Abstract
Purpose
Measure the association between the incidence of primary tumor staining and the identification of mediastinal lymph node (MLN) using cytokeratins, NM23, DCC-positive tumors, and vascular endothelial growth factor (VEGF) expression in T2 and T3/N0 colorectal cancers. The impact of MLN on both recurrence and survival was assessed.
Materials and methods
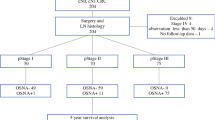
There were 153 CORC patients (T2, T3/N0) selected from a prospectively accrued database. All patients had been staged by routine histopathology after a curative resection and no patients received adjuvant chemotherapy. The primary tumors (PT) were assessed with a panel of immunohistochemical stains (cytokeratin, DCC, Nm23, and VEGF). If the PT was positive, the regional nodes were assessed with that marker(s). For any positive tumor marker, all lymph nodes (LNs, mean of 12.6±4.2) were stained for this marker.
Results
Patient age ranged from 38 to 86 years with a mean age of 61.56±25.56 years. Mean follow-up was 72.1±32.4 months. Recurrence rate of the whole group was 19/153 (12.4%) and the mean time to recurrence was 37.6±23.6 months (15 to 77 months). Crude mortality was 39.9%, while the cancer specific mortality was 11.2% after the whole follow-up period. The relationship between PT staining and MLNs was: cytokeratin-PT 143 (93.5%)/MLN 9 (6.3%); NM23-PT 51 (33.3%)/MLN 3 (5.9%); DCC-PT 79 (53%)/MLN 3 (3.8%); and VEGF-PT 72 (47%)/MLN 4 (5.6%). Nineteen (12.4%) patients experienced tumor recurrence. No correlation exist between PT and/or MLN staining and either recurrence or survival. No patient with MLN with any stain experienced a recurrence. There was no advantage to using an individual stain or all four stains.
Conclusion
Immunohistochemical stains for PT and focused analysis of regional nodes did not improve prediction of survival or recurrence. Sentinel LN evaluation and the provision of adjuvant chemotherapy in node-negative patients should be questioned and not be utilized outside of a research protocol.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Colorectal cancer is the most common gastrointestinal malignancy and the second leading cause of cancer-related deaths in the USA with a 5-year survival of about 90% in stage I, 75% in stage II, and 50% in stage III according to the American Joint committee on cancer [1]. Mortality is directly correlated with distant and regional metastases. Unfortunately, more than 30% of patients with colorectal cancer have unresectable disease at presentation and 50% of the remaining patients develop metastases and die of their disease [2]. Surgery and adjuvant chemotherapy are standard treatments for stage III of the disease as it improves the chances of survival, but adjuvant treatment is only used in clinical trials for stage II disease [3]. Accurate staging is the primary prognostic indicator for survival and is essential for stratifying patients to receive chemotherapy [3]. In the absence of established distant metastatic disease, regional lymph node involvement is the most important independent prognostic factor [4]. However, a significant percentage of patients will develop a recurrence in the absence of histologically identified lymph node metastases. This is presumed to be the result of occult metastases in liver, lymph nodes (LN), bone marrow, and other tissues [4].
Micrometastases in lymph nodes may not be detected by routine hematoxylin and eosin histopathological staining [5]. Lymph node micrometastasis represented by single cells or small clusters of tumor cells may be overlooked. There have been reports of histopathologically upstaging stage II colorectal cancer using immunohistochemical staining of these nodes, resulting in the conversion of 25–40% of patients from stage II to stage III [6–9]. Polymerase chain reaction (PCR) techniques have also been applied to this problem, with techniques including reverse transcriptase (RT)-PCR for cytokeratins and carcinoembryonic antigen (CEA) [10, 11] and mutant-allele-specific amplification (MASA)/PCR for K-ras [12]. PCR techniques lead to upstaging of 30–100% of stage II colorectal cancer patients. There is controversy in the literature regarding the optimal method of micrometastasis detection. Clinical use of PCR is limited by the inability to assess micrometastases without genetic alterations, difficulties in retrieving good-quality DNA from archived specimens, expense, and the high false positive rates. Immunostaining is far less costly and easily used on archival tissue; however, the risk of both false positive and negative results exists. Sasaki et al. 1998 [13] identified a greater sensitivity with cytokeratin immunostaining compared to PCR analysis of K-ras mutations in the nodal assessment of colorectal cancer.
Regardless of the technique used, the potential benefit of micrometastic nodal identification hinges upon a clear relationship to worsened outcome and improved survival with adjuvant treatment. Immunohistochemical identification of micrometastases has been associated with worsened survival with the use of new markers including: cytokeratins (8, 18, 19, 20, CAM5.2, and AE1:AE3) and Nm23 [12]. Liefers et al. [11] similarly suggested that inactivation of DCC gene results in early dissemination of tumor cells to locoregional lymph nodes. The identification of tumor angiogenesis with vascular endothelial growth factor (VEGF) has also been associated with increased risk of mediastinal lymph node (MLN) and a potential avenue for adjuvant treatment with antiangiogenesis factors. There is no data which clearly defines survival based upon the presence of micrometastases detected by any or multiple methods. The analysis of small numbers of cases and the use of limited numbers of markers in any individual study makes such an interpretation impossible. The need for a simultaneous analysis of a battery of putative markers is essential because a given tumor may express all potential markers. It also appears likely that a marker for MLN should be expressed by the primary tumor, thus, providing a screening approach. Finally, the presence of MLN and the immunohistochemical marker(s) pattern should be correlated with recurrence and survival.
Specific aims
The aims of this study were:
-
1.
To define the incidence of the specific tumor markers in primary T2 or T3/N0 tumors (immunohistochemical staining using panel of cytokeratin antibodies and DCC, VEGF, and NM23 antibodies)
-
2.
To identify the utility of the specific tumor markers (when present in the primary lesion) for identifying occult regional LN in stage II colorectal cancer (immunohistochemical staining using a panel of cytokeratin antibodies, and DCC, VEGF, and NM23 antibodies)
-
3.
To measure the association between the effect of the marker profiles in primary tumors and survival over the whole period of follow-up
-
4.
To measure the association between the incidence of marker profiles and the identification of micrometastases
-
5.
To measure the association between the identification of MLN and survival
Materials and methods
The study was performed on all the banked specimens of primary tumors and lymph nodes of stage I and II colorectal cancer patients performed in CORS department in the period from January 1990 to December 1995 (153 patients). All patients underwent curative resections without adjuvant therapy. Individual patients were neither identified nor separately reported.
All cases were more than 5 years post-surgery and put at ongoing follow-up based on the departmental cancer database. Patients underwent follow-up every 3 months in the first year, then twice a year for 2 years, and annually thereafter. Physical examination, proctoscopy, and evaluation of CEA level were done for follow-up.
Two 5-μm-thick sections were cut from each block for each marker and heat-adhered to positively charged glass slides. The sections were deparaffinized and rehydrated. Antigen retrieval was done by microwaving in citrate buffer. The staining was done on an automated stainer. Primary antibody was used according to marker examined (panel of antibodies for cytokeratines 8, 18, 19, and 20, CAM5.2, AE1:AE3, NM23 antibodies, and the G97-449 monoclonal antibody (Pharmingen, San Diego, CA, USA) in assessing DCC expression. The antibody has a distinct granular cytoplasmic staining pattern, and staining was performed with the diaminobenzadine kit (Venatana 250-001). After staining, slides were counterstained with hematoxylin. VEGF stain was used for scoring of tumor angiogeneis.
Primary examination of the tumor was performed first and then all the lymph nodes were examined histologically by hematoxylin and eosin H&E staining. Immunohistochemical staining of all lymph nodes was only performed with markers that proved to be positive in the primary tumor. The lymph nodes were immunostained using two sections of each node. Slides were considered to be positive if more than 25% of cells were positively stained for VEGF, Nm23, DCC, or cytokeratines. If one node stained positive for any marker, the patient was considered to have micrometastasis. Interpretation of all histology was performed by a single pathologist. The association between marker status and survival from the disease was studied.
Statistical methods
Kaplan–Meier curves are shown for time to recurrence and time to death. Curves representing patients with positive and negative stains are superimposed on each plot. Log- rank tests were used to formally compare groups with positive and negative stains.
Results
There were 153 patients included in the study. The age ranged from 38 to 86 years with a mean age of 61.56±25.56 years. All of the patients were followed at least for 5 years or until the death of the patient. The mean follow-up period was 65.123±32.41 months. Recurrence rate of the whole group was 19/153 (12.4%) and the mean period for recurrence was 37.6±23.6 months (range from 15 to 77 months). The mortality of the group over the whole period of follow-up was 61 cases (39.9%) while the cancer-specific mortality in the whole group over the total follow-up period was 17 cases (11.2%).
There were 153 tumors examined: 98 rectal tumors and 55 colonic tumors. All the lymph nodes recovered from all subjects were individually examined. The number of lymph nodes ranged from eight to 22 with a mean of 12 nodes per subject. There were 143 (93.5%) tumors positive for cytokeratins. Of these 143 tumor casess, nine (6.3%) patients proved to have positive lymph node micrometastasis. Fifty-one (33.3%) tumor cases had positive staining for NM23, and three of these patients had nodal micrometastasis. Seventy-nine (53%) tumors were positive for DCC gene; only three (3.8%) of the lymph nodes were positive for metastasis. VEGF was positive in 47% of the tumors and in only 5.6% of the nodes (Table 1).
Only 19 patients experienced recurrence. There was no evidence of any associations between positive stain of the tumor for any marker and recurrence (Fig. 1) or survival (Fig. 2). There was also no significant difference between the percentages of micrometastases detected by each individual antibody. Moreover, the use of any individual antibodies was similar in detecting micrometastases in the lymph nodes. Using more than one marker did not significantly increase the sensitivity of detecting micrometastasis.
The plots and associated log-rank tests indicate there is no association between positive stains and recurrence. Remember that only ten tumors stained negative to cytokeratines so the difference in the lower-right corner plot is not as dramatic as it looks. Log-rank test p-values are shown above each plot. Only two patients stained negative to all four stains so no comparison was made between recurrence and any positive stain
Kaplan–Meier curves for time until death are remarkably similar between the proportion staining positive and negative for each stain. Log-rank test p-values are shown above each plot. Only two patients stained negative to all four stains so no comparison was made between survival and any positive stain
No association was found between the identification of MLN (by each antibody separately) and recurrence (incidence of recurrence). There was, again, no patient with a positive LN stain who experienced recurrence during the 5-year follow-up period, and the log-rank test showed no evidence of a difference in time to recurrence (p=0.46, Fig. 3).
Neither crude survival nor cancer-related survival was affected by the presence of lymph node micrometastases identified with either an individual antibody or multiple antibodies (Figs. 4 and 5).
Correlation between the identification of LN miocrometastasis (by any antibody of the four used) and survival (crude survival). This plots shows that patients with at least one of the four antibodies staining positive in their lymph nodes experienced all-cause mortality faster than patients who tested negative with all four antibodies. The difference, however, is not statistically significant according to the log-rank test (p=0.17)
Discussion
Regional lymph node metastasis is an important adverse prognostic factor. However, even in colorectal cancer patients without node metastasis as assessed by conventional light microscopy, 20 to 30% will die of metastasis or recurrence within 5 years [14]. This unfavorable outcome may be explained by occult metastases which are not found by routine histopathological techniques. There is controversy in the literature about the impact of the presence of micrometastases detected by either the genetic method or immunohisochemistry (IHC) on the clinical outcome. Early results of immunohistochemistry showed no significant increase in the detection of lymph node metastasis than routine H&E stain [7, 14, 15], while later results showed either equal or more sensitive detection of micrometastases by IHC than PCR [13, 16]. The clinical significance is still challenged. It becomes even more controversial when the role of adjuvant treatment is considered in this group of colorectal cancer patients.
The molecular detection of lymph node metastases by PCR is very sensitive but has many disadvantages.
-
1.
The presence of pseudogenes (DNA sequence that shows a high degree of sequence similarity to a non-allelic functional gene but is non-functional). These pseudogenes are stable components of the genome that can be transcribed easily because they lack introns; consequently, the high sensitivity of PCR-based assay could amplify the pseudogene leading to false positive results [17].
-
2.
Falsepositive results can be also obtained because lymph nodes are antigen-producing centers; a degenerating tumor may produce fragments of DNA that are transported to a lymph node. These fragments may contain a target nucleic acid sequence that could be misinterpreted as neoplasia [18].
-
3.
It can detect micrometastases only if the primary tumor has p53 or k-ras mutation specially when working in a retrospective study. RT-PCR can be done for CEA, but it cannot be done on formalin-fixed paraffin blocks.
-
4.
The sensitivity of detection by MASA method is up to 0.1% tumor cell population in a background of normal cells [13] compared to higher sensitivity of detection by IHC (up to one cell in a background of many normal cells in lymph nodes, Table 2).
These potential errors can lead to a high percentage of false results with PCR and further compromises the assessment and validity of the role of these markers in making treatment decisions or evaluating prognosis.
IHC offers a particular advantage as a means of detecting occult lymph node metastasis in that its efficacy is not compromised by paraffin-embedded, formalin-fixed tissues, making it suited for retrospective studies on archival tissues. Furthermore, it is inexpensive, requires relatively little expertise, and is widely available.
In our study, we used IHC using four antibodies, as not every tumor is positive for all markers, so that we can increase the rate of detection of micrometastases. Although we examined all the lymph nodes retrieved from each tumor (mean of 12), stained two sections of each node, and used four markers for detection, the percentage of detection was not more than 11%. This percentage was less than that previously reported by Clarke et al. [19] (25%) (IHC using anti- CK), Oberge et al. [20] (32%) (IHC using anti- CK), and Adell et al. [6] (39%), while it was near to that detected by Yasuda et al. [9] (12%). This might be due to the use of a larger series of patients, selection criteria for cases in different series, operative technique of the surgeon, the dissecting technique of the pathologist, and/or the taking of multiple sections of LN during H&E study.
It has been reported that IHC staining with anti-cytokeratin antibodies increases the detection rate of metastasis in the lymph nodes in breast or colorectal carcinoma (Hayashi 1994); however, it has been reported that cytokeratin staining is positive for both reticulum cells and plasma cells which are present in lymph nodes [21]. Therefore, it is necessary to differentiate epithelial cells from non-epithelial cells to detect occult node metastasis which may be an additional source of falsely positive disease.
NM23 is an anti-metastasis gene and we expected it to be protective against micrometastases if the tumor is positive. We were not able to detect any significant differences between NM23 positive and negative tumors as regards micrometastases, recurrence, or survival. This data is similar to the results obtained by Sarris and Lee [22] who believed that the role and importance of the nm23 gene in the development of tumor metastasis in colorectal carcinoma is questionable. Conversely, Dursan et al. [23] reported a higher survival rate with NM23 positivity with a 3-year mean follow-up period. The use of 5-year follow-up may account for the difference.
Clarke et al. [19] suggested that the significant effect of lymph node micrometastases on survival—detected by immunohistochemistry—increased with the presence of extensive lymph node micrometastases and with high-level node micrometastases, while Yasuda et al. [9] suggested that the involvement of four or more lymph nodes and that stage N2 and higher are useful indicators of recurrence in patients with Dukes’ B colorectal cancer. The number of lymph nodes that were examined ranged from six to 15 in the most recent studies [11, 24].
Bilchik et al. [25] suggested that identification and focused examination of the sentinel lymph node using CK-IHC and RT-PCR can identify occult micrometastases in 53% of colorectal cancer patients whose sentinel nodes (SNs) were negative by conventional staging techniques. These ultrasensitive assays of the SN can identify patients who may be at high risk for recurrence of CRC and who, therefore, may benefit from systemic adjuvant therapy. The reliability of sentinel lymph as a detector for lymph node metastasis is still a controversial issue as it is accurate in only 55% of patients according to the most recent study [26]. It is also limited by the absence of consensus regarding the implications of micrometastases. The results in this study suggest that sentinel lymph node mapping will not have a clinical role while the detection of micrometastasis is not clinically significant.
Farnk et al. [27] suggested that tumor angiogenesis within colon cancer is an important predictor of tumor behavior and patient survival in node-negative patients. He suggested that node-negative patients with high angiogenic score are at higher risk of developing recurrences. Modulation of angiogenesis is mediated by genes that can initiate angiogenesis as well as by genes that can inhibit angiogenesis. Some studies suggest that p53 gene might be involved in this process. Tokunaga et al. [28] and Bruns et al. [29] showed a correlation with vascular endothelial growth factor which is a well-known factor that induces angiogenesis in liver metastasis in colon cancer. Other studies suggest that the recurrence rate in patients with VEGF-positive tumors was significantly higher than that of patients with VEGF-negative tumors [30]. Because angiogenesis promotes metastases, it seems plausible that there may be a correlation with the presence of lymph node micrometastases. DCC gene expression in the tumor seems to be associated with angiogenesis activity. However, we did not identify a significant relation between VEGF-positive tumors and MLN, tumor recurrence, or survival. This may be related to the particularly long follow-up period in this study, as all other studies have had short follow-up periods with a maximum of 60 months [30]. This concept is supported by the finding that recurrence in VEGF-positive tumors occurred earlier than in VEGF-negative tumor; although this did not reach significance (p=0.09).
In our study, there were no associations between IHC DCC tumor status and clinical outcome. Our results agree with those reported by Morgan et al. [31]. These results contradict the findings in previous series in which patients with colorectal and rectal cancers were examined [32–35].
Micrometastasis has been viewed as a requisite and early event in the progression to frankly metastatic disease [36–38]. If this hypothesis is correct, then the correct identification of a micrometastasis would be essential to allow administration of adjuvant chemotherapy. However, it is equally plausible that micrometastases may represent tumor cells destined to be destroyed by host immune responses before implantation, growth, and wider metastasis can occur. The small number of patients of micrometastasis reflects the absence of a significant difference between an experienced pathologist and immunostaining; however, this small number puts a limitation to the statistical analysis.
Conclusion
Data in this study support the view that lymph node micrometastases is a pathological finding that has no clinical significance for patients with colorectal cancer. Adjuvant therapy is, therefore, not recommended for this cohort, provided that all LN retrieved from the specimens are examined by an experienced pathologist. The detection of micrometastases is not improved by using more than one antibody. Neither NM23 nor DCC gene has any relation with the development of micrometastases, and VEGF does not affect the development of micrometastases or surgical outcome. Therefore, until there is a clear relationship between the identification of micrometastatic lymph nodes in colorectal cancer and survival, the use of sentinel lymph node mapping to alter treatment should remain under the supervision of a clinical study.
References
Greenlee RT, Murray T, Bolden S et al (2000) Cancer statistics, 2000. CA Cancer J Clin 50(1):7–33
Jessup JM, McGinnis LS, Steele GD Jr et al (1996) The national cancer data base. Report on colon cancer. Cancer 78(4):918–926
Gelmann A, Desnoyers R, Cagir B et al (2000) Colorectal cancer staging and adjuvant chemotherapy. Expert Opin Pharmacother 1(2):737–755
Cohen AM, Tremiterra S, Candela F et al (1991) Prognosis of node-positive colon cancer. Cancer 67(7):1859–1861
Buie W, Rothenberger D (1993) Surveillance after curative resection of colorectal cancer: individualizing follow-up. Gastrointest Endosc Clin N Am 3:691–713
Adell G, Boeryd B, Franlund B et al (1996) Occurrence and prognostic importance of micrometastases in regional lymph nodes in Dukes’ B colorectal carcinoma: an immunohistochemical study. Eur J Surg 162(8):637–642
Jeffers MD, O’Dowd GM, Mulcahy H et al (1994) The prognostic significance of immunohistochemically detected lymph node micrometastases in colorectal carcinoma. J Pathol 172(2):183–187
Greenson JK, Isenhart CE, Rice R et al (1994) Identification of occult micrometastases in pericolic lymph nodes of Duke’s B colorectal cancer patients using monoclonal antibodies against cytokeratin and CC49. Correlation with long-term survival. Cancer 73(3):563–569
Yasuda K, Adachi Y, Shiraishi N et al (2001) Pattern of lymph node micrometastasis and prognosis of patients with colorectal cancer. Ann Surg Oncol 8(4):300–304
Gunn JMJ, Yun K, Wright PA (1996) Detection of micrometastases in colorectal cancer patients by K19 and K20 reverse-transcription polymerase chain reaction. Lab Invest 75(4):611–616
Liefers GJ, Cleton-Jansen AM, van de Velde CJ et al (1998) Micrometastases and survival in stage II colorectal cancer. N Engl J Med 339(4):223–228
Hayashi N, Ito I, Yanagisawa A et al (1995) Genetic diagnosis of lymph-node metastasis in colorectal cancer. Lancet 345(8960):1257–1259
Sasaki M WH, Jass JR, Ajioka Y, Kobayashi M, Matsuda K, Hatakeyama K (1997) Occult lymph node metastases detected by cytokeratin immunohistochemistry predict recurrence in “node-negative” colorectal cancer. J Gastroenterol 32(6):758–764
Cutait R, Alves V, Lopes L et al (1991) Restaging of colorectal cancer based on the identification of lymph node micrometastases through immunoperoxidase staining of CEA and cytokeratins. Dis Colon Rectum 34(10):917–920
Davidson B, Sams V, Styles J et al (1990) Detection of occult nodal metastases in patients with colorectal carcinoma. Cancer 65(4):967–970
Miyake Y, Yamamoto H, Fujiwara Y et al (2001) Extensive micrometastases to lymph nodes as a marker for rapid recurrence of colorectal cancer: a study of lymphatic mapping. Clin Cancer Res 7(5):1350–1357
Neumaier M, Gerhard M, Wagener C (1995) Diagnosis of micrometastases by the amplification of tissue-specific genes. Gene 159(1):43–47
Sloane J (1995) Molecules and micrometastases. Lancet 345:1255–1256
Clarke G, Ryan E, O’Keane J et al (2000) The detection of cytokeratins in lymph nodes of Duke’s B colorectal cancer subjects predicts a poor outcome. Eur J Gastroenterol Hepatol 12(5):549–525
Oberg A, Stenling R, Tavelin B et al (1998) Are lymph node micrometastases of any clinical significance in Duke’s stages A and B colorectal cancer? Dis Colon Rectum 41(10):1244–1249
Gould VE, Bloom KJ, Franke WW et al (1995) Increased numbers of cytokeratin-positive interstitial reticulum cells (CIRC) in reactive, inflammatory and neoplastic lymphadenopathies: hyperplasia or induced expression? Virchows Arch 425(6):617–629
Sarris M, Lee CS (2001) nm23 protein expression in colorectal carcinoma metastasis in regional lymph nodes and the liver. Eur J Surg Oncol 27(2):170–174
Dursun A, Akyurek N, Gunel N et al (2002) Prognostic implication of nm23-H1 expression in colorectal carcinomas. Pathology 34(5):427–432
Jen J, Kim H, Piantadosi S et al (1994) Allelic loss of chromosome 18q and prognosis in colorectal cancer. N Engl J Med 331(4):213–221
Bilchik AJ, Saha S, Wiese D et al (2001) Molecular staging of early colon cancer on the basis of sentinel node analysis: a multicenter phase II trial. J Clin Oncol 19(4):1128–1136
Merrie A, van Rij A, Phillips L et al (2001) Diagnostic use of the sentinel node in colon cancer. Dis Colon Rectum 44(3):410–417
Frank RE, Saclarides TJ, Leurgans S et al (1995) Tumor angiogenesis as a predictor of recurrence and survival in patients with node-negative colon cancer. Ann Surg 222(6):695–699
Tokunaga T, Oshika Y, Abe Y et al (1998) Vascular endothelial growth factor (VEGF) mRNA isoform expression pattern is correlated with liver metastasis and poor prognosis in colon cancer. Br J Cancer 77(6):998–1002
Bruns C, Liu W, Davis D et al (2000) Vascular endothelial growth factor is an in vivo survival factor for tumor endothelium in a murine model of colorectal carcinoma liver metastases. Cancer 89(3):488–499
Cascinu S, Staccioli M, Gasparini G et al (2000) Expression of vascular endothelial growth factor can predict event-free survival in stage II colon cancer. Clin Cancer Res 6(7):2803–2807
Morgan M, Koorey D, Painter D et al (2003) P53 and DCC immunohistochemistry in curative rectal cancer. Int J Colorectal Dis 18:188–195
Fearon E, Cho K, Nigro J et al (1990) Identification of a chromosome 18q gene that is altered in colorectal cancers. Science 247:49–56
Ookawa K, Sakamoto M, Hirohashi S et al (1993) Concordant p53 and DCC alterations and allelic losses on chromosomes 13q and 14q associated with liver metastases of colorectal carcinoma. Int J Cancer 53:382–387
Shibata D, Reale M, Lavin P et al (1996) The DCC protein and prognosis in colorectal cancer. N Engl J Med 335:1727–1732
Reymond M, Dworak O, Remke S et al (1998) DCC protein as a predictor of distant metastases after curative surgery for rectal cancer. Dis Colon Rectum 41:775–760
Nordgard O, Aloysius TA, Todnem K et al (2003) Detection of lymph node micrometastases in colorectal cancer. Scand J Gastroenterol 38(2):125–132
Feezor RJ, Copeland EM 3rd, Hochwald SN (2002) Significance of micrometastases in colorectal cancer. Ann Surg Oncol 9(10):944–953
Noura S, Yamamoto H, Ohnishi T et al (2002) Comparative detection of lymph node micrometastases of stage II colorectal cancer by reverse transcriptase polymerase chain reaction and immunohistochemistry. J Clin Oncol 20(20):4232–4241
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Madbouly, K.M., Senagore, A.J., Mukerjee, A. et al. Does immunostaining effectively upstage colorectal cancer by identifying micrometastatic nodal disease?. Int J Colorectal Dis 22, 39–48 (2007). https://doi.org/10.1007/s00384-006-0098-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-006-0098-5