Abstract
Background and aims
Thymidylate synthase (TS) is an important enzyme for DNA synthesis and the target for 5-fluorouracil (5-FU). Its expression may determine the outcome of patients with gastrointestinal cancers. We examined the prognostic and predictive value of TS-protein expression in patients with ductal adenocarcinoma of the pancreas.
Methods
TS expression from 131 patients with ductal adenocarcinoma of the pancreas was analyzed by immunohistochemistry in paraffin-embedded primary tumour specimens or biopsies.
Results
The median disease-specific survival among all patients (n=131) was 13 months. The invasion depth, the presence of metastases, grading and Union Internationale Contre le Cancer [International Union Against Cancer] (UICC) stage were associated with survival. Among resected patients (n=98), a difference in median survival was seen in the group receiving postoperative adjuvant treatment (21.1 months) compared with the group treated by surgery alone (12.4 months) (p=0.025). Low- and high-TS immunoreactivity was present in 74 (56%) and 56 (43%) of the cancers, respectively. One sample was not evaluable. No difference in median survival was observed among low- and high-TS-expressing tumours. Among patients undergoing resection and receiving postoperative intra-arterial chemotherapy (n=23), a marked trend to a longer median survival was seen for the group with low-TS-expressing tumours compared with the corresponding high-TS group (25.0 vs 16.0 months) (p=0.3834). There was no difference in survival among all palliative treated patients with low- and high-TS-expressing tumours.
Conclusion
Especially patients undergoing tumour resection with low-TS values seemed to have taken advantage from an intensified postoperative chemotherapeutic protocol. However due to the heterogeneous group of patients in the present report, larger trials of more homogenous patient populations will be necessary to confirm this hypothesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Human pancreatic cancer is an aggressive and devasting disease with 1- and 5-year survival rates of 24 and 4%, respectively, combined for all stages [1]. The disease is mostly diagnosed at an advanced stage precluding operative treatment and is generally resistant to chemotherapeutic agents. But even for patients diagnosed with small and resectable primary tumours, the 5-year survival rate is only 17% [1, 2]. Systemic gemcitabine is actually considered the current standard of palliative treatment for patients with locally advanced and metastatic pancreatic cancer, although 5-fluorouracil (5-FU) for decades was the most widely used chemotherapeutic agent in this setting [3]. After surgery with curative intent, patients seem to benefit from systemic treatment with 5-FU, whereas no survival advantage was seen when radiation was added to chemotherapy over surgery alone [4]. Chemotherapy of pancreatic cancer was also attempted via the celiac axis using 5-FU-based treatment combinations. Using this regional approach, no significant survival benefit could be demonstrated, but a suppression of hepatic disease progression [5].
Fluoropyrimidines like 5-FU act by blocking thymidylate synthase (TS) after conversion to 5-fluorodeoxyuridine-monophosphate (5-FdUMP) [6]. TS catalyzes the methylation of deoxyuridine-monoposphate (dUMP) to deoxythymidine-monoposphate (dTMP) with 5,10 methylenetetrahydrofolate as a cofactor. As this reaction provides the sole intracellular de novo source of thymidylate, it is one of the rate-limiting steps of DNA synthesis. TS expression in tumours has attracted considerable attention because of its potential role as prognostic factor for survival and response to 5-FU-based chemotherapy regimens. Thus, pre-clinical studies have shown that high intracellular TS levels correlate with resistance to fluoropyrimidine treatment. These data were confirmed by several clinical studies demonstrating that high-TS levels were associated with 5-FU resistance in metastatic colorectal cancer limiting response rates to less than 25%, while response was observed in up to 67%, when low-TS levels were present [7, 8]. Similar observations have also been made for other gastrointestinal malignancies, including gastric cancer [9] and secondary liver tumours [10]. The value of TS as prognostic factor for patients with gastrointestinal malignancies undergoing potential curative surgery, particularly for colorectal cancer, was also examined. Several studies reported that high-TS levels were associated with poor survival after tumour resection in colorectal [11–14] and gastric [15] cancer. However, the role of intratumoral TS expression in the outcome of adjuvant fluoropyrimidine-based treatment after surgical resection in patients with gastrointestinal malignancies remains controversial [16]. Moreover, very little is known about TS expression in pancreatic cancer [17, 18].
In view of the recently published data favouring adjuvant 5-FU treatment for resected pancreatic cancer [European Study Group for Pancreatic Cancer-Trial 1 (ESPAC 1 Study)] [4], the aim of the present study was to determine the possible role of TS as a prognostic and predictive marker for patients with resectable and unresectable pancreatic cancers treated at our institution.
Patients and methods
Patients and tissue acquirement
We retrospectively analyzed paraffin-embedded primary tumour specimens from patients with ductal adenocarcinoma of the pancreas who were treated at our institution. Eventually, paraffin blocks from 131 patients were available. Clinical and pathological information was obtained from a review of physician charts or from the hospital tumour registry. The study was approved by the Ethics Committee of the University of Ulm (Nr. 105/98). Depending on several clinical studies running at our institution, patients with resectable and unresectable cancers were included into different chemotherapeutic trials including 5-FU-based regimens given as (1) intravenous infusion (5-FU, 425 mg/m2 + folinic acid, 20 mg/m2 delivered on days 1–5 and repeated every 4 to 6 weeks) or as (2) intra-arterial infusion via the celiac axis (5-FU, 600 mg/m2 + folinic acid, 170 mg/m2 on days 2–4 in combination with mitoxanthrone, 10 mg/m2 on day 1 and cisplatin, 60 mg/m2 on day 5 delivered every 4–6 weeks). Thirty-three patients received external beam radiation up to a dose of 54 Gy in addition to intra-arterial chemotherapy. Later on, also gemcitabine was administered as intravenous chemotherapy at a dose of 1,000 mg/m2 delivered once a week.
TS immunohistochemistry
Paraffin-embedded 5-μm tissue sections were stained using the streptavidin–peroxidase technique as described previously [19]. After deparaffinization and blocking endogenous peroxidase activity, the sections were incubated for 20 min at 23°C with 1% normal horse serum and for 24 h at 4°C with the mouse monoclonal antibody against TS (4 μg/ml). This antibody has been shown to specifically recognize TS [20, 21]. Bound antibodies were detected with biotinylated horse universal secondary antibodies and streptavidin–peroxidase complex, using diaminobenzidine tetrahydrochloride as the substrate. Sections were counterstained with Mayer’s haematoxylin. Omission of primary antibodies did not yield any immunoreactivity.
Evaluation of TS immunoreactivity
The slides were examined independently by two observers blinded to both clinical and pathological data. TS expression of the tumor cells was quantified arbitrarily using a visual grading system based on the intensity of staining from 0 to 3 [13]. The highest staining intensity found in a tumour was used for classification of the tumour. The agreement of TS intensity reached by the two independent observers was >80%, and in case of disagreement, intensity was determined by consensus. According to the intensity of staining, cancers were classified as low-TS- (0–1) or high-TS- (2–3) expressing tumours.
Statistical analysis
For descriptive statistical analysis, absolute and relative frequencies and median, minimum and maximum were calculated. Disease-specific survival time was defined as the time from the day of operation to tumour-related death (failure), to death from other reasons (censored), or until data evaluation for the patients being alive or lost to follow-up (censored). Survival data were analyzed using the Kaplan–Meier method and log-rank test. Multiple Cox regression analysis was performed to simultaneously assess the influence of several prognostic factors on survival. Backward elimination with a selection level of 5% was used for variable selection. The hazard ratio with corresponding 95% CI and the p-value were computed. Statistical analysis was performed using SAS Version 8 (SAS Institute, Carry, NC).
Results
Patients characteristics and treatment
Histological examination revealed in all patients a ductal adenocarcinoma of the pancreas (PDAC). The presence of metastases was not stated in one patient. Operative procedures consisted of resection with curative intention in 98 (75%) and palliative procedures in 33 (25%) patients. Patients’ characteristics including age, gender, UICC staging, tumour grading and operative procedures are summarized in Table 1.
Treatment consisted of surgery alone in 29 of the 131 (22%) patients. In addition to surgery 62 (47%) patients received chemotherapy alone and 33 (25%) patients received combined chemoradiation. One patient (1%) received radiotherapy alone. In six (5%) patients, no information could be acquired concerning postoperative treatment. Chemotherapeutic treatments are summarized in Table 2.
The median disease-specific survival among all patients (n=131) was 13 months. The 1-, 2-, 3- and 5-year survival rates for this population of patients were 0.58 (95% confidence interval: 0.49; 0.67), 0.26 (0.18; 0.34), 0.17 (0.10; 0.24) and 0.11 (0.05; 0.17), respectively. Kaplan–Meier curves for age (≤60 vs >60 years), sex and lymph node status (N0 vs N+) showed no association with disease-specific survival, whereas the invasion depth of the tumour (T1+T2 vs T3+T4) (p=0.026), the presence of distant metastases (M0 vs M1) (p<0.001), histological grading (G1+2 vs G3+4) (p=0.002), UICC-tumour stage (p=0.003) and the surgical treatment (resection with curative intention vs palliative procedures) (p<0.001) were associated with disease-specific survival (data not shown). Among the 98 patients with resected cancers, a difference in survival was seen in the group receiving postoperative adjuvant treatment (n=68; median survival: 21.1 months) compared with the group which was treated by surgery alone (n=26; median survival: 12.4 months) (p=0.025). Interestingly, 68% (46/68) of patients in the former group but only 54% (14/26) of patients in the latter group had an UICC stage III tumour. A similar analysis for the palliative treated group (n=33) was not performed because only three patients were treated by surgery alone whereas 28 patients received chemotherapy or chemoradiation.
Immunohistochemical analysis of TS protein expression
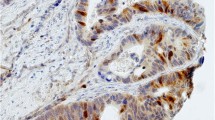
TS immunoreactivity was localized in the cytoplasm of the tumour cells and appeared in the form of a granular staining pattern. There were large variations in the TS expression levels of 131 tumours (Fig. 1). Thus, low-TS immunoreactivity was present in 74 (56%) of the cancers and high-TS immunoreacitivity in 56 (43%). In one sample, TS staining could not be determined because no tumour cells were present in the sections evaluated. In the normal pancreas surrounding the pancreatic cancer areas, faint to moderate TS immunoreactivity was present in the islet cells and in some acinar cells (data not shown).
TS protein expression in human pancreatic cancer. Moderate to strong granular (2–3) cytoplasmatic TS immunoreactivity (a) was observed in almost all cancer cells of this sample. In contrast, in several samples faint or no TS immunoreactivity was present in the cancer cells (b). The arrows depict the area of magnification of each inset. Original magnification: ×100, inset ×400
Association of TS protein expression and prognosis
In the first step, we analyzed survival and TS protein expression in all patients with available TS (n=130). No difference in median survival time was observed among low- (n=74) and high- (n=56) TS-expressing tumours. In a subsequent analysis, we separately compared TS expression in the group of resected tumours and in the group which only received palliative procedures.
Patients undergoing tumour resection
In the group of patients undergoing tumour resection (n=98), high-TS expression was associated with decreased patient survival. Median disease specific survival among all resected patients with low-TS-expressing tumours (n=53) was longer than that among patients with high-TS-expressing cancers (n=44) (18.9 vs 16.0 months). This difference was more marked when considering patients receiving postoperative adjuvant chemo- or radiochemotherapy. Thus, median survival time was 21.4 months for patients with low- (n=37) vs 17.1 months for patients with high- (n=31) TS-expression. In contrast, no difference in median disease specific survival was seen for the corresponding groups of patients treated by surgery alone. Interestingly, the analysis of TS expression and survival according to the form of treatment received, revealed that, among patients receiving intra-arterial chemotherapy (n=23), a trend to better survival was seen for the group with low-TS-expressing tumours treated by intra-arterial chemotherapy alone compared with the corresponding high-TS group (median survival: 25.0 vs 16.0 months) although these results were not statistically significant (p=0.3834). It should be noted that 80% (8/10) of patients in the low-TS group, but 62% (8/13) of patients in the high-TS group had an UICC-stage III cancer. For patients receiving radiotherapy in addition to intra-arterial chemotherapy (n=25) median survival was 20.0 months in the group with low-TS-expressing tumours (n=14) vs 19.2 months in the group with high-TS-expressing tumours (n=11). No difference was seen among low- and high-TS-expressing tumours for patients receiving systemic chemotherapy (n=19; both median survival: 22 months). Results are summarized in Table 3.
Patients undergoing palliative procedures
There was no difference in survival among all palliative treated patients with low- (n=21) and high- (n=12) TS-expressing pancreatic cancers (7.2 vs 8.2 months). Similarly, no survival benefit was seen when analyzing patients according to the treatment received.
Association of TS protein expression and clinicopathological parameters
Afterwards, a multiple Cox regression analysis was performed to identify the important risk factors and to assess their effect simultaneously. The following variables were included: age (≤ 60 vs >60 years), gender (male vs female), stage (UICC I vs II vs III vs IV), histological grading (G3–4 vs 1–2), surgical treatment (palliative procedures vs resection), postoperative treatment (no therapy vs adjuvant therapy) and TS expression (high vs low). Owing the missing values in the covariates considered, 121 patients were studied altogether. The results of variable selection are presented in Table 4 demonstrating that stage and grading were independent prognostic factors in this group.
Discussion
TS plays a key role in fluoropyrimidine resistance and catalyses the rate-limiting step of DNA de novo synthesis [22]. High intratumoral TS expression is believed to confer 5-FU resistance due to inefficient TS inhibition [23]. The value of TS as a prognostic factor has been most widely studied in colorectal cancer. In advanced unresectable colorectal cancer and other advanced gastrointestinal malignancies, numerous studies have shown the predictive value of intratumoral TS for the response to palliative 5-FU treatment. A meta-analysis including 13 studies and a total of 887 cases of advanced colorectal cancer has shown that patients with high-TS-expressing tumours have a poorer prognosis than those with low-TS-expressing tumours [24]. Furthermore, high-TS levels were associated with 0–24% response rate to 5-FU chemotherapy regimens, while response was observed in 49–67% when low-TS levels were present [7]. There is also evidence for TS as prognostic marker for survival after complete surgical tumour removal. This might be explained by the fact that TS catalyses the rate-limiting step of DNA de novo synthesis, which is essential for rapid cell proliferation [23]. Several studies showed that high-TS levels were associated with poor postoperative outcome after resection of colorectal and gastric cancer [16]. However, in contrast to palliative treatment of unresectable disease, patients with high-TS-expressing primary tumours seem to be the ones that benefit from adjuvant treatment after tumour resection, while patients with low TS might not or might even be harmed [16]. The hypothesis that high TS might predict the increased efficiency of adjuvant treatment contrasts with the inverse correlation of TS expression and response to palliative fluoropyrimidine treatment in metastasized colorectal cancer. There is no definitive explanation for that, but unlike treatment of advanced disease, the survival benefit of adjuvant therapy is due to the eradication of circulating tumour cells, an obviously different situation to that of an established tumour. High-TS levels in circulating tumour cells may render them more susceptible to chemotherapy through still unknown mechanisms or pathways different from TS inhibition [11, 14, 23].
In this study, we observed that patients who undergo resection of pancreatic cancer and have a low-TS-expressing tumour tend to have, even if marginally, a longer median survival time. This is more evident for patients receiving adjuvant postoperative treatment and particularly in the form of 5-FU-based intra-arterial chemotherapy. No benefit was seen for patients receiving 5-FU-based intra-arterial chemotherapy plus radiotherapy or systemic chemotherapy. These results, although observed on a relatively small number of patients receiving heterogeneous treatments, are consistent with reports on colorectal [11–14] and gastric [15] cancer but are in contrast with previous results on colorectal [11, 12, 21, 25, 26], gastric [15] and pancreatic cancer [17, 18] reporting a benefit of postoperative adjuvant 5-FU-based chemotherapy for high-TS-expressing tumours only. Our results are consistent with the hypothesis of the inhibition of growth in tumour cells expressing low-TS levels. The reason for the lack of the survival advantage, at least theoretically expected, when radiotherapy is added to intra-arterial chemotherapy is not known but is consistent with the data of the recent published multicentre, randomized study ESPAC-1 [4], showing no benefit for patients receiving intravenous chemotherapy (5-FU + folinic acid) plus external beam irradiation vs intravenous chemotherapy alone with survival in the former group being even harmed. As for patients receiving intravenous chemotherapy, there was no difference among high- and low-TS-expressing tumours. This might be explained by the fact that some patients in this group received gemcitabine as an adjuvant drug, and only a relatively small number of patients were treated by a 5-FU-containing regimen. It has also been reported that pancreatic cancer is known to express high dihydropyrimidine-dehydrogenase (DPD) levels, which can rapidly inactivate 5-FU [27]. Concerning patients with unresectable cancers, our results did not show any differences in survival and response to chemotherapy in terms of survival between high- and low-TS-expressing tumours. This is in contrast with the studies on advanced colorectal cancer [7, 24] but in agreement with the data of Takamura et al. [18] on unresectable pancreatic cancer.
To our knowledge only two studies examined TS expression and its correlation with clinical outcome in pancreatic cancer so far. Takamura et al. [18] examined the expression of TS and its correlation with the clinicopathological features of 102 patients with resectable and unresectable tumours. In the group with resectable cancer, overall survival was significantly better for patients with high-TS-expressing tumours and for patients receiving adjuvant 5-FU-based chemotherapy. Considering TS expression and response to 5-FU-based chemotherapy, a significant improvement of survival was seen for resected cancers with high-TS expression. In the group with unresectable cancer, overall survival was significantly different for patients with palliative surgery plus chemotherapy vs those receiving palliative surgery alone. Concerning TS expression and response to 5-FU-based chemotherapy, a trend to better survival was seen for unresectable cancers with high-TS expression. The authors concluded that high-TS immunoreactivity is a prognostic factor for patients with pancreatic cancer but its implications regarding the efficacy of 5-FU-based chemotherapy remains unclear. Hu et al. [17] studied the immunohistochemical expression of TS in a series of 132 patients, who underwent resection for pancreatic ductal adenocarcinoma with and without postoperative adjuvant treatment. High-TS expression was associated with decreased patient overall survival. Similar to the previous report, overall survival was significantly increased in patients receiving postoperative chemotherapy vs surgery alone. Adjuvant treatment significantly improved survival in patients with high-TS-expressing tumours, whereas no difference was observed for patients with low-TS-expressing tumours managed with resection vs resection plus chemotherapy. Authors concluded that high-TS expression is a marker of poor prognosis in resected pancreatic cancer, whereas patients with high intratumoral TS expression benefit from adjuvant therapy. In addition to these two studies, a recent report investigated the presence of TS promotor tandem repeats of patients participating in an adjuvant trial, however, without demonstrating differing frequencies of the three possible repeats compared to non-pancreatic cancer populations, suggesting that TS promoter heterogeneity may not play a role in the etiology of this disease [28].
In conclusion, the present study demonstrated conflicting results regarding the implication of TS protein expression in patients with pancreatic cancer for prognosis and response to 5-FU-based chemotherapy. Despite the heterogeneous group of patients receiving several chemotherapeutic protocols, our results suggest that TS expression may also play a role in the prognosis and chemosensitivity of patients with resectable pancreatic cancer. Especially, patients with low TS receiving intra-arterial 5-FU-based chemotherapy after tumour resection seem to have taken advantage from this postoperative treatment. To further address this topic, subsequent studies including more patients are needed to clarify the role of TS in the treatment and progression of human pancreatic cancer.
References
American Cancer Society (2004) Cancer facts and figures 2004. American Cancer Society, Atlanta, GA, pp 1–60
Parkin DM, Bray F, Ferlay J, Pisani P (2005) Global cancer statistics 2002. CA Cancer J Clin 55:74–108
El Kamar FG, Grossbard ML, Kozuch PS (2003) Metastatic pancreatic cancer. Emerging strategies in chemotherapy and palliative care. Oncologist 8(1):18–34
Neoptolemos J, Stocken D, Friess H et al (2004) A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med 350:1200–1210
Link KH, Leder G, Formentini A, Fortnagel G, Kornmann M, Schatz M, Beger HG (1999) Surgery and multimodal treatments in pancreatic cancer—a review on the basis of future multimodal treatment concepts. Gan To Kagaku Ryoho 26:10–40
Santi DV, McHenry CS, Sommer H (1974) Mechanism of interaction of thymidylate synthase with 5-fluorodeoxyuridylate. Biochemistry 13:471–481
Bertino JR, Banerjee D (2003) Is the measurement of thymidylate synthase to determine suitability for treatment with 5-fluoropyrimidines ready for prime time? Clin Cancer Res 9:1235–1239
Aschele C, Lonardi S, Monfardini S (2002) Thymidylate synthase expression as a predictor of clinical response to fluoropyrimidine-based chemotherapy in advanced colorectal cancer. Cancer Treat Rev 28:27–47
Lenz HJ, Leichman CG, Danenberg KD, Danenberg PV, Groschen S, Cohen H, Laine L, Crookes P, Silberman H, Baranda J, Garcia Y, Li J, Leichman L (1996) Thymidylate synthase mRNA level in adenocarcinoma of the stomach: a predictor for primary tumour response and overall survival. J Clin Oncol 14:176–182
Hillenbrand H, Formentini A, Staib L, Sander S, Salonga D, Danenberg K, Danenberg P, Kornamnn M (2004) A long term follow-up study of thymidylate synthase as a predictor for survival of patients with liver tumours receiving hepatic arterial infusion chemotherapy. Eur J Surg Oncol 30:407–413
Yamachika T, Nakanishi H, Inada K, Tsukamoto T, Kato T, Fukushima K, Inoue M, Tatematsu M (1998) A new prognostic factor for colorectal carcinoma, thymydilate synthase, and its therapeutic significance. Cancer 82:70–77
Takenoue T, Nagawa H, Matsuda K, Fujii S, Nita ME, Hatano K, Kitayama J, Tsuruo T, Muto T (2000) Relation between thymydilate synthase expression and survival in colon carcinoma, and determination of appropriate application of 5-fluorouracil by immunohistochemical method. Ann Surg Oncol 7:193–198
Edler D, Hallstrom M, Johnston PG, Magnusson I, Ragnhammar P, Blomgren H (2002) Thymidylate synthase expression: an independent prognostic factor for local recurrence, distant metastasis, disease-free survival in rectal cancer. Clin Cancer Res 6:1378–1384
Edler D, Glimelius B, Hallstrom M, Jakobsen A, Johnston PG, Magnusson I, Ragnhammar P, Blomgren H (2002) Thymidylate synthase expression in colorectal cancer: a prognostic and predictive marker of benefit from adjuvant fluorouracil-based chemotherapy. J Clin Oncol 20:1721–1728
Tsujitani S, Konishi I, Suzuki K, Oka S, Gomyo Y, Matsumoto S, Hirooka Y, Kaibara N (2000) Expression of thymidylate synthase in relation to survival and chemosensitivity in gastric cancer patients. J Exp Clin Cancer Res 19:189–195
Formentini A, Henne-Bruns D, Kornmann M (2004) Thymidylate synthase expression and prognosis of patients with gastrointestinal cancers receiving adjuvant chemotherapy: a review. Langenbecks Arch Surg 389(5):405–413
Hu YC, Komorowski RA, Graewin S, Hostetter G, Kallioniemi OP, Pitt HA, Ahrendt SA (2003) Thymidylate synthase expression predicts the response to 5-fluorouracil-based adjuvant therapy in pancreatic cancer. Clin Cancer Res 9:4165–4171
Takamura M, Nio Y, Yamasawa K, Dong M, Yamaguchi K, Itakura M (2002) Implication of thymidylate synthase in the outcome of patients with invasive ductal carcinoma of the pancreas and efficacy of adjuvant treatment using 5-fluorouracil or its derivative. Anti-Cancer Drugs 13:75–81
Kornmann M, Ishiwata T, Beger H.G., Korc M (1997) Fibroblast growth factor-5 stimulates mitogenic signaling and is overexpressed in human pancreatic cancer: evidence for autocrine and paracrine actions. Oncogene 15:1417–1424
Johnston PG, Liang CM, Henry S, Chabner BA, Allegra CJ (1991) Production and characterization of monoclonal antibodies that localize human thymydilate synthase in the cytoplasm of human cell and tissue. Cancer Res 51:6668–6676
Johnston PG, Lenz HJ, Leichmann CG, Danenberg KD, Allegra CJ, Danenberg PV, Leichmann L (1995) Thymydilate synthase gene and protein expression correlate and are associated with response to 5-fluorouracil in human colorectal and gastric tumours. Cancer Res 55:1407–1412
Danenberg PV (1977) Thynidylate synthetase—a target enzyme in cancer chemotherapy. Biochim Biophys Acta 473:73–92
Van Triest B, Pinedo H, Giaccone G, Peters J (2000) Downstream molecular determinants of response to 5-fluorouracil and antifolate thymidylate synthase inhibitors. Ann Oncol 11:385–391
Popat S, Matakidou A, Houlston RS (2004) Thymydilate synthase expression and prognosis in colorectal cancer: a systematic review and meta-analysis. J Clin Oncol 22:529–536
Tomiak A, Vincent M, Earle CC, Johnaston PG, Kocha W, Taylor M, Maroun J, Eidus L, Whiston F, Stitt L (2001) Thymidylate synthase expression in stage II and III colon cancer: a retrospective review. Am J Clin Oncol 24:597–602
Sugiyama Y, Kato T, Nakazato H, Ito K, Mizuno I, Kanemitsu T, Matsumoto K, Yamaguchi A, Nakai K, Inada K, Tatematsu M (2002) Retrospective study on thymidylate synthase as a predictor of outcome and sensitivity to adjuvant chemotherapy in patients with curatively resected colorectal cancer. Anti-cancer Drugs 13:931–938
Nagakawa T, Kayahara M, Ohta T, Kitagawa H, Mikami K, Kurata T, Otsuji S (2000) Dihydropyrimidine dehydrogenase activity in human pancreatic tumor tissues. Cancer Investig 18(6):516–20
Farrell J, Winter K, Van Rijnsoever M, Regine W, Abrams R, Safran H, Hoffman J, Schaeffer P, Mohiuddin M, Macdonald J, Bowen Benson A, Willet C, Elsaleh H (2005) Thymidylate synthase and thymidine phosphorylase: protein expression and genotype in pancreatic cancer: results from the RTOG 9704 prospective randomized adjuvant treatment trial. Pancreas 31(4):439–440
Acknowledgement
We thank Iris Schneider for assisting in the immunohistochemical analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Formentini, A., Sander, S., Denzer, S. et al. Thymidylate synthase expression in resectable and unresectable pancreatic cancer: role as predictive or prognostic marker?. Int J Colorectal Dis 22, 49–55 (2007). https://doi.org/10.1007/s00384-006-0111-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-006-0111-z