reduced epithelial injury caused by intestinal inflammation and improved the survival rate of mice with lethal colitis. This indicates that CSF mediates the protective effect of B. subtilis and is potentially useful for treating intestinal inflammation. Probiotics, including Lactobacillus, Bifidobacterium, and B. subtilis, are beneficial for maintaining intestinal homeostasis and host health [1–3], and they can be utilized to treat antibiotics-induced colitis [10, 11], necrotizing enterocolitis [12, 13], and inflammatory bowel disease (IBD) including ulcerative colitis and Crohn's disease [5–9]. However, these probiotics are not always effective for treating these intestinal disorders [46, 47] because such live probiotics are required to colonize and to maintain their activity under the various conditions of the lumen in each patient in order to exhibit their beneficial functions for host health. In most patients with intestinal disorders, intestinal conditions are diverse due to the augmentation of pathogenic bacteria and/or the administration of drugs which may be harmful for probiotics. Because CSF, a B. subtilis-derived molecule, activates the protein kinase B (Akt) and p38 mitogen-activated protein kinase pathways, induces Hsps, and increases barrier function of intestinal epithelia against oxidant stress [33], we hypothesized that CSF might function to improve epithelial cell injury in intestinal inflammation. The present study proposed that probiotic-derived molecules as well as live probiotics can be useful to regulate intestinal inflammation. A stabilizing effect is expected to regulate intestinal inflammation rather than using live probiotics because probiotic-derived molecules exhibit their physiological functions without bacterial colonization in the intestinal lumen. A further analysis of the secretions from various probiotics will identify effective molecules which have unique functions that are beneficial for host health.
The current analysis of conditioned media of Caco2/bbe cells indicated that CSF decreased epithelia-released mediators including many pro-inflammatory mediators, IL-4, IL-6, and IL-7, and CXCL-1, induced by TNF-α. Whereas some probiotics or their secretions in conditioned media regulate inflammatory-related mediators released from epithelial cells [18–31], the present study showed a bacteria-derived molecule that could regulate the release of inflammation-related mediators from the intestinal epithelia. It is noteworthy that CSF functioned as an effector not only for the downregulation of pro-inflammatory mediators but also for the upregulation of the anti-inflammatory cytokine IL-10, which is an essential cytokine with immunosuppressive properties and whose impairment causes continuous intestinal inflammation similar to that observed in Crohn's disease [45]. In addition, CSF induced Hsp 27 which is an essential molecule for epithelial cytoprotection [41–43] and whose downregulation is associated with the pathogenesis of IBD [48]. Therefore, CSF can improve epithelial cell injury in intestinal inflammation including IBD through both immunomodulation and cytoprotection. Probiotics enhance innate immunity through the induction of anti-microbial defensin peptides [14–16]. Moreover, Schröder et al. proposed that hBD-1 reduced by the thioredoxin system, which possesses free cysteine residues in the carboxy terminus, exhibits antibacterial activity for commensal bacteria and opportunistic pathogenic fungus while oxidized hBD-1, with three intramolecular disulphide bridges, exerts only minor antibiotic killing activity, suggesting the crucial role of the interaction between redox regulation and innate immune defense for an effective barrier protecting human epithelia [49]. Further analysis of the mechanism of the in vivo effects of CSF on epithelial barrier function as well as immune systems is expected to clarify the mechanism of intestinal cytoprotection in the future.
In summary, this study demonstrated that a B. subtilis-derived molecule, CSF, improved the epithelial cell injury caused by intestinal inflammation. The anti-inflammatory effect of CSF was mediated by the downregulation of pro-inflammatory mediators, the upregulation of anti-inflammatory IL-10, and the induction of cytoprotective protein Hsps in the intestinal epithelia. CSF can possibly reduce intestinal inflammations without bacterial colonization. Probiotic-derived molecules, such as CSF, are therefore potential options for the treatment of intestinal inflammation.
References
Hooper LV, Wong MH, Thelin A, Hansson L, Falk PG, Gordon JI (2001) Molecular analysis of commensal host-microbial relationships in the intestine. Science 291(5505):881–884. doi:10.1126/science.291.5505.881
Sudo N, Sawamura S, Tanaka K, Aiba Y, Kubo C, Koga Y (1997) The requirement of intestinal bacterial flora for the development of an IgE production system fully susceptible to oral tolerance induction. J Immunol 159(4):1739–1745
Guarner F, Malagelada JR (2003) Gut flora in health and disease. Lancet 361(9356):512–519. doi:10.1016/S0140-6736(03)12489-0
Rousseaux C, Thuru X, Gelot A, Barnich N, Neut C, Dubuquoy L, Dubuquoy C, Merour E, Geboes K, Chamaillard M, Ouwehand A, Leyer G, Carcano D, Colombel JF, Ardid D, Desreumaux P (2007) Lactobacillus acidophilus modulates intestinal pain and induces opioid and cannabinoid receptors. Nat Med 13(1):35–37. doi:10.1038/nm1521
Kruis W, Schutz E, Fric P, Fixa B, Judmaier G, Stolte M (1997) Double-blind comparison of an oral Escherichia coli preparation and mesalazine in maintaining remission of ulcerative colitis. Aliment Pharmacol Ther 11(5):853–858
Rembacken BJ, Snelling AM, Hawkey PM, Chalmers DM, Axon AT (1999) Non-pathogenic Escherichia coli versus mesalazine for the treatment of ulcerative colitis: a randomised trial. Lancet 354(9179):635–639
Gionchetti P, Rizzello F, Venturi A, Brigidi P, Matteuzzi D, Bazzocchi G, Poggioli G, Miglioli M, Campieri M (2000) Oral bacteriotherapy as maintenance treatment in patients with chronic pouchitis: a double-blind, placebo-controlled trial. Gastroenterology 119(2):305–309
Kato K, Mizuno S, Umesaki Y, Ishii Y, Sugitani M, Imaoka A, Otsuka M, Hasunuma O, Kurihara R, Iwasaki A, Arakawa Y (2004) Randomized placebo-controlled trial assessing the effect of bifidobacteria-fermented milk on active ulcerative colitis. Aliment Pharmacol Ther 20(10):1133–1141. doi:10.1111/j.1365-2036.2004.02268.x
Bibiloni R, Fedorak RN, Tannock GW, Madsen KL, Gionchetti P, Campieri M, De Simone C, Sartor RB (2005) VSL#3 probiotic-mixture induces remission in patients with active ulcerative colitis. Am J Gastroenterol 100(7):1539–1546. doi:10.1111/j.1572-0241.2005.41794.x
Surawicz CM, Elmer GW, Speelman P, McFarland LV, Chinn J, van Belle G (1989) Prevention of antibiotic-associated diarrhea by Saccharomyces boulardii: a prospective study. Gastroenterology 96(4):981–988
McFarland LV, Surawicz CM, Greenberg RN, Fekety R, Elmer GW, Moyer KA, Melcher SA, Bowen KE, Cox JL, Noorani Z et al (1994) A randomized placebo-controlled trial of Saccharomyces boulardii in combination with standard antibiotics for Clostridium difficile disease. JAMA: J Am Med Assoc 271(24):1913–1918
Bin-Nun A, Bromiker R, Wilschanski M, Kaplan M, Rudensky B, Caplan M, Hammerman C (2005) Oral probiotics prevent necrotizing enterocolitis in very low birth weight neonates. J Pediatr 147(2):192–196. doi:10.1016/j.jpeds.2005.03.054
Lin HC, Hsu CH, Chen HL, Chung MY, Hsu JF, Lien RI, Tsao LY, Chen CH, Su BH (2008) Oral probiotics prevent necrotizing enterocolitis in very low birth weight preterm infants: a multicenter, randomized, controlled trial. Pediatrics 122(4):693–700. doi:10.1542/peds.2007-3007
Wehkamp J, Harder J, Wehkamp K, Wehkamp-von Meissner B, Schlee M, Enders C, Sonnenborn U, Nuding S, Bengmark S, Fellermann K, Schroder JM, Stange EF (2004) NF-kappaB- and AP-1-mediated induction of human beta defensin-2 in intestinal epithelial cells by Escherichia coli Nissle 1917: a novel effect of a probiotic bacterium. Infect Immun 72(10):5750–5758. doi:10.1128/IAI.72.10.5750-5758.2004
Schlee M, Harder J, Koten B, Stange EF, Wehkamp J, Fellermann K (2008) Probiotic lactobacilli and VSL#3 induce enterocyte beta-defensin 2. Clin Exp Immunol 151(3):528–535. doi:10.1111/j.1365-2249.2007.03587.x
Mondel M, Schroeder BO, Zimmermann K, Huber H, Nuding S, Beisner J, Fellermann K, Stange EF, Wehkamp J (2009) Probiotic E. coli treatment mediates antimicrobial human beta-defensin synthesis and fecal excretion in humans. Mucosal Immunol 2(2):166–172. doi:10.1038/mi.2008.77
Yan F, Polk DB (2002) Probiotic bacterium prevents cytokine-induced apoptosis in intestinal epithelial cells. J Biol Chem 277(52):50959–50965. doi:10.1074/jbc.M207050200
Tao Y, Drabik KA, Waypa TS, Musch MW, Alverdy JC, Schneewind O, Chang EB, Petrof EO (2006) Soluble factors from Lactobacillus GG activate MAPKs and induce cytoprotective heat shock proteins in intestinal epithelial cells. Am J Physiol Cell Physiol 290(4):C1018–C1030. doi:10.1152/ajpcell.00131.2005
Morita H, He F, Fuse T, Ouwehand AC, Hashimoto H, Hosoda M, Mizumachi K, Kurisaki J (2002) Adhesion of lactic acid bacteria to caco-2 cells and their effect on cytokine secretion. Microbiol Immunol 46(4):293–297
Hosoi T, Hirose R, Saegusa S, Ametani A, Kiuchi K, Kaminogawa S (2003) Cytokine responses of human intestinal epithelial-like Caco-2 cells to the nonpathogenic bacterium Bacillus subtilis (natto). Int J Food Microbiol 82(3):255–264
Kelly D, Campbell JI, King TP, Grant G, Jansson EA, Coutts AG, Pettersson S, Conway S (2004) Commensal anaerobic gut bacteria attenuate inflammation by regulating nuclear-cytoplasmic shuttling of PPAR-gamma and RelA. Nat Immunol 5(1):104–112. doi:10.1038/ni1018
Petrof EO, Kojima K, Ropeleski MJ, Musch MW, Tao Y, De Simone C, Chang EB (2004) Probiotics inhibit nuclear factor-kappaB and induce heat shock proteins in colonic epithelial cells through proteasome inhibition. Gastroenterology 127(5):1474–1487
Jijon H, Backer J, Diaz H, Yeung H, Thiel D, McKaigney C, De Simone C, Madsen K (2004) DNA from probiotic bacteria modulates murine and human epithelial and immune function. Gastroenterology 126(5):1358–1373
Zhang L, Li N, Caicedo R, Neu J (2005) Alive and dead Lactobacillus rhamnosus GG decrease tumor necrosis factor-alpha-induced interleukin-8 production in Caco-2 cells. J Nutr 135(7):1752–1756
Otte JM, Podolsky DK (2004) Functional modulation of enterocytes by gram-positive and gram-negative microorganisms. Am J Physiol Gastrointest Liver Physiol 286(4):G613–G626. doi:10.1152/ajpgi.00341.2003
O'Hara AM, O'Regan P, Fanning A, O'Mahony C, Macsharry J, Lyons A, Bienenstock J, O'Mahony L, Shanahan F (2006) Functional modulation of human intestinal epithelial cell responses by Bifidobacterium infantis and Lactobacillus salivarius. Immunology 118(2):202–215. doi:10.1111/j.1365-2567.2006.02358.x
Broekaert IJ, Nanthakumar NN, Walker WA (2007) Secreted probiotic factors ameliorate enteropathogenic infection in zinc-deficient human Caco-2 and T84 cell lines. Pediatr Res 62(2):139–144. doi:10.1203/PDR.0b013e31809fd85e
Lopez M, Li N, Kataria J, Russell M, Neu J (2008) Live and ultraviolet-inactivated Lactobacillus rhamnosus GG decrease flagellin-induced interleukin-8 production in Caco-2 cells. J Nutr 138(11):2264–2268. doi:10.3945/jn.108.093658
Candela M, Perna F, Carnevali P, Vitali B, Ciati R, Gionchetti P, Rizzello F, Campieri M, Brigidi P (2008) Interaction of probiotic Lactobacillus and Bifidobacterium strains with human intestinal epithelial cells: adhesion properties, competition against enteropathogens and modulation of IL-8 production. Int J Microbiol 125(3):286–292. doi:10.1016/j.ijfoodmicro.2008.04.012
Zeuthen LH, Fink LN, Frokiaer H (2008) Epithelial cells prime the immune response to an array of gut-derived commensals towards a tolerogenic phenotype through distinct actions of thymic stromal lymphopoietin and transforming growth factor-beta. Immunology 123(2):197–208. doi:10.1111/j.1365-2567.2007.02687.x
Ueno N, Fujiya M, Segawa S, Nata T, Moriichi K, Tanabe H, Mizukami Y, Kobayashi N, Ito K, Kohgo Y (2011) Heat-killed body of Lactobacillus brevis SBC8803 ameliorates intestinal injury in a murine model of colitis by enhancing the intestinal barrier function. Inflamm Bowel Dis 17(11):2235–2250. doi:10.1002/ibd.21597
Lievin V, Peiffer I, Hudault S, Rochat F, Brassart D, Neeser JR, Servin AL (2000) Bifidobacterium strains from resident infant human gastrointestinal microflora exert antimicrobial activity. Gut 47(5):646–652
Fujiya M, Musch MW, Nakagawa Y, Hu S, Alverdy J, Kohgo Y, Schneewind O, Jabri B, Chang EB (2007) The Bacillus subtilis quorum-sensing molecule CSF contributes to intestinal homeostasis via OCTN2, a host cell membrane transporter. Cell Host Microbe 1(4):299–308. doi:10.1016/j.chom.2007.05.004
Yan F, Cao H, Cover TL, Whitehead R, Washington MK, Polk DB (2007) Soluble proteins produced by probiotic bacteria regulate intestinal epithelial cell survival and growth. Gastroenterology 132(2):562–575. doi:10.1053/j.gastro.2006.11.022
Alexopoulos C, Georgoulakis IE, Tzivara A, Kyriakis CS, Govaris A, Kyriakis SC (2004) Field evaluation of the effect of a probiotic-containing Bacillus licheniformis and Bacillus subtilis spores on the health status, performance, and carcass quality of grower and finisher pigs. J Vet Med A Physiol Pathol Clin Med 51(6):306–312. doi:10.1111/j.1439-0442.2004.00637.x
D'Arienzo R, Maurano F, Mazzarella G, Luongo D, Stefanile R, Ricca E, Rossi M (2006) Bacillus subtilis spores reduce susceptibility to Citrobacter rodentium-mediated enteropathy in a mouse model. Res Microbiol 157(9):891–897. doi:10.1016/j.resmic.2006.06.001
Solomon JM, Lazazzera BA, Grossman AD (1996) Purification and characterization of an extracellular peptide factor that affects two different developmental pathways in Bacillus subtilis. Genes Dev 10(16):2014–2024
Kunst F, Ogasawara N, Moszer I, Albertini AM, Alloni G, Azevedo V, Bertero MG, Bessieres P, Bolotin A, Borchert S, Borriss R, Boursier L, Brans A, Braun M, Brignell SC, Bron S, Brouillet S, Bruschi CV, Caldwell B, Capuano V, Carter NM, Choi SK, Codani JJ, Connerton IF, Danchin A et al (1997) The complete genome sequence of the gram-positive bacterium Bacillus subtilis. Nature 390(6657):249–256. doi:10.1038/36786
Lazazzera BA, Solomon JM, Grossman AD (1997) An exported peptide functions intracellularly to contribute to cell density signaling in B. subtilis. Cell 89(6):917–925
Tam NK, Uyen NQ, Hong HA, le Duc H, Hoa TT, Serra CR, Henriques AO, Cutting SM (2006) The intestinal life cycle of Bacillus subtilis and close relatives. J Bacteriol 188(7):2692–2700. doi:10.1128/JB.188.7.2692-2700.2006
Musch MW, Kaplan B, Chang EB (2001) Role of increased basal expression of heat shock protein 72 in colonic epithelial c2BBE adenocarcinoma cells. Cell Growth Differ: Mol Biol J Am Assoc Cancer Res 12(8):419–426
Ropeleski MJ, Tang J, Walsh-Reitz MM, Musch MW, Chang EB (2003) Interleukin-11-induced heat shock protein 25 confers intestinal epithelial-specific cytoprotection from oxidant stress. Gastroenterology 124(5):1358–1368
Ropeleski MJ, Riehm J, Baer KA, Musch MW, Chang EB (2005) Anti-apoptotic effects of L-glutamine-mediated transcriptional modulation of the heat shock protein 72 during heat shock. Gastroenterology 129(1):170–184
Berg DJ, Davidson N, Kuhn R, Muller W, Menon S, Holland G, Thompson-Snipes L, Leach MW, Rennick D (1996) Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4(+) TH1-like responses. J Clin Invest 98(4):1010–1020. doi:10.1172/JCI118861
Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W (1993) Interleukin-10-deficient mice develop chronic enterocolitis. Cell 75(2):263–274
McFarland LV (2006) Meta-analysis of probiotics for the prevention of antibiotic associated diarrhea and the treatment of Clostridium difficile disease. Am J Gastroenterol 101(4):812–822. doi:10.1111/j.1572-0241.2006.00465.x
Rahimi R, Nikfar S, Rahimi F, Elahi B, Derakhshani S, Vafaie M, Abdollahi M (2008) A meta-analysis on the efficacy of probiotics for maintenance of remission and prevention of clinical and endoscopic relapse in Crohn's disease. Dig Dis Sci 53(9):2524–2531. doi:10.1007/s10620-007-0171-0
Hu S, Ciancio MJ, Lahav M, Fujiya M, Lichtenstein L, Anant S, Musch MW, Chang EB (2007) Translational inhibition of colonic epithelial heat shock proteins by IFN-gamma and TNF-alpha in intestinal inflammation. Gastroenterology 133(6):1893–1904. doi:10.1053/j.gastro.2007.09.026
Schroeder BO, Wu Z, Nuding S, Groscurth S, Marcinowski M, Beisner J, Buchner J, Schaller M, Stange EF, Wehkamp J (2011) Reduction of disulphide bonds unmasks potent antimicrobial activity of human beta-defensin 1. Nature 469(7330):419–423. doi:10.1038/nature09674
Acknowledgments
The study was supported by a Grant-in-Aid for Scientific Research no. 20590734 (M.F.) and the Intractable Disease, the Health and Labour Sciences Research Grants from the Ministry of Health, Labor and Welfare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Okamoto, K., Fujiya, M., Nata, T. et al. Competence and sporulation factor derived from Bacillus subtilis improves epithelial cell injury in intestinal inflammation via immunomodulation and cytoprotection. Int J Colorectal Dis 27, 1039–1046 (2012). https://doi.org/10.1007/s00384-012-1416-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-012-1416-8