Abstract
Objectives Although overweight body mass is an established risk factor for colorectal cancer incidence, few studies have examined the association between body mass index (BMI) and mortality after colorectal cancer diagnosis. We examined survival in a group of postmenopausal women according to BMI.
Methods Using the Wisconsin Cancer Reporting System we identified and enrolled 633 postmenopausal women aged 38–74 years who were diagnosed with colorectal cancer in 1988–1991. These women were interviewed by telephone; vital status was ascertained via Wisconsin death certificates. Cox proportional hazards regression was used to estimate multivariate risks of colorectal cancer-specific and all-cause mortality.
Results Both underweight (BMI <20.0 kg/m2) (Hazard Ratio (HR) 2.3, 95% Confidence Interval (CI) 1.0–5.4) and obese (BMI ≥ 30.0 kg/m2) women (HR 2.1, 95% CI 1.1–3.8) were at increased risk of colon cancer death, as compared to normal weight women (BMI 20.0–24.9 kg/m2). No association was observed for those with rectal cancer. Approximately 50% increases in all-cause mortality were observed among underweight and obese women with colorectal cancer. Postmenopausal hormone use did not appear to modify these associations.
Conclusions Underweight and obese postmenopausal women with colon cancer were at increased risk of death, though comorbidities may partially account for this risk.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multiple studies have reported an increased risk of colorectal cancer with increasing body mass index (BMI) [1–24]. However, this association has been found more consistently in men than in women, and further to be stronger in magnitude in men [2, 3, 7, 8, 15, 16, 18, 20, 22–24]. The weaker association in women may be due to the modifying effects of endogenous and exogenous estrogen [3, 4]. Since heavier women have higher levels of estrogen [25], and because estrogen is inversely associated with colorectal cancer [26–28], the higher circulating levels of estrogen in overweight and obese postmenopausal women may partially counteract the negative consequences of elevated BMI.
Fewer studies have examined the relationship between BMI and survival following the diagnosis of colorectal cancer [29–31], and results of these studies have been less consistent. For colon cancer, Slattery et al. [31] found the highest risk of all-cause mortality among both the thinnest and the heaviest individuals, while Meyerhardt et al. [29] reported an increased risk of death in underweight men, and an increasing risk with increasing BMI in women. In a similar analysis of rectal cancer cases, Meyerhardt et al. [30] found an increased risk of overall mortality among underweight men and women, but no suggestion of increased mortality among those who were overweight and obese. To our knowledge, no previous study has examined colorectal cancer-specific mortality following cancer diagnosis according to BMI.
We sought to examine the impact of BMI on colorectal cancer-specific and all-cause mortality in a population-based sample of postmenopausal women with colorectal cancer who had participated in a case-control study in Wisconsin. Further, we investigated the potentially modifying influence of postmenopausal hormone (PMH) use on BMI-related survival.
Methods
Study subjects were women aged less than 75 years who had a diagnosis of colorectal cancer reported to the Wisconsin Cancer Reporting System in 1990 or 1991 (including cancers diagnosed in 1988–1991), and who participated in a case–control study of risk factors for incident colorectal cancer [11]. The women completed a telephone interview that elicited information regarding basic demographics and risk factors for colorectal cancer, including weight 5 years prior to interview and tallest height attained. A woman’s current weight at the time of interview was not ascertained. Interviews were conducted between 1990 and 1992, and occurred a median of 13 months (range 4–28 months) following diagnosis. Overall participation of the cases was approximately 75%; about 15% of cases died prior to attempted contact, and the remainder of non-participants represent either physician or subject refusals [11].
Data were available from 714 cases. Of these, 21 cases did not report their height and/or weight at the interview and were excluded. An additional 60 women were premenopausal at the time of diagnosis and were also excluded, as the sample size did not permit a thorough evaluation of BMI and subsequent mortality among premenopausal colorectal cancer cases. The remaining 633 cases, ranging in age from 38 to 74 years, were included in this analysis.
Body mass index (BMI) was categorized as less than 20.0, 20.0–24.9, 25.0–29.9, and 30.0 or more kilograms per meter squared. These categories correspond roughly to “underweight”, “normal weight”, “overweight”, and “obese” as defined by the World Health Organization [32]. Additional data needed for analysis was obtained from the Wisconsin Cancer Reporting System, including stage of colorectal cancer at diagnosis and tumor location within the colorectum. Vital status and cause of death were ascertained using Wisconsin death certificates through 31 December 2002. Linkage of study participants’ data to data from the death certificate files was accomplished using the same criteria used by the National Death Index (i.e. matches based on social security number, name, and/or birthdate), which has been shown to be an accurate, efficient method of identifying deaths among study participants [33]. Cause of death information was determined using codes according to the International Classification of Diseases (version 9 for deaths through 1998, version 10 thereafter).
The association between BMI and all-cause and colorectal cancer-specific mortality was assessed using the Kaplan–Meier product-limit estimator. Cox proportional hazards models were used to assess the risk of death according to BMI while adjusting for potentially confounding factors. For all Cox models, the reference category for BMI was the normal weight group. There was some suggestion that the proportional hazards assumption was violated for the age at diagnosis and stage of disease variables, so we stratified on these variables in the Cox models (age <60, 60–69, or ≥70 years; localized, regional, distant, or unknown stage). Stratification allows the baseline hazard function to vary for differing levels of a covariate, allowing Cox models to accommodate covariates for which the proportional hazards assumption is violated [34]. All models were also adjusted for PMH use and smoking (never, former, or current for both variables). Potentially confounding factors were decided upon a priori based on their possible associations with both BMI and colorectal cancer and/or all-cause mortality. In addition to the above variables, family history, sigmoidoscopy history, marital status, education, race, and alcohol consumption were considered as potentially confounding factors, but none had much effect on the BMI risk estimates, and were therefore not retained in the final models. Separate Cox models for colon and rectal cancer and also for each tumor stage were fit to assess whether the association between body mass and survival differed by tumor site or stage. Finally, we considered Cox models that evaluated PMH use as an effect modifier of the BMI-survival association. The reference category for the interaction model included women of normal weight who had never used postmenopausal hormones. Due to small sample size, the overweight and obese BMI categories were combined for the model with BMI-PMH interaction terms. The significance of the interaction was evaluated using the likelihood ratio test.
Results
The 633 cases of colorectal cancer were followed for mortality over a mean of 9.4 years (range 0.9–14.7 years). During the course of follow-up there were 280 deaths, of which 147 were due to colorectal cancer. Characteristics of the cases are shown in Table 1. PMH use, smoking, and history of screening by sigmoidoscopy tended to decrease with increasing body mass. The cancer stage distribution was most advanced among those of normal weight. There was a tendency for underweight individuals to be slightly older, and obese individuals to be less educated and somewhat less likely to consume alcohol. Family history of colorectal cancer was more common in those of greater than normal weight. The cases were almost exclusively white, and the proportion of married individuals was similar across BMI categories.
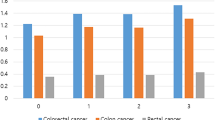
For both colorectal cancer-specific and all-cause mortality, underweight and obese women were at the greatest risk of death, and normal weight women were least likely to die during the follow-up period (Figure 1). In the multivariate Cox proportional hazards models that were stratified on age and stage and adjusted for smoking and PMH use, the same overall pattern of survival was observed, with 50–60% increases in the risk of death for both underweight and obese as compared to normal weight women (Table 2). However, the increased risk of death among underweight women was present only among those who had never smoked. Underweight/never-smokers were at a greatly increased risk of both all-cause (HR 3.6, 95% CI 1.8–7.0) and colorectal cancer mortality (HR 4.4, 95% CI. 1.9–10.1), while underweight ever-smokers showed no such increased risk, with HRs of approximately one.
The remaining analyses focused on colorectal cancer-specific mortality. Similar patterns were observed when the models were restricted to those with either localized or regional cancers (there was insufficient sample size to evaluate the associations with distant disease). For those with localized disease, the risk of colorectal cancer death was increased in both underweight (HR 1.3, 95% CI 0.3–6.7) and obese women (HR 1.8, 95% CI 0.5–6.4). The associations were somewhat stronger for those with regional colorectal cancer (HR 2.3, 95% CI 1.0–5.0 for underweight women, HR 2.1, 95% CI 1.1–4.0 for obese women).
The increased risk of colorectal cancer death among both the leanest and the heaviest women was driven by cancers of the colon; obese and underweight women with colon cancer experienced a greater than twofold increase in risk of death due to their cancer (Table 3). This pattern was present in both left- and right-sided colon cancers (data not shown). BMI was not associated with rectal cancer mortality (Table 3).
Cox proportional hazards models that included an interaction term between BMI and current PMH use were also considered (Table 4). For both women who had ever or never used PMH, underweight and overweight/obese women were at greater risk of colorectal cancer death than normal weight women. There was no evidence of an interaction between BMI and PMH use (p for interaction=0.75).
Discussion
In these data, there was a suggestion of an increase in both cancer-specific and all-cause mortality among postmenopausal women with colorectal cancer who were either underweight (for nonsmokers only) or obese. The increased risk of cancer death was found exclusively among those who had colon cancer. These differences in survival occurred despite a more advanced stage distribution in those of normal weight. There was no evidence that the association was strongest among women who were using postmenopausal hormones, as has been previously noted in studies examining BMI and colorectal cancer incidence [3, 4]; this finding should be interpreted cautiously, however, as a relatively small number of women had used PMH.
There are several limitations to this study that should be considered in interpreting these findings. First, we had no information available to us regarding several potentially confounding or modifying variables. For example, it has been suggested that physical activity may modify the BMI-colorectal cancer incidence association, with active individuals who are overweight experiencing smaller increases in their risk of colorectal cancer than sedentary ones [35]. Also, the increased risk of death that we observed may be due at least in part to the presence of comorbid conditions. If, for example, those with other chronic conditions experienced weight loss associated with their disease(s), they may be overrepresented in the lowest BMI class [36], and further it is possible that these individuals may be less able to withstand the stressors of cancer surgery and adjuvant therapy. Neither of these variables was available in these data.
Second, the cause of death was determined solely by Wisconsin death certificates. It is possible that misclassification of the cause of death occurred, either by recording deaths from other causes as deaths from colorectal cancer or vice versa. In a study from Minnesota, the sensitivity of the death certificate in designating colorectal cancer as the cause of death was 90%, as compared to a gold standard of medical record review by an expert panel; the corresponding specificity was 99.7% [37]. However, unless misclassification of the cause of death differed by BMI, which we consider less likely, this would tend to bias the hazard ratios towards the null. Additionally, those dying after leaving the state of Wisconsin would have failed to have their deaths captured. Migration from Wisconsin does not appear to have had a large impact on these results, though, as a prior analysis (which examined deaths through 1998) suggested a loss of approximately 4% of deaths when death certificate data only were used, as compared to both death certificates and the National Death Index (unpublished data).
Third, in order for cases to be included in this study, they had to survive long enough to be interviewed. Approximately 15% of cases died prior to contact, which occurred, on average, about one year after diagnosis. If the association between BMI and mortality differed for these early deaths, then this would serve to bias our results. However, the same general pattern, with both underweight and overweight/obese patients at increased risk of death, was observed for all stages of cancer, including those with localized cancers, very few of whom would have died prior to contact.
And finally, due to sample size, we had limited statistical power to detect associations of the magnitude that were observed. This was especially problematic in assessing the potential interaction between BMI and PMH use, since the number of women who had used postmenopausal hormones was rather small.
There have been few prior studies examining body size and risk of death or cancer recurrence following the diagnosis of colorectal cancer. Tartter et al. [38] reported 5-year recurrence rates of colon cancer of about 25% of female cases who were below the median value for BMI in their hospital-based sample, and 50% for those above the median (p<0.01). A smaller, non-significant increase in recurrence was seen among males who were above the median BMI.
Three previous studies have examined overall survival following the diagnosis of colon [29, 31] or rectal [30] cancer. A small study by Slattery et al. (n=411 cases) [31] found a U-shaped relationship with overall mortality that was similar to ours, with 30% increases in the risk of death in men and women for both the first and fourth quartiles of BMI, as compared to the second quartile. Separate hazard ratios for men and women were not provided. The other two studies, by Meyerhardt et al., were conducted in participants in randomized trials of different chemotherapy regimens in stage II and III colorectal cancer patients. There was no evidence of differing survival between the treatment arms of the trials, and therefore the survival of all participants by BMI was compared. In the trial of colon cancer patients (n=3,438) [29], there was a trend towards increasing risk of all-cause mortality among normal weight to obese women (HR for obese women 1.34, 95% CI 1.07–1.67); a similar though somewhat less pronounced trend for disease recurrence was also observed. No increase in risk was observed among underweight women. Men with colon cancer had the opposite pattern, with an increased risk of death among underweight men, but no increase in risk associated with overweight/obesity. Within the rectal cancer trial (n=1,688) [30], there was a suggestion of an increased risk of death among underweight women (HR 1.29, 95% CI 0.87–1.91), but no increase in local recurrence, which led the authors to speculate that death from non-cancer related causes was driving this finding. Overweight and obese women with rectal cancer did not experience higher rates of either death or local recurrence. For men, on the other hand, there was an increased risk of local rectal cancer recurrence among those who were of greater than normal weight.
Our results are consistent with these previous findings of an increased risk of death for those with colon, but not rectal, cancer among those who were overweight/obese. However, as opposed to studies that were based on data from randomized controlled trials, we also observed an increased risk of death among underweight patients. Several factors may account for this difference. First, ours was a population-based sample of colorectal cancer cases, while trial participants are a highly selected group. Those with comorbid conditions that might interfere with treatment were specifically excluded from trial participation. Thus, as mentioned above, the increased risk we observed among underweight individuals may have been due at least in part to comorbid illness. This may have been reflected in the association in underweight women that was restricted to those who had never smoked, both for colorectal cancer-specific and all-cause mortality. Because those who smoke are generally leaner than those who do not [36], it is possible that those who are underweight despite being non-smokers may be an unusual group, and one that is on average less healthy (i.e. a group more likely to be underweight as a result of comorbidities that impact upon mortality). Second, we had access to certain relevant confounding variables, including smoking and PMH use, that were not adjusted for in prior studies of this question. Third, we focused primarily on deaths due to colorectal cancer, rather than all-cause mortality and/or local recurrence. And finally, our study ascertained weight 5 years prior to diagnosis, which has both advantages and disadvantages. Weight was ascertained for a time that was more likely to be prior to when occult cancer would have caused unintentional weight loss, and therefore was a more representative measurement of long-term energy balance. Because the association between obesity and colorectal cancer may be due to insulin resistance and the resulting hyperinsulinemia that occur in a condition of long-term positive energy balance [39], past weight may be the more relevant measurement for assessing this association. On the other hand, any short-term impact of body mass at the time of diagnosis on mortality outcomes could be obscured by the use of pre-disease weight.
In conclusion, similar to studies of BMI and incident colorectal cancer, our results and those of others support an association between overweight and mortality following the diagnosis of colon cancer. However, there are important questions that remain. The differing associations between the sexes, between pre- and post-menopausal women, and the risks for underweight individuals need to be better characterized, so that the underlying biological mechanisms behind this association can be better understood. And on a public health level, it is not yet clear whether weight management after cancer diagnosis could lead to improved survival. This is perhaps the most relevant information for patients who wish to make lifestyle changes that will enhance their chances of surviving their cancer.
References
J Lin SM Zhang NR Cook et al. (2004) ArticleTitleBody mass index and risk of colorectal cancer in women (United States) Cancer Causes Control 15 581–589 Occurrence Handle15280637 Occurrence Handle10.1023/B:CACO.0000036168.23351.f1
N Shimizu C Nagata H Shimizu et al. (2003) ArticleTitleHeight, weight, and alcohol consumption in relation to the risk of colorectal cancer in Japan: a prospective study Br J Cancer 88 1038–1043 Occurrence Handle1:STN:280:DC%2BD3s7lsVWmsw%3D%3D Occurrence Handle12671701 Occurrence Handle10.1038/sj.bjc.6600845
ML Slattery R Ballard-Barbash S Edwards BJ Caan JD Potter (2003) ArticleTitleBody mass index and colon cancer: an evaluation of the modifying effects of estrogen (United States) Cancer Causes Control 14 75–84 Occurrence Handle1:STN:280:DC%2BD3s7ovFCgtg%3D%3D Occurrence Handle12708728
PD Terry AB Miller TE Rohan (2002) ArticleTitleObesity and colorectal cancer risk in women Gut 51 191–194 Occurrence Handle1:STN:280:DC%2BD38znt1Wqsw%3D%3D Occurrence Handle12117878 Occurrence Handle10.1136/gut.51.2.191
P Terry E Giovannucci L Bergkvist L Holmberg A Wolk (2001) ArticleTitleBody weight and colorectal cancer risk in a cohort of Swedish women: relation varies by age and cancer site Br J Cancer 85 346–349 Occurrence Handle1:STN:280:DC%2BD3MvmvVyisw%3D%3D Occurrence Handle11487263
ES Ford (1999) ArticleTitleBody mass index and colon cancer in a national sample of adult US men and women Am J Epidemiol 150 390–398 Occurrence Handle1:STN:280:DyaK1MzotFKiug%3D%3D Occurrence Handle10453815
A Russo S Franceschi C La Vecchia et al. (1998) ArticleTitleBody size and colorectal-cancer risk Int J Cancer 78 161–165 Occurrence Handle1:STN:280:DyaK1cvisFKitQ%3D%3D Occurrence Handle9754646 Occurrence Handle10.1002/(SICI)1097-0215(19981005)78:2<161::AID-IJC7>3.0.CO;2-X
L Le Marchand LR Wilkens LN Kolonel JH Hankin LC Lyu (1997) ArticleTitleAssociations of sedentary lifestyle, obesity, smoking, alcohol use, and diabetes with the risk of colorectal cancer Cancer Res 57 4787–4794 Occurrence Handle1:CAS:528:DyaK2sXnt12gu70%3D Occurrence Handle9354440
ME Martinez E Giovannucci D Spiegelman et al. (1997) ArticleTitleLeisure-time physical activity, body size, and colon cancer in women. Nurses’ Health Study Research Group J Natl Cancer Inst 89 948–955 Occurrence Handle1:STN:280:DyaK2szlslentw%3D%3D Occurrence Handle9214674 Occurrence Handle10.1093/jnci/89.13.948
E Giovannucci A Ascherio EB Rimm et al. (1995) ArticleTitlePhysical activity, obesity, and risk for colon cancer and adenoma in men Ann Intern Med 122 327–334 Occurrence Handle1:STN:280:DyaK2M7ltVejug%3D%3D Occurrence Handle7847643
AT Dietz PA Newcomb PM Marcus BE Storer (1995) ArticleTitleThe association of body size and large bowel cancer risk in Wisconsin (United States) women Cancer Causes Control 6 30–36 Occurrence Handle1:STN:280:DyaK2M3jsVyktw%3D%3D Occurrence Handle7718733 Occurrence Handle10.1007/BF00051678
RM Bostick JD Potter LH Kushi et al. (1994) ArticleTitleSugar, meat, and fat intake, and non-dietary risk factors for colon cancer incidence in Iowa women (United States) Cancer Causes Control 5 38–52 Occurrence Handle1:STN:280:DyaK2c7mvFartQ%3D%3D Occurrence Handle8123778 Occurrence Handle10.1007/BF01830725
IM Lee RS Paffenbarger SuffixJr. (1992) ArticleTitleQuetelet’s index and risk of colon cancer in college alumni J Natl Cancer Inst 84 1326–1331 Occurrence Handle1:STN:280:DyaK38zls1eqtg%3D%3D Occurrence Handle1495102
L Le Marchand LR Wilkens MP Mi (1992) ArticleTitleObesity in youth and middle age and risk of colorectal cancer in men Cancer Causes Control 3 349–354 Occurrence Handle1:STN:280:DyaK38zhsVeitg%3D%3D Occurrence Handle1617122
M Gerhardsson de Verdier U Hagman G Steineck A Rieger SE Norell (1990) ArticleTitleDiet, body mass and colorectal cancer: a case-referent study in Stockholm Int J Cancer 46 832–838 Occurrence Handle1:STN:280:DyaK3M%2FjsValug%3D%3D Occurrence Handle2172171
GA Kune S Kune LF Watson (1990) ArticleTitleBody weight and physical activity as predictors of colorectal cancer risk Nutr Cancer 13 9–17 Occurrence Handle1:STN:280:DyaK3c7jvVamtQ%3D%3D Occurrence Handle2300499
DW West ML Slattery LM Robison et al. (1989) ArticleTitleDietary intake and colon cancer: sex- and anatomic site-specific associations Am J Epidemiol 130 883–894 Occurrence Handle1:STN:280:DyaK3c%2FltlGjuw%3D%3D Occurrence Handle2554725
S Graham J Marshall B Haughey et al. (1988) ArticleTitleDietary epidemiology of cancer of the colon in western New York Am J Epidemiol 128 490–503 Occurrence Handle1:STN:280:DyaL1czisVekug%3D%3D Occurrence Handle2843038
AL Klatsky MA Armstrong GD Friedman RA Hiatt (1988) ArticleTitleThe relations of alcoholic beverage use to colon and rectal cancer Am J Epidemiol 128 1007–1015 Occurrence Handle1:STN:280:DyaL1M%2FkslensQ%3D%3D Occurrence Handle3189277
AH Wu A Paganini-Hill RK Ross BE Henderson (1987) ArticleTitleAlcohol, physical activity and other risk factors for colorectal cancer: a prospective study Br J Cancer 55 687–694 Occurrence Handle1:STN:280:DyaL2s3pvFCjuw%3D%3D Occurrence Handle3620314
A Nomura LK Heilbrun GN Stemmermann (1985) ArticleTitleBody mass index as a predictor of cancer in men J Natl Cancer Inst 74 319–323 Occurrence Handle1:STN:280:DyaL2M7jsFGmsA%3D%3D Occurrence Handle3856045
EE Calle C Rodriguez K Walker-Thurmond MJ Thun (2003) ArticleTitleOverweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults N Engl J Med 348 1625–1638 Occurrence Handle12711737 Occurrence Handle10.1056/NEJMoa021423
EA Lew L Garfinkel (1979) ArticleTitleVariations in mortality by weight among 750,000 men and women J Chronic Dis 32 563–576 Occurrence Handle1:STN:280:DyaE1M3jvFKmtw%3D%3D Occurrence Handle468958 Occurrence Handle10.1016/0021-9681(79)90119-X
RL Phillips DA Snowdon (1985) ArticleTitleDietary relationships with fatal colorectal cancer among Seventh-Day Adventists J Natl Cancer Inst 74 307–317 Occurrence Handle1:STN:280:DyaL2M7jsFGmsw%3D%3D Occurrence Handle3856044
TJ Key NE Allen PK Verkasalo E Banks (2001) ArticleTitleEnergy balance and cancer: the role of sex hormones Proc Nutr Soc 60 81–89 Occurrence Handle1:CAS:528:DC%2BD3MXitlKmt7s%3D Occurrence Handle11310427 Occurrence Handle10.1079/PNS200068
RT Chlebowski J Wactawski-Wende C Ritenbaugh et al. (2004) ArticleTitleEstrogen plus progestin and colorectal cancer in postmenopausal women N Engl J Med 350 991–1004 Occurrence Handle1:CAS:528:DC%2BD2cXhvFGjs7g%3D Occurrence Handle14999111 Occurrence Handle10.1056/NEJMoa032071
F Grodstein PA Newcomb MJ Stampfer (1999) ArticleTitlePostmenopausal hormone therapy and the risk of colorectal cancer: a review and meta-analysis Am J Med 106 574–582 Occurrence Handle1:STN:280:DyaK1M3ms1OlsA%3D%3D Occurrence Handle10335731 Occurrence Handle10.1016/S0002-9343(99)00063-7
PA Newcomb BE Storer (1995) ArticleTitlePostmenopausal hormone use and risk of large-bowel cancer J Natl Cancer Inst 87 1067–1071 Occurrence Handle1:STN:280:DyaK2MzktFOisw%3D%3D Occurrence Handle7616598
JA Meyerhardt PJ Catalano DG Haller et al. (2003) ArticleTitleInfluence of body mass index on outcomes and treatment-related toxicity in patients with colon carcinoma Cancer 98 484–495 Occurrence Handle12879464 Occurrence Handle10.1002/cncr.11544
JA Meyerhardt JE Tepper D Niedzwiecki et al. (2004) ArticleTitleImpact of body mass index on outcomes and treatment-related toxicity in patients with stage II and III rectal cancer: findings from Intergroup Trial 0114 J Clin Oncol 22 648–657 Occurrence Handle14966087
ML Slattery TK French MJ Egger JL Lyon (1989) ArticleTitleDiet and survival of patients with colon cancer in Utah: is there an association? Int J Epidemiol 18 792–797 Occurrence Handle1:STN:280:DyaK3c7ltlSitA%3D%3D Occurrence Handle2559896
World Health Organization Website, (http://www.euro.who.int/nutrition/20030507_1), updated 1 November 2004.
EE Calle DD Terrell (1993) ArticleTitleUtility of the National Death Index for ascertainment of mortality among cancer prevention study II participants Am J Epidemiol 137 235–241 Occurrence Handle1:STN:280:DyaK3s7pvVyltg%3D%3D Occurrence Handle8452128
DW Hosmer S Lemeshow (1999) Applied Survival Analysis: Regression Modeling of Time to Event Data John Wiley & Sons, Inc New York 243–248
ML Slattery J Potter B Caan et al. (1997) ArticleTitleEnergy balance and colon cancer-beyond physical activity Cancer Res 57 75–80 Occurrence Handle1:CAS:528:DyaK2sXjvFOisQ%3D%3D Occurrence Handle8988044
JE Manson MJ Stampfer CH Hennekens WC Willett (1987) ArticleTitleBody weight and longevity. A reassessment JAMA 257 353–358 Occurrence Handle1:STN:280:DyaL2s%2FoslSisA%3D%3D Occurrence Handle3795418 Occurrence Handle10.1001/jama.257.3.353
F Ederer MS Geisser SJ Mongin TR Church JS Mandel (1999) ArticleTitleColorectal cancer deaths as determined by expert committee and from death certificate: a comparison. The Minnesota Study J Clin Epidemiol 52 447–452 Occurrence Handle1:STN:280:DyaK1M3oslSrtw%3D%3D Occurrence Handle10360340 Occurrence Handle10.1016/S0895-4356(99)00016-5
PI Tartter G Slater AE Papatestas AH Aufses SuffixJr. (1984) ArticleTitleCholesterol, weight, height, Quetelet’s index, and colon cancer recurrence J Surg Oncol 27 232–235 Occurrence Handle1:STN:280:DyaL2M%2FlvFCiuw%3D%3D Occurrence Handle6503298
E Giovannucci (2003) ArticleTitleDiet, body weight, and colorectal cancer: a summary of the epidemiologic evidence J Womens Health (Larchmt) 12 173–182 Occurrence Handle10.1089/154099903321576574
Acknowledgements
The authors wish to acknowledge Drs. Henry Anderson and Patrick Remington for their advice and support throughout this study; Laura Stephenson and the staff of the Wisconsin Cancer Reporting System for assistance with data; and the participants and study staff of the Wisconsin Women’s Health Study for their commitment.
Author information
Authors and Affiliations
Corresponding author
Additional information
Grant Support: Supported in part by the Alcoholic Beverage Medical Research Foundation, grant CA47147 from the National Institutes of Health, and American Cancer Society grant SIG-15.
Rights and permissions
About this article
Cite this article
Doria-Rose, V.P., Newcomb, P.A., Morimoto, L.M. et al. Body Mass Index and the Risk of Death Following the Diagnosis of Colorectal Cancer in Postmenopausal Women (United States). Cancer Causes Control 17, 63–70 (2006). https://doi.org/10.1007/s10552-005-0360-0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10552-005-0360-0