Abstract
Purpose
To engraft bladder organoids (BO) on de-epithelialized mouse colon using an epithelial replacement technique.
Methods
BO cultured using bladder specimens from enhanced green fluorescent protein (EGFP) transgenic mice were engrafted to replace proximal colon epithelium stripped from an approximately 1 cm long target site in syngeneic wild-type recipient mice (n = 9) by exposure to ethylenediaminetetraacetic acid by infusion and flushing with phosphate buffered saline. Target sites were harvested on postoperative days 2, 7, and 28 for hematoxylin–eosin staining and immunofluorescence.
Results
Histology on postoperative days 7 and 28 showed BO derived EGFP + cells forming multiple layers on the luminal surface of the colon. Immunohistochemistry showed that EGFP + areas were positive for CK5 and CK14, markers for basal and immature subtype urothelium, respectively, but negative for CA2, a marker for colonic epithelium. Ki67 was detected predominantly in the basal parts of EGFP + areas on postoperative day 7 and day 28.
Conclusions
This is the first report of successful engraftment of BO in de-epithelialized colon with urothelial tissue reconstituted by actively proliferating cells. This technique could be developed for augmentation cystoplasty to prevent bladder calculi formation and malignant transformation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Augmentation cystoplasty using gastrointestinal tract (GIT) segments is performed in patients with neurogenic bladder dysfunction, tuberculous cystitis, and interstitial cystitis to extend dry-time by increasing compliance and capacity of the dysfunctional native bladder [1, 2]. As a result, improvement in quality of life and protection of upper urinary tract function can be expected [3]. However, prevention of serious postoperative complications such as bladder calculi and malignant transformation requires life-long monitoring because of the constant exposure of gastrointestinal epithelium to urine [1, 2, 4]. Formation of urinary tract calculi occurs in up to 40% of all augmentation cystoplasties and the incidence of malignancy ranges from 1 to 4.6% in the literature [1, 2]. In fact, 2 cases of malignancy have developed at the authors’ institute in 120 cases of augmentation cystoplasty performed over 25 years. One case was after 14 years and the other after 25 years [5, 6].
New strategies for bladder augmentation have been devised over the years to avoid using GIT segments to prevent serious complications, but few have been practically or clinically viable. For example, ureterocystoplasty [7] requires a megaureter, as a condition and is applicable only to a limited number of cases. Organoid technology has attracted close scrutiny in recent years because by culturing tissue stem cells and inducible pluripotent stem cells, tissue with specific features can be created and incorporated into recipient animals by engraftment [8, 9]. The scope for organoid technology to create useful materials is broad and expectations are high.
Methods to culture urothelial organoids from healthy murine bladders and human urothelial cancer specimens have been published [10] and undifferentiated basal urothelial cell clusters, mainly composed of cells expressing a specific marker for basal cells, cytokeratin 5 (CK5), were cultured by one protocol. Using a different protocol, urothelial clusters consisting of both undifferentiated and differentiated clusters, the latter of which expressed a specific marker for suprabasal cells, uroplakin III (UPK3) were created. This report hinted at the potential of organoid technology for treating morbidity and research has been applied to tissue engineering, regenerative medicine, and understanding epithelial disease pathogenesis [11, 12].
Colon has been used widely for experimental organoid engraftment. Yui et al. succeeded in grafting colonic epithelial organoids into chemically induced colitis lesions in recipients and reported that organoid-derived cells maintained their regenerative potential in vivo [13]. Fukuda et al. reported a different organoid engraftment model in which the luminal surface of the recipient’s rectum was temporarily exposed to ethylenediaminetetraacetic acid (EDTA), a chelating agent that loosens epithelial cell adhesions to the basement membrane. Small intestinal organoids cultured using specimens of small intestine were then engrafted to the rectum heterotopically and were found to retain many characteristics typical of small intestine [14]. This EDTA-mediated colonic de-epithelialization technique was recently applied by Sugimoto et al. [9] to show the beneficial clinical outcome of colonic epithelial replacement with small intestinal organoids. They reported that re-surfacing of a certain length of the colon with small intestinal organoids in a rat model of short bowel syndrome resulted in improvement in mortality and several clinical manifestations by compensating the loss of small intestinal functions [9].
This study was designed to develop a technique for replacing the epithelium of a segment of colon with bladder organoids to create hybrid urothelium-lined colon that could be used for augmentation cystoplasty without the intensive follow-up that is currently mandatory for life to monitor calculi formation and malignant transformation after augmentation colocystoplasty. A new protocol combining surgery and modified EDTA de-epithelialization of a target site in the proximal colon of a murine model is presented with histological assessment of how engrafted tissue behaves in a heterotopic environment (Fig. 1A).
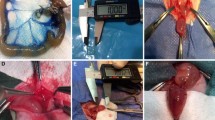
Engraftment of basal type bladder organoids onto de-epithelialized colon tissue by chemical injury. A Diagram of the experimental design. Recipient mice were sacrificed on days 2, 7, or 28 after engraftment for histologic evaluation of the characteristics of EGFP+ bladder organoid cells that had been incorporated onto recipient colon tissue. B Surgical procedure for preparing a target site in the proximal colon and engraftment of bladder organoids. Proximal colon was exposed (arrow) through a midline abdominal incision (I). 500 mM ethylenediaminetetraacetic acid was instilled into a target site of the colon approximately 1 cm in length between two clamps (II). Bladder organoid cells were infused into the de-epithelialized region between the clamps (III). Hematoxylin and eosin (H&E) staining of a longitudinally sectioned target site immediately after the epithelium had been removed chemically. muscular layers are preserved intact (IV). High-power views of the inset showing de-epithelialized colon. Scale bar: 500 \(\mu \)m [low] and 100 \(\mu \)m [high]. C Introduction of urothelial organoid culture derived from murine bladder. An example of morphological changes in a representative organoid formation from day 0 to 7 is shown on the left. Scale bar: 100 \(\mu \)m. A stereoscopic image of an organoid culture on day 7 at low magnification is shown on the right. Scale bar: 500 \(\mu \)m. D Imaging of bladder organoids derived from EGFP transgenic mice using a fluorescent microscope. High-power view of the inset is shown on the right. Scale bar: 1 mm [low] and 500 \(\mu \)m [high]. E Urothelial subtypes in (P1) pup mice, adult mice, and murine organoids characterized by immunofluorescence. Fluorescence for co-staining with CK5 and UPK3 shown with DAPI. Scale bar: 50 \(\mu \)m. F Urothelial subtypes in (P1) pup mice, adult mice, and murine organoids characterized by immunofluorescence. Fluorescence for co-staining with E-cadherin and CK14 shown with DAPI. Scale bar: 50 \(\mu \)m
Materials and methods
All animal experiments were performed with the approval of the Institutional Animal Care and Use Committee of Juntendo University (registration number: 1429, permission number: 2021155).
Mice
Donor cells for engraftment experiments were sourced from the bladders of 8–10-week-old enhanced green fluorescent protein (EGFP) transgenic mice of C57BL/6 background [15]. Recipients for engraftment experiments were 8–10-week-old C57BL/6 mice. Bladder tissue from 1-day-old pups (P1) from breeding pairs of C57BL/6 mice and adult C57BL/6 mice were applied for histological reference.
Mouse bladder organoid culture
In this study, basal type organoids were cultured from EGFP transgenic mice according to a modified technique based on a report by Mullenders et al. [10]. Briefly, minced bladder tissue was placed into a collagenase solution (2 mg/mL of collagenase from Clostridium histolyticum, Sigma-Aldrich, UK) in Advanced Dulbecco's Modified Eagle’s Medium/F-12 (AdvDMEM; Sigma-Aldrich, UK) to isolate urothelium. The tissue was incubated at 37 °C for 30 min while being rapidly shaken. After the cell suspension was filtered through a 70 μm filter, cells were plated in a 35 μL drop of Matrigel (Corning, USA) in individual wells of a prewarmed 24-well plate. The medium was changed every 3 days until the cultured cells could be used. Bladder organoids were cultured for at least 14 days and recovered from Matrigel using Cell Recovery Solution (Corning, USA). 1.0 × 106 cells were resuspended in 20 μL of diluted Matrigel/AdvDMEM (1:1) to be ready for engraftment.
Preparing the target site in a recipient mouse; stripping the colonic epithelium and engraftment
To prepare a target site, the epithelium of a segment of proximal colon was exposed to EDTA using a previously published technique [14] modified for this study. Briefly, C57BL/6 mice were anesthetized by isoflurane inhalation (2L/min) and a laparotomy was performed through a midline abdominal incision. One small incision was made at either end of an approximately 1 cm long target site in the proximal colon. A 22-gage catheter was inserted in each incision, tied with a silk suture (Fig. 1B), and 3 mL of 500 mM EDTA instilled. After 1 min of exposure to EDTA, the epithelium was stripped by flushing with 25–50 mL of PBS. The silk sutures were untied and the catheters were removed. Hemostatic forceps were used to clamp the distal incision site and just proximal to the proximal incision site (Fig. 1B) and 20 μL of resuspended organoid cells was pipetted into the de-epithelialized target site between the two clamps for 10 min. The intestinal incisions were closed and the abdomen closed. All mice were fed with DietGel® Recovery (ClearH2O, USA) for 7 days after surgery then had unrestricted access to standard feed. Recipient mice were sacrificed on postoperative days 2, 7, or 28 and target sites were harvested for hematoxylin–eosin (H&E) staining and immunofluorescence.
Histologic analyses
Each harvested target site was opened longitudinally and imaged to identify EGFP + cells using M165FC FSM, a fluorescence stereomicroscope system (Leica, Germany). Target sites, P1 bladder tissue, and adult mice bladder tissue were processed as follows. All specimens were fixed in 4% paraformaldehyde (PFA) at 4 °C for 2 h, sequentially dehydrated in 10%, 15%, and 20% sucrose in PBS, embedded in OCT mounting compound (Sakura Finetek Japan, Japan), frozen at − 80 °C, and sectioned transversely at a thickness of 8 μm. Frozen sections were stained with H&E or processed for immunofluorescence using the following primary antibodies according to manufacturer protocols: Green fluorescent protein (GFP; Invitrogen, USA, 1:250), E-cadherin (H-108, SANTA CRUZ, USA, 1:200), Cytokeratin 5 (CK5) (EP1601Y, abcam, UK, 1:250), Cytokeratin 14 (CK14) (LL002, Invitrogen, USA, 1:250), Uroplakin III (UPK3) (AU1, Progen, Germany, 1:200), CA II (H-70, SANTA CRUZ, USA, 1:200), Ki67 (16A8, BioLegend, USA, 1:200), Laminin (abcam, UK, 1:250). Slides were incubated with primary antibody at 4 °C overnight. Secondary antibody was added in PBS with Tween 20 (PBST) at room temperature for 1 h at the following dilutions: anti-chicken Alexa Flour 488 (Invitrogen, USA), anti-rabbit Alexa Flour 594 (Invitrogen, USA), anti-mouse Alexa Flour 548 (Invitrogen, USA), anti-goat Alexa Flour 548 (Invitrogen, USA): 1:200 each. Finally, slides were washed with PBS and mounted with coverslips using Vectashield containing DAPI (Vector Laboratories, USA). Fluorescent imaging was also acquired using a BZ-X 700 microscope (KEYENCE, Japan).
Whole mount staining of organoids
Organoids were fixed in 4% PFA for 30 min followed by treatment with 50 mM NH4Cl for 30 min. Permeabilization was performed with 0.1% Triton X-100 for 30 min, and then blocking was performed with 5% bovine serum albumin for 1 h. Thereafter, organoids were incubated with primary antibodies overnight at 4 °C and then with the secondary antibody for 1 h at room temperature. Primary and secondary antibodies were used in the same concentrations described earlier. All samples were treated with Hoechst 33,342 for nucleus staining. Images were acquired using a TCS-SP5 confocal laser scanning microscope (Leica, Germany).
Quantification of cell layers in graft tissue
The number of cell layers of EGFP+ and Ki67+ were counted perpendicularly from the basement membrane in 100 positions per colon specimen to quantify EGFP+ and Ki67+ cell layers in EGFP+ areas of target sites (Fig. 4B for EGFP+ and Fig. 4E for Ki67+). Data from 3 target sites on each of days 2, 7, and 28 were averaged to calculate a mean.
Statistical analysis
All data are expressed as mean value ± standard deviations (SD). Differences between two groups were tested for statistical significance using the unpaired t-test. All statistical tests were two-sided; p value of 0.05 or less was considered statistically significant.
Results
Features of cultured bladder organoids as donors for engraftment
Cells isolated from adult mouse bladders grew as spherical organoids initially (Fig. 1C) and after approximately 7 days of culture, some exhibited branch formation, a typical morphological feature of basal organoids. Cells from EGFP transgenic mice cultured using the same protocol grew the same way but expressed EGFP fluorescence (Fig. 1D).
Bladder urothelium has a well-organized multi-layered structure of epithelial cells with distinct differentiation [10]; cells in the superficial layer contain highly differentiated cells types that are positive for UPK3, while there is an abundance of undifferentiated CK5+ cells in the basal layers. Immunofluorescence of bladder tissues isolated from P1 and adult mice confirmed these features. In both tissues, UPK3+ cells were detected in the surface layer, while CK5+ cells in deeper layers on the basal side (Fig. 1E top and middle). Of note, organoids cultured from the bladders of adult mice had CK5+ cells in outer layers of their enclosed structure, while UPK3+ cells were absent (Fig. 1E bottom) which suggests that basal organoid cells are mostly undifferentiated, a finding that was also reported by Mullender et al. [10].
Basal organoid cells were further assessed using an antibody for CK14, a marker for a subpopulation of bladder urothelium that emerges during a short window of development, capable of self-renewal as well as differentiation into all urothelial lineages [16]. As reported [16], expression of CK14 was observed in nearly a half (44.8%) of E-cadherin+ epithelial cells in P1 tissues, whereas in only a minor population (5.7%) in adult tissues, respectively (Fig. 1F top and middle). These data coincide with a previous finding that CK14+ cells in mice reach a peak during the postnatal period and then decrease in number [16]. When organoids cultured for 14 days were immunostained for CK14 expression, CK14+ cells were found to be abundant and distributed broadly (Fig. 1F bottom), a previously unreported finding. Thus, cells in basal organoids that grew from adult urothelium showed phenotypical features of urothelium from a much earlier phase of development because of the abundant presence of CK14.
Bladder organoid cells engrafted on recipient colon demonstrate immature urothelium-like features
In preparation for engraftment, the epithelium of the colon was stripped from the target site leaving underlying non-epithelial tissues intact (Fig. 1B). EGFP+ organoid cells were engrafted onto the de-epithelialized colon segments. At the first histological assessment 2 days post-engraftment, clusters of EGFP+ cells were observed attached on the luminal side of the recipient colon (Fig. 2A). These EGFP+ cells expressed E-cadherin suggesting that engrafted cells on day 2 had an epithelial feature (Fig. 2B). By 28 days post-engraftment, EGFP+ cells had formed a multi-layered structure (Fig. 3A left and lower right) resembling the transitional epithelium in bladder specimens from P1 and adult mice, morphologically (Fig. 3B). EGFP+ areas were also devoid of crypts and mucus-containing goblet cells (Fig. 3A lower right), both typical of colonic epithelium (Fig. 3A upper right). Immunofluorescence demonstrated that EGFP+ cells were present immediately above the laminin+ basement membrane (Fig. 3C). CK5 and CK14 were expressed broadly in EGFP+ cells (Fig. 3D, E). However, UPK3 was not detected in EGFP+ cells or the native colon of the recipient (Fig. 3F). Expression of CA2, a colon-specific marker enzyme was negative in EGFP+ cell areas but positive in areas of native colonic epithelium (Fig. 3G). Collectively, these findings prove that EGFP+ cell areas were comprised of cells that retain the morphological features of the urothelium from which the engrafted organoids were cultured, without acquiring epithelium-like features of the colon onto which they were engrafted. Of note was the abundance of CK14 and complete absence of UPK3 in EGFP+ cells, findings typical of developing bladder tissue seen postnatally rather than adult bladder urothelium from which the organoids were cultured.
Histological evaluation for recipient colon 2 days post-engraftment. A Combined stereoscopic and EGFP fluorescent view (top) and EGFP fluorescent view alone (lower left). High-power view of the inset is shown (lower right). Bars: 1 mm. B Immunohistology of graft tissue from recipient colon on day 2. Fluorescence for EGFP and E-cadherin with DAPI staining. Scale bar: 100 μm
Engrafted bladder organoid cells demonstrate immature urothelium-like features on recipient colon. A Histologic analysis of a longitudinally sectioned target site at 28 days post-engraftment. A stereoscopic image overlaid with results of EGFP fluorescence (upper left) and H&E staining (lower left) obtained sequentially from a single section, are shown. High-power views of the inset and the dashed line indicate a colonic epithelial region of the recipient and graft region (right panel). Scale bar: 500 μm [low] and 20 μm [high]. B H&E staining of bladder tissue from a P1 pup (top) and adult mouse (bottom). Note the urothelial layer. Scale bar: 20 μm. C Multilayered structure of EGFP + cells shown with respect to the laminin + basement membrane. Image of recipient colon co-stained with EGFP and laminin antibodies at 28 days post-engraftment. Scale bar: 100 μm. D–F Immunofluorescence of a target site for EGFP and CK5 (F), CK14 (G), and UPK3 (H). Scale bar: 100 μm. (G) Immunofluorescence for EGFP and CA2 shown for different sections from a single recipient. Fluorescent images presented with DAPI staining. Scale bar: 100 μm
Active proliferation and regenerative capacity of engrafted bladder organoids
During the study period of 4 weeks, EGFP+ cells in target sites increased in thickness and the number of layers of EGFP+ cells in grafted areas was counted to measure thickness, quantitatively. Thickness was a mean of 1.68 ± 1.04 layers (Fig. 4A top, B) on day 2 post-engraftment, mean of 2.64 ± 1.73 layers on day 7 (Fig. 4A middle, B) and a mean of 4.58 ± 1.3 layers on day 28 (Fig. 4A bottom, B). Some grafted areas were actually 8 layers thick on day 28, exceeding the 2–7 layers seen in normal bladder urothelium of healthy adult mice [17]. These findings suggest that EGFP+ urothelial cells continue to proliferate on recipient colon after engraftment.
Active proliferation and tissue regeneration of engrafted bladder organoid cells. A Co-staining for EGFP and Ki67 in a target site: 2 days (top), 7 days (middle), 28 days (bottom) days post-engraftment. Arrowhead indicates a Ki67+ cell. Scale bar: 50 μm. B Graph of EGFP+ cell layers on recipient colon: 2 days (left), 7 days (middle), 28 days (right) post-engraftment. C Immunofluorescence for Ki67+ cells on E-cadherin+ urothelium in a P1 pup (top) and an adult mouse (bottom). The arrow indicates a Ki67+ cell. Scale bar: 50 μm. D Graph of Ki67+ cells in E-cadherin+ urothelium from a P1 pup and an adult mouse and in E-cadherin+ cells of a EGFP+ region on days 2, 7, and 28. In recipient colon, E-cadherin+ cells of graft tissue are synonymous with EGFP+ cells. **p < 0.001 and *p < 0.05. (E) Graph of Ki67+ cell layers in EGFP+ regions of recipient colon: 2 days (left), 7 days (middle), 28 days (right) post-engraftment
Target site specimens were examined by immunofluorescence to Ki67 to assess the pattern of actively proliferating cells in urothelium quantitatively. As expected, expression of Ki67 in E-cadherin+ urothelial cells in P1 bladder (15.9 ± 3.51%) was significantly higher than in adult mice (2.0 ± 0.43%; Fig. 4C, D), indicating that proliferation of urothelium postnatally is more active than in adults. Specifically, Ki67 expression in E-cadherin+ graft tissues on day 2 was almost negative (0.27 ± 0.61%) (Fig. 4D), indicating that graft proliferative activity was poor at that stage. However, on day 7, Ki67 was 26.0 ± 13.0%, a level of expression higher than for both P1 and adult mice (Fig. 4D). Although it decreased to 18.4 ± 7.4% on day 28, this level was still comparable with P1 pup levels (Fig. 4D). Next, the distribution of actively proliferating Ki67 cells was investigated to determine if they were distributed broadly throughout grafted areas or were localized in a non-uniform pattern. The number of Ki67+ cells in the layers of grafted areas on days 2, 7, and 28 post-engraftment were counted and used to calculate the distribution of Ki67+ cells. On day 2, there were very few Ki67+ cell layers in grafted areas; a mean of 0.03 ± 0.17 of a layer on day 2 (Fig. 4A top, E) that increased just adjacent to the basement membrane in EGFP+ regions to a mean 1.48 ± 1.19 layers on day 7 (Fig. 4A middle, E) and mean of 1.8 ± 1.26 layers on day 28 (Fig. 4A bottom, E). Localization of Ki67+ cells in these layers was discovered in this study.
Discussion
The protocol presented in this study successfully engrafted basal type bladder organoids onto a segment of de-epithelialized proximal colon in recipient mice, the first-time bladder urothelial cells were incorporated stably onto a non-epithelial component of colon using a modified epithelial replacement technique. This result expands the application of organoid technology from engraftment of intestinal columnar cells onto colon [9, 13, 14] and has abundant potential for growing organoids originated from different types of tissue, ectopically. This study was limited to post-engraftment follow-up of 28 days, but the structure and growth of grafted organoids was suggestive of healthy urothelium and further follow-up is required to confirm that engrafted bladder organoids can grow into phenotypically true tissue when engrafted heterotopically. Once long-term stability is confirmed, the presented protocol could be used to create hybrid urothelium-lined colon, ideal for augmentation cystoplasty with less risk for complications such as calculi formation and malignant transformation and adapted to create GIT segments lined by tissue grown from engrafted urothelial organoids.
Grafted EGFP+ cells formed multi-layered tissue, characteristic of bladder urothelium, as shown by the presence of CK5+ urothelial cells and absence of CA2+ colonic cells in grafted tissue. However, grafted urothelium differed from the adult bladder urothelium it originated from which contains terminally differentiated UPK3+ cells in addition to undifferentiated cells. Grafted tissue was mostly composed of undifferentiated cells, but with greater proliferative capacity than adult bladder urothelium in adults; in other words, the turnover rate of grafted tissue was faster than adult bladder urothelium, more akin to turnover seen in early phases of development [18,19,20]. As proof of this, the population of Ki67 + cells in graft tissues and bladder urothelial cells were compared quantitatively. As reported, Ki67+ cells in bladder urothelium of P1 pups was significantly higher than bladder urothelium from adults. Notably, Ki67+ cells were significantly increased in 7-day-old grafts compared with P1 pups and remained close to levels seen in P1 pups throughout the study despite decreasing in grafts after 28 days. This suggests that an undetermined mechanism predominantly active in early developmental phases must be acting to induce proliferation of urothelium in grafted tissue even though the organoids were cultured from adult bladder urothelium with no such function. Interestingly, grafted cells expressing Ki67 were distributed immediately above the basement membrane clearly demarcated by laminin, without any of the uncontrolled patterns of distribution of Ki67+ cells commonly observed in hyperplasia, dysplasia, or malignant transformation [21], similar to the distribution seen in developing stages of urothelium or during the rapid regeneration to tissue damage by induction of Ki67 expression in the very bottom layers of basal cells reported in the literature [16, 22]. In other words, active proliferation of graft tissues would seem to occur preferentially close to the basement membrane between days 7 and 28 post-engraftment and the behavior of Ki67+ cells would suggest that proliferation of graft cells on colon tissue is activated by a mechanism resembling the regeneration of bladder urothelium after injury.
Similarly, CK14 expression in bladder urothelium, known to be linked to its proliferative capacity during development was abundant consistently throughout the study although CK14 expression in bladder urothelium has been reported as 32% in 5-day-old pups, 3% in 8-week-old mice, and 0.8% in 24-week-old mice [16, 18, 23]. However, CK14 is also induced markedly in bladder urothelium in response to injury, suggesting it contributes to tissue regeneration [16, 23]. Thus, CK14 expression in grafted cells would also suggest re-activation of some mechanism that controls active proliferation of bladder urothelium during development and in response to tissue injury, by the process of organoid culture and engraftment. Further research on how grafted urothelium derived from adult mouse bladder becomes highly proliferative when placed in a heterotopic microenvironment will provide valuable insight for understanding the mechanism of urothelial tissue morphogenesis during development as well as regeneration after injury.
To date, several studies have proposed unique strategies for augmentation cystoplasty to overcome complications. A technical report published by the authors described how bladder tissue derived from a donor rat could be embedded in a pedicle of omentum in a recipient for 14 days, then anastomosed successfully to the bladder of the recipient [24]. This modified bladder augmentation technique decreased the development of bladder calculi in the long-term compared with conventional augmentation cystoplasty using a GIT segment, indicating that tissue other than GIT was advantageous. However, this allogenic transplantation strategy required the use of one-third of the donor’s bladder, so was not practical. Another recent study reported harvesting a colonic segment from a rat donor, chemically decellularizing it, and anastomosing it to the bladder of a recipient rat as a patch for augmentation cystoplasty [25]. The patch became lined by transitional epithelial cells, presumably by migration from surrounding areas of bladder to the decellularized colon patch, after surgery. This strategy required reconstruction of non-epithelial components of the patch, such as blood vessels and neuronal elements, after anastomosis of the patch to the bladder of the recipient with full postoperative recovery taking some 3 months. As alternatives to augmentation colocystoplasty using a segment of GIT are not readily forthcoming, the clinical application of the protocol presented in this study could be advantageous because culture of bladder organoids from a small amount of a patient’s own bladder may allow generation of large areas of urothelialized colon tissues sufficient enough for autologous augmentation cystoplasty and in contrast to decellularized colon used for augmentation cystoplasty, urothelialized colon would be well-structured and fully mature at the time of surgery, if bladder organoid engraftment is performed appropriately, beforehand.
In conclusion, the presented protocol could find clinical application as a novel strategy to decrease risks for calculi formation and malignant transformation after augmentation cystoplasty. Further research on engraftment in larger animals and assessment of outcome over longer periods will consolidate the potential of organoid technology for clinical use.
Data availability statement
Data are available on reasonable request from the authors.
References
Çetinel B, Kocjancic E, Demirdağ Ç (2016) Augmentation cystoplasty in neurogenic bladder. Investig Clin Urol 57(5):316–323
Kispal ZF, Kardos D, Jilling T, Kereskai L, Isaacs M, Balogh DL et al (2015) Long-term histological and mucin alterations in the neobladder mucosa following urinary bladder augmentation or substitution with gastrointestinal segment. J Pediatr Urol 11(6):349.e1–6
Hayashi Y, Nishimura E, Shimizu S, Miyano G, Okawada M, Nagae I et al (2017) Sigmoidocolocystoplasty for neurogenic bladder reviewed after 20 years. J Pediatr Surg 52(12):2070–2073
Peycelon M, Szymanski KM, Francesca Monn M, Salama AK, Risk H, Cain MP et al (2020) Adherence with bladder irrigation following augmentation. J Pediatr Urol 16(1):33.e1-e8
Kitamura K, Isotani S, Muto S, Horie S (2022) Efficacy of pembrolizumab in a rare type of bladder cancer arising 25 years after augmentation cystoplasty. BMJ Case Rep 15(3)
Hayashi Y, Shiyanagi S, Okawada M, Koga H, Fujimura J, Nagae I et al (2015) Undifferentiated sarcoma developing 14 years after colocystoplasty: our experience and literature review. J Ped Surg Case Rep 3:385–388
Tekgül S, Oge O, Bal K, Erkan I, Bakkaloğlu M (2000) Ureterocystoplasty: an alternative reconstructive procedure to enterocystoplasty in suitable cases. J Pediatr Surg 35(4):577–579
Clevers H, Conder RK, Li VSW, Lutolf MP, Vallier L, Chan S et al (2019) Tissue-engineering the intestine: the trials before the trials. Cell Stem Cell 24(6):855–859
Sugimoto S, Kobayashi E, Fujii M, Ohta Y, Arai K, Matano M et al (2021) An organoid-based organ-repurposing approach to treat short bowel syndrome. Nature 592(7852):99–104
Mullenders J, de Jongh E, Brousali A, Roosen M, Blom JPA, Begthel H et al (2019) Mouse and human urothelial cancer organoids: a tool for bladder cancer research. Proc Natl Acad Sci USA 116(10):4567–4574
Date S, Sato T (2015) Mini-gut organoids: reconstitution of the stem cell niche. Annu Rev Cell Dev Biol 31:269–289
Nakamura T, Sato T (2018) Advancing intestinal organoid technology toward regenerative medicine. Cell Mol Gastroenterol Hepatol 5(1):51–60
Yui S, Nakamura T, Sato T, Nemoto Y, Mizutani T, Zheng X et al (2012) Functional engraftment of colon epithelium expanded in vitro from a single adult Lgr5+ stem cell. Nat Med 18(4):618–623
Okabe M, Ikawa M, Kominami K, Nakanishi T, Nishimune Y (1997) “Green mice” as a source of ubiquitous green cells. FEBS Lett 407(3):313–319
Fukuda M, Mizutani T, Mochizuki W, Matsumoto T, Nozaki K, Sakamaki Y et al (2014) Small intestinal stem cell identity is maintained with functional Paneth cells in heterotopically grafted epithelium onto the colon. Genes Dev 28(16):1752–1757
Papafotiou G, Paraskevopoulou V, Vasilaki E, Kanaki Z, Paschalidis N, Klinakis A (2016) KRT14 marks a subpopulation of bladder basal cells with pivotal role in regeneration and tumorigenesis. Nat Commun 7:11914
Abelson B, Sun D, Que L, Nebel RA, Baker D, Popiel P et al (2018) Sex differences in lower urinary tract biology and physiology. Biol Sex Differ 9(1):45
Sun W, Wilhelmina Aalders T, Oosterwijk E (2014) Identification of potential bladder progenitor cells in the trigone. Dev Biol 393(1):84–92
Khandelwal P, Abraham SN, Apodaca G (2009) Cell biology and physiology of the uroepithelium. Am J Physiol Renal Physiol 297(6):F1477–F1501
Park JH, Kotani T, Konno T, Setiawan J, Kitamura Y, Imada S et al (2016) Promotion of intestinal epithelial cell turnover by commensal bacteria: role of short-chain fatty acids. PLoS ONE 11(5):e0156334
Majewski T, Lee S, Jeong J, Yoon DS, Kram A, Kim MS et al (2008) Understanding the development of human bladder cancer by using a whole-organ genomic mapping strategy. Lab Invest 88(7):694–721
Shin K, Lee J, Guo N, Kim J, Lim A, Qu L et al (2011) Hedgehog/Wnt feedback supports regenerative proliferation of epithelial stem cells in bladder. Nature 472(7341):110–114
Volkmer JP, Sahoo D, Chin RK, Ho PL, Tang C, Kurtova AV et al (2012) Three differentiation states risk-stratify bladder cancer into distinct subtypes. Proc Natl Acad Sci USA 109(6):2078–2083
Yamataka A, Wang K, Kato Y, Okada Y, Kobayashi H, Lane GJ et al (2005) Long-term outcome of bladder augmentation using living-related partial bladder transplantation in rats. Pediatr Res 57(5 Pt 1):738–743
Kajbafzadeh AM, Khorramirouz R, Sabetkish S, Ataei Talebi M, Akbarzadeh A, Keihani S (2016) In vivo regeneration of bladder muscular wall using decellularized colon matrix: an experimental study. Pediatr Surg Int 32(6):615–622
Acknowledgements
We thank the Laboratory of Morphology and Image Analysis and the Laboratory of Molecular and Biochemical Research, Research Support Center, Juntendo University Graduate School of Medicine for technical assistance. This study was supported by Japan Society for the Promotion of Science KAKENHI grants (20K21637 and 19K09083), GlaxoSmithKline Japan Research Grant 2019 (D-17), GlaxoSmithKline Japan Research Grant 2021 (AS2021A000149025), and Juntendo University Project Grant (No. 2020-19). Tetsuya Nakamura belongs to the Department of Research and Development for Organoids, which is endowed by the Japanese pharmaceutical group, Eisai Co., Ltd.
Author information
Authors and Affiliations
Contributions
KS and AY designed the study. TO, HK, NH, and TN provided the conceptual advice. KS, YM, and TN performed the organoid culture and engraftment experiments. KS, YM, TN, and AY analyzed the data. KS, GL, TN, and AY wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Suda, K., Matsumoto, Y., Ochi, T. et al. Successful engraftment of bladder organoids in de-epithelialized mouse colon. Pediatr Surg Int 39, 14 (2023). https://doi.org/10.1007/s00383-022-05294-w
Accepted:
Published:
DOI: https://doi.org/10.1007/s00383-022-05294-w