Abstract
Background and aims
Recent studies have demonstrated the relationship between Helicobacter pylori infection and the risk of colorectal carcinoma. However, the results of these studies remain controversial as the studies were relatively small in size and partially differed in designs, and so we reviewed the published studies and carried out a meta-analysis to further explore this relationship.
Materials and methods
We performed an extensive systematic review to find all the published case–control studies up to Jan. 2007 using electronic searching, hand searching, and reference lists of retrieved articles. Odds ratio (OR) was employed to evaluate the relationship of H. pylori infection and risk of colorectal cancer. Summary estimates were obtained using random effect models according to the result of a statistical test for heterogeneity across the studies. The presence of possible publication bias was assessed using different statistical approaches.
Results
Thirteen studies were included, and summary OR 1.49 (95% confidence interval [CI] 1.17–1.91) was estimated for the association between H. pylori infection and colorectal cancer. Summary OR 1.56 (95% CI 1.14–2.14) was estimated for the association between immunoglobulin G antibody and colorectal cancer risk. By trimming and filling, the number of inputted studies was zero, and summary OR was still 1.49 (95% CI 1.17–1.91). The graphical funnel plot appeared asymmetrical, but there was no statistical evidence of publication bias. The method of fail-safe suggested that the effect of publication bias was small.
Conclusion
Current evidence, though limited, suggests that there is a possible increase in risk of colorectal cancer because of H. pylori infection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer (CRC) is one of the leading causes of cancer-related deaths worldwide. The morbidity and mortality increased quickly in both developing and developed countries [1–4]. In America, it was estimated that 148,610 new cases of colorectal cancer would be diagnosed and 55,170 patients would die from colorectal cancer in 2006 [3]. In China, from 1992/1993 to 2000/2001, CRC incidence had increased from 13.06 to 19.37 per 100,000, and the morbidity and mortality in China will be 26.12 and 17.43 per 100,000 respectively in 2006 [4]. Given the substantial injury for human quality of life, cancer prevention in its forms emerges as a very attractive approach [5]. Study results supported the colorectal cancer etiological hypothesis of “deficiency of dietary fiber and vegetables”, excessive intake of fatty food, cigarette smoking, alcohol consumption, and other environment factors [6–9]. The potent role of genetic instability in initiation and progression of colorectal cancers also has been well defined. Germline mutations of DNA mismatch repair genes are considered responsible for the development of colorectal cancer [10–12]. Recent publications reported higher levels of Helicobacter pylori antibodies in patients with colorectal cancer than that in control subjects. However, this is quite controversial for different studies hold various results [13–16].
H. pylori are Gram-negative bacterium. Its infection has been recognized as a major risk factor for gastric cancer by the International Agency for Research on Cancer in 1994 [17], and infected people are also at risk of developing a rare B cell tumor, so-called gastric mucosal-associated lymphoid tissue lymphoma [18–20]. Several studies suggested that H. pylori can increase plasma level of gastrin and stimulate mucosal cell proliferation. Persistent exposure to H. pylori for several years may result in gastric atrophy, cell mutation, and transformation of gastric mucosal cells into gastrin-producing cells, which also express gastrin receptors serving to stimulate cell proliferation and tumor growth. During this processes, the overexpression of cyclooxygenase-2 also stimulates the cells to release excessive amount of prostaglandin E2, leading to further proliferation [21, 22]. Whereas, how H. pylori predisposes gastric epithelial cells to become cancerous is not fully understood [23], the role of H. pylori in the development of colorectal cancer is still uncertain.
Therefore, we carried out a systematic review and meta-analysis of published studies to confirm this association between H. pylori infection and the risk of colorectal cancer.
Materials and methods
Literature search and standard of selection
Literature search was conducted in PubMed, EBSCO, HighWire Press, and other databases for relevant articles published up to Jan. 2007 about the association of H. pylori infection and colorectal cancer and/or colorectal adenoma (known as the precancer). We used the following medical subject heading (Mesh) terms and/or text words: Helicobacter pylori, colorectal carcinoma, or colorectal cancer to look for case–control studies. Only original articles in English were used and the cited references in these published articles were also reviewed. Since the quality of studies is important for meta-analysis, they should provide the description of how patients and control subjects were selected, information on data collection, sample size, matching procedures, prevalence of each group, statistical methods, and how to control the potential confounding. All the studies in our review are full articles.
Two reviewers independently applied eligibility criteria, assessed the studies for methodological quality, and finished data extraction simultaneously. The results were combined and cross-checked, and any differences were resolved by reviewing the original until conformity. Information abstracted included the author, year of publication, country of origin, type of design, number of patients and controls, matching condition, prevalence of H. pylori in each group, odds ratio (OR), adjustment of confounders, etc.
Assessment of homogeneity and treatment effect
OR with 95% confidence interval (CI) was computed for each study, and pooled estimates were generated by MIX (Meta-analysis with interactive explanations—version 1.51; http://www.mix-for-meta-analysis.info). Statistical homogeneity between studies was assessed using the Cochrane Q value. Since there was an obvious heterogeneity between studies (Q = 31.11, P = 0.0033, I 2 = 58.21%) [24], the random effect model was used, which has the effect of giving more weight to the smaller studies than the fixed effect model [25].
Graphical evaluation of publication bias and statistical analysis
One of the great problems in systematic review is that not all studies can be published. Those studies with statistically significant results are more likely to be submitted and published than studies without significant results. If smaller studies without significant results remain unpublished, publication bias may occur in meta-analysis [26]. The funnel plot is used as the main graphical method for identifying publication bias, which is a scatter plot of the effect estimates from individual studies (horizontal axis) against sample size or some other indicators of the estimates (vertical axis). The most precise estimates (those from larger studies) are at the top of the funnel and those from less precise or smaller studies are more likely spread at the base of the funnel because of their larger standard errors, but they should also be symmetrically around the average. In the absence of bias, the plot resembles a symmetrical inverted funnel plot. When the gap at the bottom of the graph and the asymmetry of funnel are detected, a causal relationship must be claimed with caution.
As the supplement of the funnel plot, another statistical approach “trim and fill” was used as a formal procedure [27] (first, the trimmed funnel plot is used to estimate the center of the funnel, and then the omitted studies and their missing counterparts around the center are replaced, and finally, received the true center and the adjusted effect, including the “filled” studies) to adjust the summary OR in our study. Furthermore, the possible presence of publication bias was assessed by rank correlation method, linear regression approach, and fail-safe method [28, 29].
Results
Study characteristics
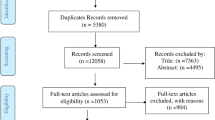
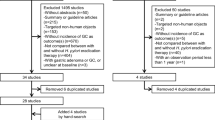
From the primary electronic database (PubMed), a total of 68 studies have been identified. Fifty-nine of them were considered ineligible for our review. The reasons were as follows: 43 studies were irrelevant to association of H. pylori infection and colorectal cancer; there was one case study, one animal study, nine clinical case reports, four comments, and one meta-analysis. After excluding, nine case–control studies met the inclusion criteria. In addition, we conducted a manual search for the other four cited references in these articles in PubMed, EBSCO, HighWire Press and other databases, which met the inclusion criteria. Therefore, 13 studies published from Dec. 1991 to Jan. 2007 were included in the final analysis. Table 1 shows the main characteristics of these studies.
Of the 13 studies, two were nested case–control studies [30], and one only chose male smokers as subjects [16]. All the cases were matched by age, gender, or other factors (race, social class, education, etc.). But three studies did not offer any information for matching [14, 31, 32], and only convenience samples were chosen (convenience sample means the person that is easy to find). Since one study used two groups of control subjects [33] (both of the two groups of control subjects were enrolled in this paper), 14 groups of data from 13 studies were obtained.
Overall analysis
Data from 13 studies with a total of 1,709 patients were included in the first meta-analysis. Since heterogeneity was detected, the random effect model was used and summary OR for colorectal cancer related to H. pylori infection was 1.49 (95% CI 1.17–1.91). Figure 1 shows the ORs, 95% CIs of each study, and summary OR. The assessment result of heterogeneity was: Q = 31.11, P = 0.0033, I 2 = 58.21%.
There are serials of measures to detect the H. pylori infection status, such as the enzyme-linked immunosorbent assay (ELISA), C-urea breath test (UBT), rapid urease test, and histological diagnosis of biopsy specimens. One or more of them were used in the 13 studies. The detected results were indicated by different indicators, which represent different infection status of H. pylori. Therefore, carefully restricted analyses for certain determined assays of H. pylori status seem necessary.
As we have known, immunoglobulin G (IgG) demonstrates the previous infection of H. pylori; the results of other methods demonstrate the present infection status of H. pylori. Three studies with the determination of H. pylori status not measured with IgG antibody were excluded in the second meta-analysis. Summary OR of 11 data from ten studies was 1.56 in the random effect model (95% CI 1.14–2.14; the assessment result of heterogeneity: Q = 28.54, P = 0.0015, I 2 = 64.96%). Comparing with 1.49 (95% CI 1.17–1.91) in Fig. 1, summary OR 1.56 (95% CI 1.14–2.14) is a little higher. It seems that there is a strong relationship between H. pylori infection and colorectal cancer while using IgG as indicator of H. pylori infection (Fig. 2).
Figure 3 shows the funnel plot of publication bias with OR value as the horizontal axis and SE of OR as the vertical axis. Judged by means of eyeballing, the graphical funnel plot of the 13 case–control studies appears asymmetry. We can assume the possibility of publication bias. After adjustment by trimming and filling, summary OR was still 1.49 (95% CI 1.17–1.91). Therefore, H. pylori infection may increase the risk of colorectal cancer.
Additionally, in any case, eyeballing examination remains a qualitative and subjective matter and therefore prone to bias. We also used the rank correlation method proposed by Begg et al. and the linear regression approach proposed by Egger et al. to evaluate the publication bias. Both of the two approaches can be used to assess the relationship between treatment effects and SE, which are similar statistical methods with the funnel plot. The regression method is more sensitive than the rank correlation approach, but the sensitivity of both of the two methods is generally lower in meta-analysis based on less than 20 trials [29]. Neither the Begg’s rank correlation (P = 0.324, two-tailed) nor the Egger’s regression tests (P = 0.257, two-tailed) show any evidence of publication bias in our study. Based on the classical fail-safe method, there must be another 72 null studies in our analysis, which can make the summary OR nonsense.
In meta-analysis, any single study may influence the final result. We evaluated the stability of the result by calculating the summary OR and one-leave-out ORs. This method is used to exclude each study and see what happens to the combined value. In our analysis, ORs of 14 data ranged from 0.73 to 3.78. After excluding any one, ORs ranged from 1.36 (95% CI 1.10–1.68) to 1.58 (95% CI 1.23–2.02). No single study markedly influenced summary OR as shown in Fig. 4.
The vertical solid line represents the original combined value when all studies are included, and the interrupted vertical line represents its confidence interval limitation. The solid plots represent combined values of one-leave-out ORs, not values of individual studies (inverse variance graph of ORs and confidence interval limitations for combined studies). Numbers and weights of the leave-out studies are indicated on the figure
Discussion
It is estimated that about 50% of the world’s population carry H. pylori in their stomach [34]. Hypergastrinemia is originating initially from G cells activated by H. pylori to release gastrin, leading to excessive production of ammonia and proinflammatory cytokines (IL-1, IL-8, TNF-α, etc.) and overexpression of growth factors such as TGF-α and EGF. During this process, cyclooxygenase-2 interacts with cytokines and growth factors and stimulates inflamed tissue or cancer tissue to release excessive amount of prostaglandin E2, which caused increased cell proliferation, angiogenesis, mutagenesis, and decreased apoptosis. Their joint action leads to the development of cancer [21].
It has been reported that H. pylori-infected people have a four- to sixfold increased risk of subsequently developing gastric adenocarcinoma [35, 36]. Several epidemiology studies have demonstrated the association between H. pylori seropositive and colorectal cancer risk. However, the results from these studies remain inconclusive [16, 30, 33]. Until now, there was no systematical review on the relationship between H. pylori infection and colorectal neoplasia concerning the difference of methods for testing H. pylori infection with satisfactory analysis. The purposes of this study were therefore to assess the association between H. pylori infection and the risk of colorectal carcinoma and to clarify any test-based differences.
A total of 13 studies with 14 data have been cited in this paper (due to one study used two groups of control subjects). In total, 72.3% (1,236/1,709) of patients and 60.8% (1,139/1,872) of control subjects were infected with H. pylori. As can be seen in Fig. 1, ORs of these 14 data ranged from 0.73 (95% CI 0.30–1.79) to 3.78 (95% CI 1.50–9.51) and summary OR was 1.49 (95% CI 1.17–1.91). It indicated that H. pylori infection may increase the risk of colorectal cancer. Although the graphical funnel plot appeared asymmetrical, neither rank correlation method nor linear regression approach supported statistical evidence of publication bias, and the result of fail-safe also suggested that the effect of publication bias was small.
Current diagnostic tests for H. pylori involve histological diagnosis of biopsy specimens, UBT, rapid urease test, serological tests, etc. The test for H. pylori serum IgG is a rapid, accurate, and reliable method. Moreover, the IgG antibody presents the previous infection of H. pylori to some extent, and other indicators illustrate that H. pylori exists while colorectal carcinoma was detected. If a person is infected with H. pylori ahead of neoplasia, it will be more reasonable to presume that H. pylori may be a risk factor of carcinoma. If we could not ensure the temporal relationship between H. pylori infection and neoplasia, the causal relationship cannot be assessed. In the meta-analysis of Zumkeller et al., different testing methods (ELISA assay, UBT, urease test, histological diagnosis of biopsied gastric specimens) were combined to determine the H. pylori infection status, which raised doubts about their conclusions [37]. In our meta-analysis, the IgG antibody was detected in ten of the 13 cited studies. Studies of detecting IgG antibody were further analyzed, the results of which showed a little more strength of the association between H. pylori infection and colorectal cancer (OR increased from 1.49 to 1.56).
Some case reports suggested the regression of colorectal cancer after eradication of H. pylori [38–40] and disappearance of rectal mucosa-associated lymphoid tissue lymphoma following antibiotic therapy [41]. van de Wouw et al. reported that after antibiotic therapy the number of H. pylori decreased and consequently led to a negative result of UBT and some other tests [42, 43]. Kosunen et al. pointed out that after 6 weeks of eradication therapy, the IgG titers had fallen by 20–30%, and 6 to 12 months after treatment the titer was 50% or less [44]. Little information is mentioned about whether the patients included in this meta-analysis used antibiotic therapy or not. Compared with control subjects, colorectal cancer patients were more possible to use antibiotic therapy. If the colorectal cancer patients who have been treated with H. pylori eradication therapy were also recruited in these case–control studies, and at the same time H. pylori infection status was detected without using IgG antibody, a false negative result of the relationship would be concluded.
Heterogeneity of these studies may be partially explained by one or more of the following design features of most reviewed studies: clinic- or hospital-based (rather than community-based) subject populations, small sample sizes, and inadequate consideration of potential confounding variables in the data analyses. Meta-analysis is a statistical procedure which is susceptible to various biases. First, the studies reviewed in this paper were carried out in different countries (three in America, two in Israel, two in Japan, two in western Europe, and one in eastern Europe). The race, environment, geography, or other conditions may influence the prevalence of H. pylori. Second, the selection of control subjects in some case–control studies may distort the results because hospital-based controls may not be as representative as population-based controls. Third, development of colorectal carcinoma is a long lasting process and is effected by multiple factors, such as body mass index, dairy foods, nutrients intake, and physical exercise [45–48]. It has been found that men were more susceptible to H. pylori infection compared to women, which may be caused by the propensity of smoking and drinking [49, 50], heavy physical labor in men, and different work environment between men and women. The estimate ORs were adjusted for age and gender only in the studies of Talley et al. [31] and Siddheshwar et al. [51]. The estimate ORs were adjusted for body mass index, nutritional level, etc. only in the studies of Breuer-Katschinski et al. [33] and Limburg et al. [16].
We excluded the studies published in non-English language and letters and clinical reports about the association of H. pylori infection and colorectal cancer. Since the data provided usually were not sufficient for assessing the quality of their studies, bias may result and influence the analysis result.
With these limitations in mind, all analyses in this paper supported the hypothesis of the relationship between H. pylori and colorectal cancer. Moreover, both of the two meta-analyses before and after concerning the difference of testing methods for H. pylori infection revealed that there are small risk increases of H. pylori infection in colorectal cancer (OR increased from 1.49 to 1.56). With the possible presence of publication bias, this result should be interpreted cautiously. We recommended that the important role of H. pylori in the development of colorectal cancer should not be disregarded. Larger and methodologically rigorous analytical studies with confounders being controlled using reliable outcomes and exposure measures are needed to further confirm this association.
References
Remontet L, Esteve J, Bouvier AM, Grosclaude P, Launoy G, Menegoz F, Exbrayat C, Tretare B, Carli PM, Guizard AV, Troussard X, Bercelli P et al (2003) Cancer incidence and mortality in France over the period 1978–2000. Rev Epidemiol Sante Publique 51:3–30
Yiu HY, Whittemore AS, Shibata A (2004) Increasing colorectal cancer incidence rates in Japan. Int J Cancer 109:777–781
From: American Cancer Society: Cancer Facts and Figures (2006) Atlanta, Ga: American Cancer Society, 2006. Also available online. Last accessed October 10, 2006
Sun XW, Wu SL, Lin YJ, Bo W, Han HL, Dai XD (2004) Trend of morbidity and mortality of colorectal carcinoma in Nangang District of Harbin from 1992 to 2001. World Chin J Digestol 12:2302–2306
Gustin DM, Brenner DE (2002) Chemoprevention of colon cancer: current status and future prospects. Cancer Metastasis Rev 21:323–348
Giovannucci E, Colditz GA, Stampfer MJ, Hunter D, Rosner BA, Willett WC, Speizer FE (1994) A prospective study of cigarette smoking and risk of colorectal adenoma and colorectal cancer in U.S. women. J Natl Cancer Inst 86:192–199
Potter JD, Slattery ML, Bostick RM, Gapstur SM (1993) Colon cancer: a review of the epidemiology. Epidemiol Rev 15:499–545
Cho E, Smith-Warner SA, Ritz J, van den Brandt PA, Colditz GA, Folsom AR, Freudenheim JL, Giovannucci E, Goldbohm RA, Graham S, Holmberg L, Kim DH et al (2004) Alcohol intake and colorectal cancer: a pooled analysis of 8 cohort studies. Ann Intern Med 140:603–613
Astorg P, Boutron-Ruault MC, Andrieux C, Astorg P, Blachier F, Blottiere H, Bonithon-Kopp C, Boutron-Ruault MC, Cassand P, Chaumontet C, Cherbut C, Clavel-Chapelon F et al (2002) Dietary fibers and colorectal cancer. Experimental studies, epidemiology, mechanisms. Gastroenterol Clin Biol 26:893–912
Papadopoulos N, Lindblom A (1997) Molecular basis of HNPCC: mutations of MMR genes. Hum Mutat 10:89–99
Liu B, Parsons R, Papadopoulos N, Nicolaides NC, Lynch HT, Watson P, Jass JR, Dunlop M, Wyllie A, Peltomaki P, de la Chapelle A, Hamilton SR et al (1996) Analysis of mismatch repair genes in hereditary non-polyposis colorectal cancer patients. Nat Med 2:169–174
Whitehouse A, Meredith DM, Markham AF (1998) DNA mismatch repair genes and their association with colorectal cancer. Int J Mol Med 1:469–474 Review
Penman ID, el-Omar E, Ardill JE, McGregor JR, Galloway DJ, O'Dwyer PJ, McColl KE (1994) Plasma gastrin concentrations are normal in patients with colorectal neoplasia and unaltered following tumor resection. Gastroenterology 106:1263–1270
Fireman Z, Trost L, Kopelman Y, Segal A, Sternberg A (2000) Helicobacter pylori: seroprevalence and colorectal cancer. Isr Med Assoc J 2:6–9
Hartwich A, Konturek SJ, Pierzchalski P, Zuchowicz M, Labza H, Konturek PC, Karczewska E, Bielanski W, Marlicz K, Starzynska T, Lawniczak M, Hahn EG (2001) Helicobacter pylori infection, gastrin, cyclooxygenase-2, and apoptosis in colorectal cancer. Int J Colorectal Dis 16:202–210
Limburg PJ, Stolzenberg-Solomon RZ, Colbert LH, Perez-Perez GI, Blaser MJ, Taylor PR, Virtamo J, Albanes D (2002) Helicobacter pylori seropositivity and colorectal cancer risk: a prospective study of male smokers. Cancer Epidemiol Biomark Prev 11:1095–1099
Infection with Helicobacter pylori. IARC Monogr Eval Carcinog Risks Hum 1994; 61:177–240
Suzuki T, Matsuo K, Ito H, Hirose K, Wakai K, Saito T, Sato S, Morishima Y, Nakamura S, Ueda R, Tajima K (2006) A past history of gastric ulcers and Helicobacter pylori infection increase the risk of gastric malignant lymphoma. Carcinogenesis 27:1391–1397
Isaacson PG, Du MQ (2004) MALT lymphoma: from morphology to molecules. Nat Rev Cancer 4:644–653
Parsonnet J, Isaacson PG (2004) Bacterial infection and MALT lymphoma. N Engl J Med 350:213–215
Konturek SJ, Konturek PC, Hartwich A, Hahn EG (2000) Helicobacter pylori infection and gastrin and cyclooxygenase expression in gastric and colorectal malignancies. Regulatory Pept 93:13–19
Hartwich J, Konturek SJ, Pierzchalski P, Zuchowicz M, Konturek PC, Bielanski W, Marlicz K, Starzynska T, Lawniczak M (2001) Molecular basis of colorectal cancer—role of gastrin and cyclooxygenase-2. Med Sci Monit 7:1171–181
Stoicov C, Saffari R, Cai X, Hasyagar C, Houghton J (2004) Molecular biology of gastric cancer: Helicobacter infection and gastric adenocarcinoma: bacterial and host factors responsible for altered growth signaling. Gene 341:1–17
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327:557–560
Copas J, Shi JQ (2000) Meta-analysis, funnel plots and sensitivity analysis. Biostatistics 1:247–262
Schork MA (2003) Publication bias and meta analysis. J Hypertens 21:243–245
Duval S, Tweedie R (2000) Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 56:455–463
Song F, Khan KS, Dinnes J, Sutton AJ (2002) Asymmetric funnel plots and publication bias in meta-analyses of diagnostic accuracy. Int J Epidemiol 31:88–95
Sterne JA, Egger M, Smith GD (2001) Systematic reviews in health care: investigating and dealing with publication and other biases in meta-analysis. BMJ 323:101–105
Thorburn CM, Friedman GD, Dickinson CJ, Vogelman JH, Orentreich N, Parsonnet J (1998) Gastrin and colorectal cancer: a prospective study. Gastroenterology 115:275–280
Talley NJ, Zinsmeister AR, Weaver A, DiMagno EP, Carpenter HA, Perez-Perez GI, Blaser MJ (1991) Gastric adenocarcinoma and Helicobacter pylori infection. J Natl Cancer Inst 83:1734–1739
Shmuely H, Passaro D, Figer A, Niv Y, Pitlik S, Samra Z, Koren R, Yahav J (2001) Relationship between Helicobacter pylori CagA status and colorectal cancer. Am J Gastroenterol 96:3406–3410
Breuer-Katschinski B, Nemes K, Marr A, Rump B, Leiendecker B, Breuer N, Goebell H, Colorectal Adenoma Study Group (1999) Helicobacter pylori and the risk of colonic adenomas. Digestion 60:210–215
Williams MP, Pounder RE (1999) Helicobacter pylori: from the benign to the malignant. Am J Gastroenterol 94:S11–S116
Marshall B (2002) Helicobacter pylori: 20 years on. Clinical Medicine (London, England) 2:147–152
Pinto-Santini D, Salama NR (2005) The biology of Helicobacter pylori infection, a major risk factor for gastric adenocarcinoma. Cancer Epidemiol Biomark Prev 14:1853–1858
Zumkeller N, Brenner H, Zwahlen M, Rothenbacher D (2006) Helicobacter pylori infection and colorectal cancer risk: a meta-analysis. Helicobacter 11:75–80
Inoue F, Chiba T (1999) Regression of MALT lymphoma of the rectum after anti-H. pylori therapy in a patient negative for H. pylori. Gastroenterology 117:514–515
Matsumoto T, Iida M, Shimizu M (1997) Regression of mucosa-associated lymphoid-tissue lymphoma of rectum after eradication of Helicobacter pylori. Lancet 350:115–116
Raderer M, Pfeffel F, Pohl G, Mannhalter C, Valencak J, Chott A (2000) Regression of colonic low grade B cell lymphoma of the mucosa associated lymphoid tissue type after eradication of Helicobacter pylori. Gut 46:133–135
Hori K, Suguro M, Koizuka H, Sakagami T, Tomita T, Kosaka T, Fukuda Y (2004) Disappearance of rectal mucosa-associated lymphoid tissue lymphoma following antibiotic therapy. Dig Dis Sci 49:413–416
Meucci G, Tatarella M, Vecchi M, Ranzi ML, Biguzzi E, Beccari G, Clerici E, de Franchis R (1997) High prevalence of Helicobacter pylori infection in patients with colonic adenomas and carcinomas. J Clin Gastroenterol 25:605–607
van de Wouw BA, de Boer WA, Hermsen HW, Valkenburg JG, Geuskens LM, Tytgat GN (1997) Usefulness of the 14C urea breath test as a semi-quantitative monitoring instrument after therapy for Helicobacter pylori infection. Scand J Gastroenterol 32:112–117
Kosunen TU, Seppala K, Sarna S, Sipponen P (1992) Diagnostic value of decreasing IgG, IgA, and IgM antibody titres after eradication of Helicobacter pylori. Lancet 339:893–895
Harris DM, Go VL (2004) Vitamin D and colon carcinogenesis. J Nutr 134:3463S–3471S
Doria-Rose VP, Newcomb PA, Morimoto LM, Hampton JM, Trentham-Dietz A (2006) Body mass index and the risk of death following the diagnosis of colorectal cancer in postmenopausal women (United States). Cancer Causes Control 17:63–70
Wei EK, Giovannucci E, Selhub J, Fuchs CS, Hankinson SE, Ma J (2005) Plasma vitamin B6 and the risk of colorectal cancer and adenoma in women. J Natl Cancer Inst 97:684–692
Longnecker MP, Chen MJ, Probst-Hensch NM, Harper JM, Lee ER, Frankl HD, Haile RW (1996) Alcohol and smoking in relation to the prevalence of adenomatous colorectal polyps detected at sigmoidoscopy. Epidemiology 7:275–280
Kuepper-Nybelen J, Rothenbacher D, Brenner H (2005) Relationship between lifetime alcohol consumption and Helicobacter pylori infection. Ann Epidemiol 15:607–613
Cardenas VM, Graham DY (2005) Smoking and Helicobacter pylori infection in a sample of U.S. adults. Epidemiology 16:586–590
Siddheshwar RK, Muhammad KB, Gray JC, Kelly SB (2001) Seroprevalence of Helicobacter pylori in patients with colorectal polyps and colorectal carcinoma. Am J Gastroenterol 96:84–88
Moss SF, Neugut AI, Garbowski GC, Wang S, Treat MR, Forde KA (1995) Helicobacter pylori seroprevalence and colorectal neoplasia: evidence against an association. J Natl Cancer Inst 87:762–733
Mizuno S, Morita Y, Inui T, Asakawa A, Ueno N, Ando T, Kato H, Uchida M, Yoshikawa T, Inui A (2005) Helicobacter pylori infection is associated with colon adenomatous polyps detected by high-resolution colonoscopy. Int J Cancer 117:1058–1059
Fujimori S, Kishida T, Kobayashi T, Sekita Y, Seo T, Nagata K, Tatsuguchi A, Gudis K, Yokoi K, Tanaka N, Yamashita K, Tajiri T et al (2005) Helicobacter pylori infection increases the risk of colorectal adenoma and adenocarcinoma, especially in women. J Gastroenterol 40:887–893
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was supported by grants from the National Natural Foundation (2004–2006 No. 30371243).
Rights and permissions
About this article
Cite this article
Zhao, Ys., Wang, F., Chang, D. et al. Meta-analysis of different test indicators: Helicobacter pylori infection and the risk of colorectal cancer. Int J Colorectal Dis 23, 875–882 (2008). https://doi.org/10.1007/s00384-008-0479-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-008-0479-z