Abstract
Background and aims
Colorectal cancer (CRC) ranks as the fourth most frequently diagnosed cancer worldwide. CRCs that arise proximally or distally to the splenic flexure show differences in epidemiologic incidence, morphology, and molecular alterations, suggesting the existence of two categories of CRC based on the site of origin. The aim of the present work is to investigate the histological and molecular differences between CRCs located proximally and distally to the splenic flexure, and their potential involvement in tumor prognosis and therapeutic strategies.
Methods
We evaluated 120 patients affected by sporadic CRC for clinicopathologic features, microsatellite instability (MSI), loss of heterozygosity (LOH) of chromosomes 18q, 8p, and 4p; they were also investigated for hMlh1, hMsh2, Fhit, p27, and Cox-2 immunostaining.
Results
The mucinous histotype was more frequent in the proximal than in the distal CRCs (p<0.004). The frequency of MSI phenotype was higher in proximal than in distal tumors (p<0.001); moreover, reduced or absent hMlh1, Fhit, p27 immunohistochemical expressions were more frequent in proximal than in distal tumors (p<0.001 and 0.01 for p27). In contrast, the frequency of LOH in 18q was higher in distal than in proximal tumors (p=0.002). No significant differences were observed between proximal and distal tumors in the frequency of LOH in 8p and altered expression of hMsh2 and p53 protein.
Conclusion
These different features may reflect different genetic pathways of carcinogenesis and support the hypothesis of a different mechanism of cancer development between the proximal and the distal colon, with potential implications in the therapeutic approach.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Worldwide, colorectal cancer (CRC) is the fourth most common malignancy, after carcinoma of the lung, breast, and prostate, accounting in 2002 for nearly 148,000 new cases and 56,000 deaths in the United States and approximately 125,000 mortalities in Europe [1, 2]. More than a decade ago, the existence was proposed of two broadly different categories of CRCs according to whether the tumor is located proximally (right) or distally (left) to the splenic flexure [3–9]. This hypothesis has been supported by recent epidemiologic studies showing that the incidence of proximal tumors in Western countries has steadily increased, while that of distal tumors has shown a corresponding decrease [7, 10]. Moreover, cancers with a proximal location were found more frequently in elderly patients [11–13] and in the female gender [11]. It is well known that two different genetic pathways are involved in the carcinogenesis of CRC, one involving chromosomal instability and the other involving microsatellite instability (MSI) [14–16]. The first pathway includes mutation in p53 and K-ras and loss of heterozygosity (LOH) of chromosome regions such as 4p, 5q, 8p, 17p, and 18q, 22q [17–20]. The second pathway is characterized by disruption of the DNA mismatch repair system (MMR) that normally maintains sequence fidelity during DNA replication [21].
The FHIT (fragile histidine triad) gene mapped at 3p14.2 functioning as tumor suppressor [22] encodes for a protein which low expression is associated with MMR deficiency in human advanced colorectal carcinoma [23].
More recently, an epigenetic model that is not accompanied by changes in DNA sequence has been characterized as an alternative mechanism for CRC initiation and progression [16, 24]. Epidemiologic studies have also shown a pathogenetic role in colonic tumorigenesis for p27kip−1, a cyclin-dependent kinase inhibitor [25], with putative tumor suppressor function and for cyclooxygenase-2 (Cox-2), an enzyme catalyzing the biosynthesis of prostaglandins and thromboxanes [26]. The loss of p27 protein expression may result in tumor development and/or progression; however, this loss of expression does not appear to result from gene mutations [27, 28]. A large number of studies have characterized p27 as a prognostic factor in various human cancers, including colorectal adenocarcinoma [25, 29–31]. Multiple studies indicate that Cox-2 is overexpressed in many human malignant tumors [32–34] and is linked to the process of carcinogenesis [35], tumor survival [36], invasion [37], and metastasis [38].
Whether the pathogenetic role of Cox-2 and p27 differs with respect to proximal and distal CRCs is unknown. The aim of the present work is to investigate the possible differences between CRCs that arise proximally (right) and distally (left) to the splenic flexure from a histological and molecular point of view, and their potential involvement in the prognosis and therapeutic strategies.
Materials and methods
Patient population
The study population consisted of 133 unselected consecutive patients who had undergone curative colorectal resection for sporadic CRC between July 1996 and April 1999 at the our surgical unit. All cases were deemed sporadic, based on the absence of relevant family history as recorded prospectively at initial patient interview. A curative operation was defined as one in which no macroscopic tumor remained at the end of surgery and in which histopathologic examination of the operative specimen showed no tumor at the margins of resection. Distant metastases at the time of resection were excluded by preoperative liver ultrasonography, chest X-ray, and intraoperative exploration. Six patients were excluded on the basis of insufficient tissue for analysis, five patients because they were lost at follow-up, and two because of a double location of metachronous tumors (one located in the right colon and the other in the left colon), leaving 120 patients for study (63 males and 57 females).
During the study period, a uniform surgical management protocol was adopted. Proximal colon was defined as the large bowel proximal to the splenic flexure, and distal colon was defined as the large bowel distal to the splenic flexure.
All specimens underwent histopathological analysis by the same gastrointestinal pathologist (Bordi C.), who was unaware of the interim results of molecular genetic and immunohistochemical analysis. In accordance with the classification of tumors by the World Health Organisation [39], tumors were defined as mucinous when 50% or more of the tumor mass consisted of accumulated mucin, mostly extracellular; the other tumors were classified as “adenocarcinoma, not otherwise specified”. Tumors were staged in accordance with the Tumour-Node-Metastasis (TNM) system [40].
Patients with stage III colon cancer under 75 years received adjuvant chemotherapy [41]. Patients with rectal cancer received irradiation therapy administered before surgery [42].
Patients were observed at 3-month interval for 24 months after the completion of therapy, then every 6 months for 3 years, and then yearly. History and physical examination, complete blood cell and platelet count, liver chemistries, ultrasound, and carcinoembryonic antigen measurement were performed at each visit, and chest X-ray, colonoscopy, and computed tomography (CT) were performed once a year.
Local recurrence was defined as the regrowth of the tumor in and around the tumor bed—including the pericolic fat, the adjoining mesentery, and lymph nodes—or in the suture or staple line of the bowel anastomosis, occurring either alone or in conjunction with generalized recurrence. We adopted the methodology to be followed in the reporting of studies of recurrences after resection of colorectal tumors recently suggested by Dent et al. [43].
Ethical approval for the study was obtained from the Human Ethics Committee of the University of Parma.
DNA preparation, LOH, and MSI testing
Specimens of freshly resected colorectal carcinomas were snap-frozen in liquid nitrogen and subsequently stored at −80°C. In all cases, fresh specimens of normal colon mucosa were also collected and used as matching controls. Only tumor samples containing at least 80% of neoplastic cells were included in the study. To verify this condition, one 10-μm-thick cryostat section from each tumor sample was stained with hematoxylin and microscopically examined by a pathologist. Fifteen to 25 cryostat sections (20-μm-thick) from the tumors included in the study and from matching normal samples were submitted to DNA extraction by QIAGEN DNeasy tissue kit (QIAGEN, Hilden, Germany).
Polymerase chain reaction (PCR)
The following panel of six polymorphic microsatellite markers located on chromosomal regions potentially involved in CRC development and progression was used: D18S58 (18q22-23), D18S61 (18q22) [44], BAT26 (2p16), BAT40 (1p13) [45], D8S254 (8p22) [20], and D4S2397 (4p14-16) [17]. The markers were selected from the Genome database (http://www.gdb.org) on the basis of chromosomal location and heterozygosity. The PCR conditions and fragment analysis have been described in more detail previously [46].
Definition of allelic loss (LOH)
An imbalance factor was calculated as the ratio of relative allelic peak area in the tumor DNA to relative allelic peak area in the corresponding normal DNA on the basis of the following formula:
For informative markers LOH was scored when signal reduction for one allele was 40% or more [47].
Microsatellite instability (MSI)
The novel appearance in the tumor DNA of one or more alleles, i.e., new peaks in the electropherogram, not present in its paired normal DNA, was the indicator of MSI [48].
Tumors were classified as high frequency MSI (MSI-H) when instability was detected in at least 30% of the interpretable microsatellite markers investigated, or as low-frequency MSI (MSI-L) when instability was found in less than 30% of the markers, in accordance with international criteria [49]. Tumors without MSI were defined as microsatellite-stable (MSS) [49]. For the purposes of this study, MSS and MSI-L cases were considered together [50].
Immunohistochemical staining
For immunohistochemical analysis the specimens containing tumor and normal glands of the same snap frozen tumors were routinely fixed in buffered 10% formalin and embedded in paraffin. Sections of 5 μm were stained with hematoxylin and eosin for histological diagnosis and with the following primary antibodies: anti-hMsh2 (Clone FE11, Oncogene Research Products, Cambridge, Massachusetts, USA; working dilution:1/20); anti-hMlh1 (clone G168-728, Pharmingen, San Diego, California, USA working dilution:1/75), anti-Fhit clone (Polyclonal-ZR44, Zymed Laboratories, San Francisco, California, USA, working dilution: 1/50), with anti-p27 (clone SX53G8, Dako, Glostrup, Denmark; working dilution:1/50), anti-p53 (clone DO7, Dako, Glostrup, Denmark; working dilution: 1/50), and with anti-Cox-2 (Clone 4H12, Novocastra Laboratories, Benton Lane, Newcastle upon Tyne, UK, working dilution: 1/100).
The antibodies (Ab), clones, pretreatments, working dilutions, incubation time, and localisation of the immunostaining are listed in Table 1.
For antigen retrieval, sections were treated with 10 mM citrate at pH 6.0, in a 750-W microwave oven for three 5-min cycles. The sections were immunostained with the streptavidin-biotin kit (LSAB2, Dako) in accordance with the manufacturer’s specifications and counterstained with haematoxylin. Positive controls were the normal glands of the intestinal crypts for anti-hMsh2, hMlh1, Fhit; peritumoral lymphocytes for anti-p27, and Cox-2; colorectal carcinomas strongly positive for anti p53. Negative controls consisted of substituting the primary antibodies with the normal serum.
Semiquantitative analysis
The immunostaining for each antibody except for p53 and Cox-2 was estimated on a semiquantitative score according to the number of positive tumor cells as follows: 0% (0),<10%(1), 10 to 50% (2), 51 to 80% (3), or >80% (4). The intensity of staining was also evaluated as weak (1+), moderate (2+), or strong (3+). For each tumor case, the values of the two parameters were multiplied, resulting in scores ranging from 0 to 12 [51]. For the purposes of the study, staining of tumor nuclei for hMlh1 and hMsh2 and cytoplasms for Fhit was evaluated as absent (no protein) or present (any evidence). The 0–6 scores were considered as altered expression, 7–12 as preserved expression.
In addition, among tumors with preserved gene expression, two groups were distinguished, with a low (<50% of positive cells, or scores of 1 to 6) and high (>50%, or scores of 8 to 12) expression, respectively [52]. Moreover, for the expression of p27 scores <6 (cut off 50%) were considered as low protein expression [53]. The expression of Cox-2 and p53 was analyzed on the basis of the frequency of positive cells as + low expression; ++ and +++ high expression [54].
Real time FHIT analysis
RNA extraction and cDNA synthesis
RNA was extracted from paraffin-embedded specimens using TriZol Reagent as described by the manufacturer (Life Technologies, Gaithersburg, MD).
Reverse transcription of RNA was performed in a final volume of 20 μl containing 5× buffer (Tris, KCl, MgCl2) 0.1 mM DTT, 1.5 mM total deoxynucleotide triphosphate, 5 U of RNase inhibitor (Roche, Penzberg, Germany), 10× Random Hexamer primers (Boehringer, Mannheim), 20 U of M-MuLV reverse transcriptase (Sigma, San Louis, Missouri, USA), and 5 μl (1 μg) of extracted RNA. The samples were incubated at 37°C for 1 h.
PCR conditions
PCR amplification was performed in the presence of specific target, fluorogenic probes (TaqMan probe) that allowed an automated quantification of the amplified products in real-time with the MJ Opticon. Primers and probes were chosen with the assistance of the Primer Amplify computer program to confirm the gene specificity of the chosen nucleotidic sequences for FHIT. The Taqman probe carrying a 5′ FAM reporter label and a 3′ MGB nonflourogenic quencher group was synthesized by PE Applied Biosystems.
For each PCR, 10 ng of cDNA template was used. The polymerase amplification was performed in a total volume of 20 μl containing 2× TaqMan Universal Master Mix, 2× Target Assay Mix (FHITprobe) or Endogenous Control Assay Mix (β-Actin probe).
The thermal cycling conditions were: 2 min at 50°C, 10 min at 95°C and then 45 cycles at 95°C for 15 s and 60°C for 1 min.
Quality control assessment was performed in standardized PCR conditions, including in each experiment a negative control (with no template) and the housekeeping gene (β-Actin) used for data normalization.
Data analysis
Calculation of the amounts of DNA is based on the cycle number, where fluorescence of each reaction passes the cycle threshold, which is set to the geometric phase of the amplification above the background. The amount of gene expression was determined using the comparative method of the ΔΔCT, always using a normal sample as control and a housekeeping gene (β-actin) as reference. Data analysis was based on the following formula:
Statistical analysis
Molecular data, immunohistochemical results, recurrence frequency, and patient survival were analyzed statistically in relation to the proximal or distal subsite location of the tumors.
Contingency tables and the χ2 test were used to evaluate differences between percentages. The association of overall, local and distant recurrences with prognostic factors was evaluated by means of multivariable logistic regression.
The statistical analyses for the recurrences and for survival were performed excluding patients with palliative surgical treatment.
Disease-free interval in patients who had recurrence was measured as the interval between the date of resection and the date of diagnosis of recurrence.
Duration of survival was measured from the date of resection until the date of death from any cause or until the censoring date of April 30, 2004. In the survival analysis, deaths due to postoperative complications within 30 days were excluded. Survival curves were drawn according to the method of Kaplan and Meier, and differences in survival and disease-free interval were evaluated by means of the log-rank test. The simultaneous effect of more than one prognostic factor was estimated by the Cox proportional hazards model. Mortality rate ratios were used to assess the difference in deaths due to colorectal cancer.
Cohen’s Kappa test was used to evaluate the concordance between the status of chromosomes 18q, 8p, and 4p.
All reported P values are two tailed. Statistical significance was set at an alpha level of 0.05.
Results
The clinical and histological data of 120 CRCs distributed in the proximal and distal colon are described in Table 2. From a histological point of view the carcinomas of mucinous type were more frequent in the proximal (57%) than in the distal (30%) colon (p=0.004) (Table 2). No significant correlations were found between tumor location and sex, mean age, distribution of TNM stage, or tumor grading, respectively.
Several molecular features evaluated in this study resulted as being different from proximal to distal tumors. The incidence of the microsatellite instability (MSI) phenotype showed marked regional differences with a frequency of up to sixfold higher in proximal than in distal tumors (39 vs 6%, p<0.001) (Fig. 1). Moreover, reduced or absent hMlh1 immunohistochemical expression was more frequent in proximal than in distal tumors (46 vs 9%, p<0.001) (Fig. 2). The frequency of reduced or absent Fhit expression was fourfold higher in proximal than in distal tumors (28 vs 6%, p=0.001) (Fig. 3). This result was confirmed by reverse transcriptase polymerase chain reaction (RT-PCR), which showed significantly lower gene Fhit expression levels in proximal tumors (p=0.019) (Fig. 4). Results of immunohistochemical analysis of Fhit expression significantly agree with those of RT-PCR (p=0.002) (Fig. 5).
Results of immunohistochemical analysis of Fhit expression significantly agree with gene FHIT expression by real time PCR (p=0.002 ).The thicker horizontal line identifies the median sample value and the ends of the box are the 25th and 75th quantiles. The whiskers extend from the ends of the box to the outer-most data point and are calculated according to: upper or lower quartile + 1.5* (interquartile range). Points represent outliers
Tumor loss of expression of cyclin-dependent kinase inhibitor, protein p27, was twice as frequent in proximal as in distal localizations (44 vs 20%; p=0.01) (Fig. 6). In contrast, the frequency of allelic losses at chromosomal regions in 18q (18qLOH) was twice as high in distal as in proximal tumors (57 vs 27%, p=0.002) (Fig. 7). The immunohistochemical expression of Cox-2 was more frequent in proximal (88%) than in distal tumors (72%), although the difference was not statistically significant (p=0.188).
No significant differences were observed between proximal and distal tumors in the frequency of allelic losses at chromosomal regions in 8p (17 vs 29%, p=0.209) and 4p (15 vs 18%, p=0.711), in the frequency of altered expression of hMsh2 protein (14 vs 8%, p=0.109). The immunohistochemical expression of p53 were found in 63% of proximal colon tumors and in 48% of the distal colorectal tumors without any statistical difference (p=0.29).
In proximal tumors the MSI phenotype was significantly associated with low levels of hMlh1 (p<0.001) and Fhit expression (p<0.001), the absence of 18qLOH (p=0.020), and with mucinous histotype (p=0.030). Reduced or absent Fhit expression was significantly associated also with reduced or absent hMlh1 expression (p=0.004). Reduced or absent hMlh1 expression was correlated with altered hMsh2 expression (p=0.003). Loss of p27 expression was more frequently associated with MSI phenotype (p=0.002) and mucinous histology (p=0.001).
In distal colorectal tumors 18qLOH was more frequently associated with normal hMlh1 expression (p=0.023). MSI+ phenotype was more frequently associated with reduced or absent hMlh1 expression (p<0.001), and with mucinous histotype (p=0.034); reduced or absent Fhit expression was more frequently associated with altered hMsh2 expression (p=0.003) and with mucinous histotype (p=0.052); reduced or absent hMlh1 expression was more frequently associated with altered hMsh2 expression (p<0.001); loss of p27 expression was more frequently associated with mucinous histotype (p=0.002).
Frequency of recurrence and survival curves
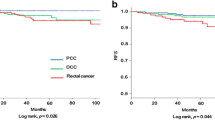
The analyses relating to frequency of recurrence of the disease and to survival were carried out using data relating to 99 patients (82.5%) who had undergone curative surgery. Mean follow-up was 65 months (range 2–85). The frequency of neoplastic recurrence was 17% for proximal localizations and 29.8% for distal ones, although the difference is not statistically significant (p=0.128). Cumulative survival curves (p=0.712) (Fig. 8) and those for disease-free survival (p=0.143) (Fig. 9) do not differ significantly in the two groups. However, the difference between the survival curve of patients who had undergone curative colorectal resection of MSI + CRC and that of patients who had undergone curative colorectal resection of CRC with 18qLOH is close to statistical significance (p=0.0521), independently of the localization of the tumor (Fig. 10).
Discussion
Our study demonstrates several significant differences between proximal and distal CRCs from a histological and molecular point of view. The incidence of mucinous carcinomas resulted as being greater in the proximal colon, and approximately twice as low in frequency from the proximal to the distal colon. This result is in agreement with several studies [55–58], whereas other authors report a higher frequency of mucinous carcinomas in the rectum and/or distal localizations [59, 60], or do not find significant differences [56]. The frequency of mucinous adenocarcinomas in the sample examined in the present study accounts for 40% of all colorectal cases and is higher than that reported in other studies [55–58]. The well-known marked geographical variations in the epidemiological characteristics of colorectal cancer [11] probably account for these differences as well as for the discrepancy between our data and that previously reported. Indeed, the association of increasing age or female gender with proximal colorectal cancer shown in some other studies [11, 61, 62] was not observed in the present investigation, which was in keeping with another recent study that does not reveal differences in sex and age between patients with proximal and distal CRCs [63, 64].
In the sample of patients examined in this study, the pattern of genetic alterations is different in proximally and distally located tumors. In particular, MSI+ phenotype, reduced or absent hMlh1 expression, reduced or absent Fhit expression, and altered espression of p27 were significantly more frequent in proximal than in distal colonic tumors. The observation that MSI+ tumors are located predominantly in the proximal colon is in agreement with most previous investigations [45, 65–67]. The majority of sporadic MSI+ tumors arise following hypermethylation of the hMLH1 gene [68]; it is not surprising, therefore, that MSI+ phenotype resulted as being correlated with reduced or absent hMlh1 expression. Even reduced or absent Fhit protein expression and FHIT gene expression evaluated by real time PCR resulted as being correlated with MSI+ phenotype and with reduced or absent hMlh1 expression. Moreover, the close correlation between Fhit protein expression evaluated by immunohistochemistry and FHIT gene expression evaluated by real time PCR has confirmed the reliability of the immunohistochemical analysis in the assessment of the FHIT gene alterations. In this regard, we previously suggested that a deficiency of an MMR gene could be responsible for the high frequency of altered tumor expression of Fhit, and FHIT gene alteration may be part of the MSI-associated genetic pathway of colonic carcinogenesis [52].
The correlation between proximal colorectal cancer with MSI and adenocarcinoma with mucinous phenotype, revealed in the present study, has already been reported in previous studies [50, 67, 69, 70].
In our CRCs the immunohistochemical expression of p27 is altered more frequently in proximal than in distal localization, in agreement with the results of Zhang [30] but in contrast with those of Manne et al. [31]. Several lines of evidence suggest that decreased p27 expression in tumors is associated with a more aggressive tumor phenotype such as poor histologic grade, the presence of lymphovascular invasion and higher growth fraction [71]. The absence of p27 protein expression is a predictor of poor prognosis in proximal CRCs [30] and a powerful negative prognostic marker in colorectal carcinomas, particularly in stage II tumors, thus helping in the selection of patients who will benefit from adjuvant therapy [25]. Our results also showed a significant correlation of the reduced expression of p27 with the mucinous type of colorectal adenocarcinoma and with the MSI+ phenotype. The latter result may suggest that also altered expression of p27 may be part of the MSI-associated genetic pathway of colonic carcinogenesis.
Distal colorectal tumors were characterized by significantly more frequent 18qLOH, normal hMlh1 expression, normal p27 expression, and normal Fhit expression. The higher frequency of allelic losses on 18q in distal CRC is in agreement with previous results [46, 62].
No significant differences in the Cox-2 expression were found between proximal and distal colorectal tumors. Nor were any correlations found between Cox-2 and the other parameters analyzed. Cox-2 expression is commonly involved in colorectal tumorigenesis [26], although its interrelationship and clinicopathological effects remain inconclusive. In a recent work, Nasir [72] found frequent expression of Cox-2 in left-sided CRCs, although this report is only based on 36 patients. The alterated immunohistochemical expression of the protein p53 was more frequent in proximal tumors but it did not reach the statistical significance in accordance with other works in which the mutations of the p53 gene were detected at similar frequencies in proximal and distal tumors [73, 74].
It has been suggested that MSI in CRC is a predictor of improved survival [45, 46, 75], whereas 18qLOH has been associated with an adverse clinical outcome [46, 62, 76–80]. It would be expected, therefore, that the outcome of proximal colonic cancers, which are more frequently MSI+, is better than that of distal CRCs, which are more often characterized by 18qLOH. Indeed, several studies conclude that patients with distal tumors have a poorer survival rate than those with tumors on the right side [44, 76, 81, 82]. Our results on the frequency of recurrent disease and on disease-free survival were in agreement with these observations, although the differences were not statistically significant, possibly because of the small size of the sample examined.
In conclusion, the present study identifies the existence of at least two major groups of colorectal cancer based on histologic and molecular features. One group occurs predominantly in the proximal colon and is characterized by histologic mucinous type, MSI, altered expression of Mlh1, Fhit, and p27. The other group occurs predominantly in the left colon and is characterized by 18qLOH. These differing features may reflect two different genetic pathways of carcinogenesis in the proximal and the distal colon, as suggested by Ikeda et al. [83].
In this regard, we agree with Iacopetta [9] in considering that the anatomic site of origin of CRC tumors may provide a convenient discriminator for two subgroups of tumors having important biological and clinical differences with potential repercussions on therapeutic choices. They could, for example, lead to the identification of a subset of patients who benefit most from 5-fluorouracil-based chemotherapy. Recent literature reports differences in the response to 5-fluorouracil on the part of colorectal tumors with differing genetic profiles [84]. Results are not univocal [84–87], and further studies on wide sample sizes will be needed to identify more clearly the role of the differing genetic profiles in defining different responses to adjuvant chemotherapy treatment. On the basis of our results, we maintain that for this purpose, it would be useful to compare the response to chemotherapy of patients with proximal tumors and those with distal ones; these groups of patients, in fact, could constitute two large populations with important differences in distribution of tumor genetic profiles, one with a higher frequency of tumors with MSI and the others with a higher frequency of tumors with 18qLOH.
References
Society AC, http://www.cancer.org/docroot/NWS/content/NWS_1_1x_ACS_Updates_Colorectal_Screening_Guidelines_.asp (2003)
Berrino F, Capocaccia R, Estève J (1999) Survival of cancer patients in Europe: the EUROCARE-2 study. In: IARC Scientific Publications. International Agency for Research on Cancer, Lyon
Bufill JA (1990) Colorectal cancer: evidence for distinct genetic categories based on proximal or distal tumor location. Ann Intern Med 113:779–788
Ponz de Leon M, Sacchetti C, Sassatelli R, Zanghieri G, Roncucci L, Scalmati A (1990) Evidence for the existence of different types of large bowel tumor: suggestions from the clinical data of a population-based registry. J Surg Oncol 44:35–43
Pocard M, Salmon RJ, Muleris M, Remvikos Y, Bara J, Dutrillaux B, Poupon MF (1995) Two colons–two cancers? Proximal or distal adenocarcinoma: arguments for a different carcinogenesis. Bull Cancer 82:10–21
Distler P, Holt PR (1997) Are right- and left-sided colon neoplasms distinct tumors? Dig Dis 15:302–311
Bonithon-Kopp C, Benhamiche AM (1999) Are there several colorectal cancers? Epidemiological data. Eur J Cancer Prev 8:S3–S12
Lindblom A (2001) Different mechanisms in the tumorigenesis of proximal and distal colon cancers. Curr Opin Oncol 13:63–69
Iacopetta B (2002) Are there two sides to colorectal cancer? Int J Cancer 101:403–408
Cucino C, Buchner A, Sonnenberg (2002) Continued rightward shift of colorectal cancer. Dis Colon Rectum 45:1035–1040
Gonzalez EC, Roetzheim RG, Ferrante JM, Campbell R (2001) Predictors of proximal vs. distal colorectal cancers. Dis Colon Rectum 44:251–258
Koketsu S, Watanabe T, Tada T, Kanazawa T, Ueda E, Nagawa H (2003) Sporadic colorectal cancer in elderly people. Hepatogastroenterology 50:1749–1752
Gannon CJ, Malone DL, Royal RE, Schreiber M, Bass BL, Napolitano LM (2002) Advanced proximal colon cancer. Surg Endosc 16:446–449
Lengauer C, Kinzler KW, Vogelstein B (1997) Genetic instability in colorectal cancers. Nature 386:623–627
Martin L, Assem M, Piard F (1999) Are there several types of colorectal carcinomas? Correlations with genetic data. Eur J Cancer Prev 8:S13–S20 (Dec)
Haydon AM, J.R. J (2002) Emerging pathways in colorectal-cancer development. Lancet Oncol 3:83–88
Arribas R, Ribas M, Risques RA, Masramon L, Tortola S, Marcuello E, Aiza G, Miro R, Capella G, Peinado MA (1999) Prospective assessment of allelic losses at 4p14-16 in colorectal cancer: two mutational patterns and a locus associated with poorer survival. Clin Cancer Res 5:3454–3459
Fearon ER, Vogelstein B (1990) A genetic model for colorectal tumorigenesis. Cell 61:759–767
Tejpar S, Van Cutsem E (2002) Molecular and genetic defects in colorectal tumorigenesis. Best Pract Res Clin Gastroenterol 16:171–185
Halling KC, French AJ, McDonnell SK, Burgart LJ, Schaid DJ, Peterson BJ, Moon-Tasson L, Mahoney MR, Sargent DJ, O’Connell MJ, Witzig TE, Farr GHJ, Goldberg RM, Thibodeau SN (1999) Microsatellite instability and 8p allelic imbalance in stage B2 and C colorectal cancers. J Natl Cancer Inst 91:1295–1303
Modrich P (1991) Mechanisms and biological effects of mismatch repair. Annu Rev Genet 25:229–253
Siprashvili Z, Sozzi G, Barnes LD, McCue P, Robinson AK, Eryomin V, Sard L, Tagliabue E, Greco A, Fusetti L, Schwartz G, Pierotti MA, Croce CM, Huebner K (1997) Replacement of Fhit in cancer cells suppresses tumorigenicity. Proc Natl Acad Sci USA 94:13771–13776
Andachi H, Yashima K, Koda M, Kawaguchi K, Kitamura A, Hosoda A, Kishimoto Y, Shiota G, Ito H, Makino M, Kaibara N, Kavasaki H, Murawaki Y (2002) Reduced FHIT expression is associated with mismatch repair deficiency in human advanced colorectal carcinoma. Br J Cancer 87:441–445
Nephew KP, Huang TH (2003) Epigenetic gene silencing in cancer initiation and progression. Cancer Lett 190:125–133
Loda M, Cukor B, Tam SW, Lavin P, Fiorentino M, Draetta GF, Jessup JM, Pagano M (1997) Increased proteasome-dependent degradation of the cyclin-dependent kinase inhibitor p27 in aggressive colorectal carcinomas. Nat Med 3:231–234
Sheehan KM, Sheahan K, O’Donoghue DP, MacSweeney F, Conroy RM, Fitzgerald DJ, Murray FE (1999) The relationship between cyclooxygenase-2 expression and colorectal cancer. JAMA 282:1254–1257
Morosetti R, Kawamata N, Gombart AF, Miller CW, Hatta Y, Hirama T, Said JW, Tomonaga M, Koeffler HP (1995) Alterations of the p27KIP1 gene in non-Hodgkin’s lymphomas and adult T-cell leukemia/lymphoma. Blood 86:1924–1930
Spirin KS, Simpson JF, Takeuchi S, Kawamata N, Miller CW, Koeffler HP (1996) p27/Kip1 mutation found in breast cancer. Cancer Res 56:2400–2404
Tenjo T, Toyoda M, Okuda J, Watanabe I, Yamamoto T, Tanaka K, Ohtani M, Nohara T, Kawasaki H, Tanigawa N (2000) Prognostic significance of p27(kip1) protein expression and spontaneous apoptosis in patients with colorectal adenocarcinomas. Oncology 58:45–51
Zhang H, Sun XF (2001) Loss of p27 expression predicts poor prognosis in patients with Dukes’ B stage or proximal colorectal cancer. Int J Oncol 19:49–52
Manne U, Jhala NC, Jones J, Weiss HL, Chatla C, Meleth S, Suarez-Cuervo C, Grizzle WE (2004) Prognostic significance of p27(kip-1) expression in colorectal adenocarcinomas is associated with tumor stage. Clin Cancer Res 10:1743–1752
Sano H, Kawahito Y, Wilder RL, Hashiramoto A, Mukai S, Asai K, Kimura S, Kato H, Kondo M, Hla T (1995) Expression of cyclooxygenase-1 and -2 in human colorectal cancer. Cancer Res 55:3785–3789
Wolff H, Saukkonen K, Anttila S, Karjalainen A, Vainio H, Ristimaki A (1998) Expression of cyclooxygenase-2 in human lung carcinoma. Cancer Res 58:4997–5001
Soslow RA, Dannenberg AJ, Rush D, Woerner BM, Khan KN, Masferrer J, Koki AT (2000) COX-2 is expressed in human pulmonary, colonic, and mammary tumors. Cancer 89:2637–2645
Liu CH, Chang SH, Narko K, Trifan OC, Wu MT, Smith E, Haudenschild C, Lane TF, Hla T (2001) Overexpression of cyclooxygenase-2 is sufficient to induce tumorigenesis in transgenic mice. J Biol Chem 276:18563–18569
Tsujii M, Kawano S, Tsuji S, Sawaoka H, Hori M, DuBois RN (1998) Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell 93:705–716
Tsujii M, Kawano S, DuBois RN (1997) Cyclooxygenase-2 expression in human colon cancer cells increases metastatic potential. Proc Natl Acad Sci USA 94:3336–3340
Tsujii M, DuBois RN (1995) Alterations in cellular adhesion and apoptosis in epithelial cells overexpressing prostaglandin endoperoxide synthase 2. Cell 83:493–501
Jass JR, Sobin LH (1989). Springer, Berlin Heidelberg New York
Compton C, Fenoglio-Preiser CM, Pettigrew N, Fielding LP (2000) American Joint Committee on Cancer Prognostic Factors Consensus Conference: Colorectal Working Group. Cancer 88:1739–1757
Moertel CG, Fleming TR, Macdonald JS, Haller DG, Laurie JA, Goodman PJ, Ungerleider JS, Emerson WA, Tormey DC, Glick JH et al (1990) Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma. N Engl J Med 322:352–358
Pahlman L (1998) Preoperative treatment of rectal cancer. In: Bleiberg H, Rougier P, Wilke H-J (eds) Management of colorectal cancer. Martin Dunitz, London, pp 156–166
Dent OF, Chapuis PH, Bokey EL, Newland RC (2001) Methodology and reporting in studies of local recurrence after curative excision of the rectum for cancer. Br J Surg 88:1476–1480
Jen J, Kim H, Piantadosi S, Liu ZF, Levitt RC, Sistonen P, Kinzler KW, Vogelstein B, Hamilton SR (1994) Allelic loss of chromosome 18q and prognosis in colorectal cancer. N Engl J Med 331:213–221
Thibodeau SN, Bren G, Schaid D (1993) Microsatellite instability in cancer of the proximal colon. Science 260:816–819
Sarli L, Bottarelli L, Bader G, Iusco D, Pizzi S, Costi R, D’Adda T, Bertolani M, Roncoroni L, Bordi C (2004) Association between recurrence of sporadic colorectal cancer, high level of microsatellite instability, and loss of heterozygosity at chromosome 18q. Dis Colon Rectum 47:1467–1482
Beckmann MW, Picard F, An HX, van Roeyen CR, Dominik SI, Mosny DS, Schnurch HG, Bender HG, Niederacher D (1996) Clinical impact of detection of loss of heterozygosity of BRCA1 and BRCA2 markers in sporadic breast cancer. Br J Cancer 73:1220–1226
Mueller JD, Haegle N, Keller G, Mueller E, Saretzky G, Bethke B, Stolte M, Hofler H (1998) Loss of heterozygosity and microsatellite instability in de novo versus ex-adenoma carcinomas of the colorectum. Am J Pathol 153:1977–1984
Thibodeau SN, French AJ, Cunningham JM, Tester D, Burgart LJ, Roche PC, McDonnell SK, Schaid DJ, Vockley CW, Michels VV, Farr GHJ, O’Connell MJ (1998) Microsatellite instability in colorectal cancer: different mutator phenotypes and the principal involvement of hMLH1. Cancer Res 58:1713–1718
Ward R, Meagher A, Tomlinson I, O’Connor T, Norrie M, Wu R, Hawkins N (2001) Microsatellite instability and the clinicopathological features of sporadic colorectal cancer. Gut 48:821–829
Peiro´ G, Diebold J, Lohse P, Ruebsamen H, Lohse P, Baretton GB, Lohrs U (2002) Microsatellite Instability, Loss of Heterozygosity, and Loss of hMLH1 and hMSH2 Protein Expression in Endometrial Carcinoma. Hum Pathol 33:347–354
Sarli L, Bottarelli L, Azzoni C, Campanini N, Di Cola G, Bader G, Iusco D, Salvemini C, Caruso G, Donadei E, Pizzi S, D’Adda T, Renato C, Roncoroni L, Bordi C (2004) Abnormal Fhit protein expression and high frequency of microsatellite instability in sporadic colorectal cancer. Eur J Cancer 40:1581–1588
Tsihlias J, Kapusta L, Slingerland J (1999) The prognostic significance of altered cyclin-dependent kinase inhibitors in human cancer. Annu Rev Med 50:401–423
Boland GP, Butt IS, Prasad R, Knox WF, Bundred NJ (2004) COX-2 expression is associated with an aggressive phenotype in ductal carcinoma in situ. Br J Cancer 90:423–429
Du W, Mah JT, Lee J, Sankila R, Sankaranarayanan R, Chia KS (2004) Incidence and survival of mucinous adenocarcinoma of the colorectum: a population-based study from an Asian country. Dis Colon Rectum 47:78–85
Green JB, Timmcke AE, Mitchell WT, Hicks TC, Gathright JBJ, Ray JE (1993) Mucinous carcinoma–just another colon cancer? Dis Colon Rectum 36:49–54
Wu CS, Tung SY, Chen PC, Kuo YC (1996) Clinicopathological study of colorectal mucinous carcinoma in Taiwan: a multivariate analysis. J Gastroenterol Hepatol 11:77–81
Lanza GJ, Gafa R, Dubini A, Maestri I, Cavazzini L (1995) Clinico-pathological features and biological characterization of mucoid colorectal carcinoma. Pathologica 87:631–639
Symonds DA, Vickery AL (1976) Mucinous carcinoma of the colon and rectum. Cancer 37:1891–1900
Secco GB, Fardelli R, Campora E, Lapertosa G, Gentile R, Zoli S, Prior C (1994) Primary mucinous adenocarcinomas and signet-ring cell carcinomas of colon and rectum. Oncology 51:30–34
Fleshner P, Slater G, Aufses AHJ (1989) Age and sex distribution of patients with colorectal cancer. Dis Colon Rectum 32:107–111
Lanza G, Matteuzzi M, Gafa R, Orvieto E, Maestri I, Santini A, del Senno L (1998) Chromosome 18q allelic loss and prognosis in stage II and III colon cancer. Int J Cancer 79:390–395
Mensink PB, Kolkman JJ, Van Baarlen J, Kleibeuker JH (2002) Change in anatomic distribution and incidence of colorectal carcinoma over a period of 15 years: clinical considerations. Dis Colon Rectum 45:1393–1396
Gomez D, Dalal Z, Raw E, Roberts C, Lyndon PJ (2004) Anatomical distribution of colorectal cancer over a 10 year period in a district general hospital: is there a true “rightward shift”? Postgrad Med J 80:667–669
Ionov Y, Peinado MA, Malkhosyan S, Shibata D, Perucho M (1993) Ubiquitous somatic mutations in simple repeated sequences reveal a new mechanism for colonic carcinogenesis. Nature 363:558–561
Lothe RA, Peltomaki P, Meling GI, Aaltonen LA, Nystrom-Lahti M, Pylkkanen L, Heimdal K, Andersen TI, Moller P, Rognum TO, et al (1993) Genomic instability in colorectal cancer: relationship to clinicopathological variables and family history. Cancer Res 53:5849–5852
Gafà R, Maestri I, Matteuzzi M, Santini A, Ferretti S, Cavazzini L, Lanza G (2000) Sporadic colorectal adenocarcinomas with high-frequency microsatellite instability. Cancer 89:2025–2037
Liu B, Nicolaides NC, Markowitz S, Willson JK, Parsons RE, Jen J, Papadopolous N, Peltomaki P, de la Chapelle A, Hamilton SR, et al (1995) Mismatch repair gene defects in sporadic colorectal cancers with microsatellite instability. Nat Genet 9:48–55
Raut CP, Pawlik TM, Rodriguez-Bigas MA (2004) Clinicopathologic features in colorectal cancer patients with microsatellite instability. Mutat Res 568:275–282
Mori S, Ogata Y, Shirouzu K (2004) Biological features of sporadic colorectal carcinoma with high-frequency microsatellite instability: special reference to tumor proliferation and apoptosis. Int J Clin Oncol 9:322–329
Lloyd RV, Erickson LA, Jin L, Kulig E, Qian X, Cheville JC, Scheithauer BW (1999) p27kip1: a multifunctional cyclin-dependent kinase inhibitor with prognostic significance in human cancers. Am J Pathol 154:313–323
Nasir A, Kaiser HE, Boulware D, Hakam A, Zhao H, Yeatman T, Barthel J, Coppola D (2004) Cyclooxygenase-2 expression in right- and left-sided colon cancer: a rationale for optimization of cyclooxygenase-2 inhibitor therapy. Clin Colorectal Cancer 3:243–247
Catalano T, Curia MC, Aceto G, Verginelli F, Cascinu S, Cama A, Mariani-Costantini R, Teti D, Battista P (2005) Mutations in the p53 and Ki-ras genes, microsatellite instability and site of tumor origin in colorectal cancer. Oncol Rep 14:625–631
Russo A, Bazan V, Iacopetta B, Kerr D, Soussi T, Gebbia N (2005) The TP53 colorectal cancer international collaborative study on the prognostic and predictive significance of p53 mutation: influence of tumor site, type of mutation, and adjuvant treatment. J Clin Oncol 23:7518–7528
Lukish JR, Muro K, DeNobile J, Katz R, Williams J, Cruess DF, Drucker W, Kirsch I, Hamilton SR (1998) Prognostic significance of DNA replication errors in young patients with colorectal cancer. Ann Surg 227:51–56
Martinez-Lopez E, Abad A, Font A, Monzo M, Ojanguren I, Pifarre A, Sanchez JJ, Martin C, Rosell R (1998) Allelic loss on chromosome 18q as a prognostic marker in stage II colorectal cancer. Gastroenterology 114:1180–1187
Watanabe T, Wu TT, Catalano PJ, Ueki T, Satriano R, Haller DG, Benson ABr, Hamilton SR (2001) Molecular predictors of survival after adjuvant chemotherapy for colon cancer. N Engl J Med 344:1196–1206
McLeod HL, Murray GI (1999) Tumour markers of prognosis in colorectal cancer. Br J Cancer 79:191–203
Kato M, Ito Y, Kobayashi S, Isono K (1996) Detection of DCC and Ki-ras gene alterations in colorectal carcinoma tissue as prognostic markers for liver metastatic recurrence. Cancer 77:1729–1735
Jernvall P, Makinen MJ, Karttunen TJ, Makela J, Vihko P (1999) Loss of heterozygosity at 18q21 is indicative of recurrence and therefore poor prognosis in a subset of colorectal cancers. Br J Cancer 79:903–908
Laurie JA, Moertel CG, Fleming TR, Wieand HS, Leigh JE, Rubin J, McCormack GW, Gerstner JB, Krook JE, Malliard J, et al (1989) Surgical adjuvant therapy of large-bowel carcinoma: an evaluation of levamisole and the combination of levamisole and fluorouracil. The North Central Cancer Treatment Group and the Mayo Clinic. J Clin Oncol 7:1156–1447
Diep CB, Thorstensen L, Meling GI, Skovlund E, Rognum TO, Lothe RA (2003) Genetic tumor markers with prognostic impact in Dukes’ stages B and C colorectal cancer patients. J Clin Oncol 21:820–829
Ikeda Y, Mori M, Koyanagi N, Minagawa S, Kondo N, Fujimaru R, Kojima Y, Kondo A, Sugimachi K (1998) Possibility of different cancer development between the proximal and distal colon: comparison of the distribution between adenomatous polyps and cancer. Hepatogastroenterology 45:1583–1586
Barratt PL, Seymour MT, Stenning SP, Georgiades I, Walker C, Birbeck K, Quirke P, Study UAtcAX-raFI (2002) DNA markers predicting benefit from adjuvant fluorouracil in patients with colon cancer: a molecular study. Lancet 360:1381–1391
Elsaleh H, Joseph D, Grieu F, Zeps N, Spry N, Iacopetta B (2000) Association of tumour site and sex with survival benefit from adjuvant chemotherapy in colorectal cancer. Lancet 355:1745–1750
Wright CM, Dent OF, Barker M, Newland RC, Chapuis PH, Bokey EL, Young JP, Leggett BA, Jass JR, Macdonald GA (2000) Prognostic significance of extensive microsatellite instability in sporadic clinicopathological stage C colorectal cancer. Br J Surg 87:1197–1202
Ahnen DJ, Feigl P, Quan G, Fenoglio-Preiser C, Lovato LC, Bunn PAJ, Stemmerman G, Wells JD, Macdonald JS, Meyskens FLJ (1998) Ki-ras mutation and p53 overexpression predict the clinical behavior of colorectal cancer: a Southwest Oncology Group study. Cancer Res 58:1149–1158
Acknowledgement
This work was supported in part by a grant from “ Lega Italiana per la lotta contro i tumori”.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Azzoni, C., Bottarelli, L., Campanini, N. et al. Distinct molecular patterns based on proximal and distal sporadic colorectal cancer: arguments for different mechanisms in the tumorigenesis. Int J Colorectal Dis 22, 115–126 (2007). https://doi.org/10.1007/s00384-006-0093-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-006-0093-x