Abstract
Background/aim
The behavior of enteric neural crest-derived cells (ENCC) during enteric nervous system (ENS) development is being gradually understood with the introduction of live-cell imaging. However, many of the analyses to date are two-dimensional and the precise multidirectional migration of ENCC has been challenging to interpret. Mice lacking the endothelin-B receptor gene, Ednrb −/− mice, are widely used as a model for Hirschsprung’s disease (HD). We have recently developed a Sox10 transgenic (Tg) mouse to visualize ENCC with enhanced green fluorescent protein (Venus). By breeding these two models, we have created a Venus-positive, Sox10 Tg mouse with a deletion of the Ednrb gene, Sox10-Venus+/Ednrb −/− mouse, to investigate the ENS in HD. The aim of this study was to investigate the behavior of migrating ENCC in the hindgut of the Sox10-Venus+/Ednrb −/− mouse using three-dimensional and four-dimensional image analysis software.
Methods
To compare the ENCC behavior when the wavefront of ENCC reaches the mid-hindgut between HD mouse and control, we harvested the fetal hindguts of Sox10-Venus+/Ednrb −/− mice on embryonic day 15.5 (E15.5) and Sox10-Venus+/Ednrb +/+ mice on E12.5, which was used as control. Dissected hindguts were cultured for 360 min and the time-lapse images were obtained using a confocal laser-scanning microscope. Each ENCC at the wavefront was tracked after adjusting the longitudinal axis of the gut to the Y axis and analyzed using Imaris software.
Results
Track displacement (TD)-Y indicates ENCC advancement in a rostral-caudal direction. TD-X and TD-Z indicate ENCC advancement perpendicular to the rostral–caudal axis. Mean TD-Y was 34.56 µm in HD, but 63.48 µm in controls. TD-Y/TD-XZ was not significantly different in both groups. However, the mean track speeds were significantly decreased in HD (72.87 µm/h) compared to controls (248.29 µm/h).
Conclusions
Our results showed that the track speed of ENCC advancement was markedly decreased in the HD mice compared to controls. This technique provides added information by tracking ENCC with depth perception, which has potential for further elucidating the altered behavior of ENCC in HD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The enteric nervous system (ENS) is established by the migration of neural crest derived cells along the gastrointestinal tract. It is understood that there is rostral-to-caudal migration of enteric neural crest derived cells (ENCC) that colonize the entire gut [1–3]. The failure of ENCC to colonize the terminal gut results in aganglionic bowel, seen in patients with Hirschsprung’s disease (HD).
The behavior of ENCC during ENS development is being gradually understood with the introduction of live-cell imaging. However, many of the analyses to date are two-dimensional and the precise multidirectional migration of ENCC has been still challenging to interpret.
Sox10 is highly expressed in vagal neural crest cells as they emerge from the neural tube [4]. Recently, we developed Sox10 transgenic (Tg) mice whose ENCC can be visualized with modified enhanced green fluorescent protein (Venus) [5, 6]. This mouse model can visualize the dynamic behavior of ENCC without any bias associated with conventional immunohistochemistry staining.
Much of our understanding of HD comes from studies of animal models. Endothelin receptor B (Ednrb) knockout mice, which present aganglionic colon, have been widely used to investigate the pathogenesis of HD. By breeding these two mouse models, we have created a Venus-positive, Sox10 Tg mouse with a deletion of the Ednrb gene, Sox10-Venus+/Ednrb −/− mouse, to investigate the ENS in HD. The aim of this study was to investigate the behavior of migrating ENCC in the hindgut of the Sox10-Venus+/Ednrb −/− mouse using three-dimensional and four-dimensional (3D/4D) image analysis software.
Materials and methods
Animal model
We created and raised Sox10-Venus Tg mice according to a protocol described elsewhere to visualize neural crest lineage cells [5]. Endothelin receptor B-null Ednrb tm1Ywa was originally obtained from Jackson Laboratory (Bar Harbor, ME, USA). The heterozygote littermates (we refer to these as Ednrb +/− and the homozygotes are referred as Ednrb −/− in this article) were used as breeding pairs. Sox10-Venus+/Ednrb −/− mice were raised at the Juntendo University School of Medicine in Tokyo (Japan) by crossing Ednrb +/− mice with Sox10-Venus Tg mice and repeat crossing with Sox10-Venus+/Ednrb +/− mice.
The genotypes of mice and embryos were determined by a polymerase chain reaction. The day when a vaginal plug was detected was classified as embryonic day 0.5 (E0.5). Sox10-Venus+/Ednrb +/− mice were killed on E12.5 and 15.5 by cervical dislocation. Each embryo was harvested and the gut was dissected under a stereoscopic microscope (M165FC FSM, Leica, Germany). All animal procedures were reviewed and approved by the Animal Care and Use Committee (Institutional review board No. 260019).
Tissue culture and image acquisition
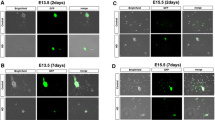
Dissected specimens were placed in tissue culture medium [DMEM/F12 with 1 % N2 supplement (Gibco, USA) and 100 ng/ml of NGF (Roche Applied Science, USA)] as described elsewhere [7]. Time-lapse images were obtained for 360 min at 10 min intervals using a confocal laser-scanning microscope (TCS-SP5, Leica, Germany).
3D/4D analysis of migratory ENCC
To compare the ENCC behavior when the wavefront of ENCC reaches the mid-hindgut between HD mouse and control, we analyzed fetal hindguts of Sox10-Venus+/Ednrb −/− mice on E15.5, and Sox10-Venus+/Ednrb +/+ mice on E12.5 which was used as control. Time-lapse images were imported into Imaris software (Bitplane, Zurich, Switzerland) for further analysis. Ten ENCC, all within 400 µm of the most caudal cell, were tracked along the three spatial axes (X, Y, Z) over time, after adjusting the longitudinal axis of the gut to the Y axis. Track displacement (TD)-X, Y and Z, and track speed were measured. TD-Y, the distance between the first and last position along the Y axis, represents rostral to caudal advancement of ENCC. TD in the perpendicular plane to the rostral–caudal axis (TD-XZ) was calculated with the formula below.
Statistical analysis
Differences between the two groups were tested using an unpaired t test. Statistical significance was defined as <0.05.
Results
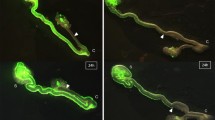
We analyzed the behavior of migratory ENCC in HD mice on E15.5 (Fig. 1a), and control mice on E12.5 (Fig. 1b), since the ENCC wavefront was located in the mid-hindgut, which is relatively straight in structure.
TD-X, Y and Z in HD and control were shown in Fig. 2a, b. The mean TD-Y, representing rostral to caudal advancement of ENCC, was 34.56 µm in HD mice, but 63.48 µm in controls (p < 0.05) (Fig. 3a). The mean TD-XZ, representing ENCC advancement in the perpendicular plane to the rostral-caudal axis, was also decreased in HD mice compared to controls, 38.70 and 98.84 µm, respectively (p < 0.001) (Fig. 3b). However, TD-Y/TD-XZ was not significantly different in both groups, suggesting directionality of ENCC advancement was not altered in HD mice compared to controls (Fig. 3c). The mean track speed was significantly decreased in HD mice (72.87 µm/h) compared to controls (248.29 µm/h) (p < 0.05) (Fig. 3d).
Discussion
The introduction of live-cell imaging has provided significant advancement in understanding the behavior of ENCC during ENS development. However, many of the analyses to date are two-dimensional and the precise multidirectional migration of ENCC has remained challenging to interpret.
Much of our information on the etiology and pathogenesis of HD have been obtained from analyses of ENCC development in genetically mutant mice. Ednrb, expressed by migrating ENCC and the mesenchyme of the gut, plays an important role in ENS development [8]. The mutation in the gene encoding Ednrb affects the development of ENCC, resulting in aganglionosis in distal colon. It is reported that the delay of ENCC migration in the Ednrb −/− mice has been detected as early as E10.5 [9]. By E14.5, the wavefront of ENCC reaches the terminal colon in control mice, however, it only reaches the proximal colon and fails to colonize distal colon in Ednrb −/− mice. It is still not clear whether this delay is caused by a slower speed of ENCC advancement or/and the altered directionality, for instance, advancing in a circumferential direction rather than rostral to caudal direction in the gut.
In this study, we analyzed the ENCC behavior in our newly created HD mouse model, Sox10-Venus+/Ednrb −/− mice, which can visualize the altered advancement of ENCC resulting in aganglionic colon. Using 3D/4D image analysis software which enables us to monitor temporal changes in 3D images over time, we succeeded to obtain added information on multidirectional displacement of ENCC.
Our results showed that the track speed of ENCC was markedly decreased in HD mice compared to controls. TD-Y, caudal advancement of ENCC, and TD-XY, circumferential advancement of ENCC on the transverse plane, were both decreased in HD mice compared to controls. However, TD-Y/TD-XZ was not significantly different between groups. These results suggest that although the directionality of ENCC advancement is not altered in HD, the speed is significantly decreased in HD mice compared to controls. These results are supported by published data showing that a pharmacological inhibitor of Ednrb reduces the speed of ENCC migration but does not affect the directionality [10].
In this study, we observed ENCC behavior in the mid-hindgut which is the terminal portion of ENCC migration in HD mouse. The mid-colon is relatively straight in structure and therefore suitable to analyze directionality with less structural bias compared to the ileum and proximal colon including cecum. To compare ENCC behavior in the same part of colon, the samples were taken at different stages of ENS development between the two groups. One is on the way to colonize the gut and the other is almost stopping migration resulting in aganglionic colon. From this point of view, the shorter displacement of rostral to caudal migration in HD seems to be predictable. However, we demonstrated that the ENCC in HD mouse on E15.5 were still advancing into the mid-hindgut but very slowly, with comparable directionality as that seen in controls on E12.5, when the ENCC reaches the mid-hindgut.
The track speeds of ENCC obtained in our series were much faster than those previously reported [10]. We expect that this is mainly because we analyzed added information including TD-Z, depth perception.
In this study, we provide convincing evidence that this technique can further our understanding of ENCC behavior by tracking multidirectional advancement of ENCC in three dimensional coordinate. This technique has potential for further elucidating the altered behavior of ENCC in HD.
References
Le Douarin NM, Teillet MA (1973) The migration of neural crest cells to the wall of the digestive tract in avian embryo. J Embryol Exp Morphol 30:31–48
Okamoto E, Satani M, Kuwata K (1982) Histologic and embryologic studies on the innervation of the pelvic viscera in patients with Hirschsprung’s disease. Surg Gynecol Obstet 155:823–828
Young HM, Bergner AJ, Anderson RB et al (2004) Dynamics of neural crest-derived cell migration in the embryonic mouse gut. Dev Biol 270:455–473
Heanue TA, Pachnis V (2007) Enteric nervous system development and Hirschsprung’s disease: advances in genetic and stem cell studies. Nat Rev Neurosci 8:466–479
Shibata S, Yasuda A, Renault-Mihara F et al (2010) Sox10-Venus mice: a new tool for real-time labeling of neural crest lineage cells and oligodendrocytes. Mol Brain 3:31
Miyahara K, Kato Y, Koga H et al (2011) Visualization of enteric neural crest cell migration in SOX10 transgenic mouse gut using time-lapse fluorescence imaging. J Pediatr Surg 46:2305–2308
Nakazawa N, Miyahara K, Okawada M et al (2013) Laminin-1 promotes enteric nervous system development in mouse embryo. Pediatr Surg Int 29:1205–1208
Leibl MA, Ota T, Woodward MN et al (1999) Expression of endothelin 3 by mesenchymal cells of embryonic mouse caecum. Gut 44:246–252
Druckenbrod NR, Epstein ML (2009) Age-dependent changes in the gut environment restrict the invasion of the hindgut by enteric neural progenitors. Development 136:3195–3203
Young HM, Bergner AJ, Simpson MJ et al (2014) Colonizing while migrating: how do individual enteric neural crest cells behave? BMC Biol 26:12–23
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nakazawa-Tanaka, N., Miyahara, K., Fujiwara, N. et al. Three- and four-dimensional analysis of altered behavior of enteric neural crest derived cells in the Hirschsprung’s disease mouse model. Pediatr Surg Int 32, 3–7 (2016). https://doi.org/10.1007/s00383-015-3806-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00383-015-3806-9