Abstract
Background/Aim
Neuronal development is regulated by extracellular environmental factors including nerve growth factor (NGF) and laminin. We have previously demonstrated that laminin-1 promotes neurite outgrowth of dorsal root ganglion cells by modulating NGF and integrin signaling. However, information about their effects on the enteric nervous system (ENS) is limited. Recently, we succeeded in visualizing enteric neural crest-derived cell (ENCC) migration using SOX10-Venus transgenic mice, in which ENCC are labeled with a green fluorescent protein, Venus. In this study, we examine the effects of NGF and laminin-1 in ENCC migration using SOX10-Venus mice gut.
Methods
Pregnant SOX10-Venus mice were killed on day 12.5 of gestation. The colorectum was dissected from embryos (n = 10) and placed in culture medium including NGF with or without laminin-1 for 12 h. Extension rates of ENCC migration, colorectum and ENCC migration per colorectum were calculated.
Results
Venus positive-ENCC extension rate was significantly higher in the laminin group (n = 5) compared to control (n = 5), 22.84 and 13.96 %, respectively (p < 0.05). The extension rate of the colorectum was not significantly different between the two groups.
Conclusions
Our results suggest that laminin promotes ENCC migration in mice. This technique allowed us to visualize the effects of extracellular molecules on ENCC migration and it potentially provides us with an insight into the pathophysiology of developmental disorders of the ENS, such as Hirschsprung’s disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neuronal development is regulated by extracellular environment factors including growth factors and extracellular matrix (ECM). Laminin-1 is a heterotrimeric ECM molecule composed of α1-, β1- and γ1- chains, and is the most important isoform in early development [1]. We have previously demonstrated that laminin-1 promotes neurite outgrowth in the presence of nerve growth factor (NGF) using dorsal root ganglion and PC12 cells. Our results showed that the percentage of cells with neurite outgrowth was increased as a result of treatment with both NGF and laminin-1 compared to treatment with NGF alone [2].
The enteric nervous system (ENS) is established by the migration of neural crest-derived cells (NCC) along the gastrointestinal tract. It is understood that there is rostral-to-caudal migration of vagal NCC that colonize the entire gut [3–5]. It has been suggested that it is the microenvironment encountered along the pathways leading to the target regions that determines the fate of migrating NCC [6].
The failure of enteric NCC (ENCC) to colonize the terminal gut results in aganglionic bowel, seen in patients with Hirschsprung’s disease (HD). Accumulated laminin-1 has been found in the presumptive aganglionic tissue of endothelin-3 deficient (Edn3 ls/ls) mice [7]. It is also reported that laminin-1 expression is increased in the aganglionic segment in HD patients [8, 9]. Although this evidence suggests that laminin-1 is a candidate molecule suspected to play a role in aganglionosis, the exact effect of laminin-1 on ENS development is not fully understood.
SOX10 is highly expressed in vagal neural crest cells as they emerge from the neural tube [10]. Recently, we used bacterial artificial chromosome transgenesis to develop SOX10 transgenic (Tg) mice whose ENCC can be visualized with modified enhanced green fluorescent protein (VENUS). This mouse model can be used to visualize the dynamic behavior of ENCC without any bias associated with conventional immunohistochemistry staining [11, 12].
In this study, we examined the effect of laminin-1 on ENCC migration using SOX10-Venus mice gut.
Materials and methods
Animal model
We created and raised transgenic SOX10-Venus Tg mice according to a protocol described elsewhere to visualize neural crest lineage cells [11]. The genotype of mice and embryos were determined by a PCR protocol described elsewhere. The day a vaginal plug was detected was classified as embryonic day 0.5 (E0.5). SOX10-Venus positive mice were killed on E12.5 by cervical dislocation. Each embryo was harvested, and hindgut with cecum was dissected under a stereoscopic microscope (M165FC FSM, Leica, Germany). All animal procedures were reviewed and approved by the Juntendo University School of Medicine Animal Care and Use Committee (Institutional review board No. 230033).
Tissue culture and image acquisition
Each dissected bowel was cultured in labeling buffer [DMEM/F12 with 1 % N2 supplement (Gibco, USA) and 100 ng/ml of NGF (Roche Applied Science, USA)] with or without 10 μg/ml of laminin-1 as described elsewhere [2]. The specimens were incubated for 12 h and the images were taken by laser scanning confocal microscopy (LSM 510, Carl Zeiss, Germany). The average distance from the cecum to the wavefront of Venus positive ENCC along the mesenteric and the anti-mesenteric side was defined as ‘the length of ENCC migration’. The distance from the cecum to the distal end of the gut was defined as ‘the length of colorectum’. The lengths above were measured using LSM viewer software (Carl Zeiss, Germany). Extension rates of ENCC migration, colorectum and ENCC migration per colorectum were calculated.
Statistical analysis
Differences between the two groups were tested using an unpaired t test. Statistical significance was defined as <0.05.
Results
We observed Venus positive ENCC in the hindgut cultured with or without laminin-1 for 12 h from E12.5. As previously reported [12], at 11.5, ENCC are colonizing the cecum and it is difficult to accurately determine the distal end of migrating ENCC. Furthermore, by E14.0 ENCC reach the terminal colon and integrate with sacral ENCC. Thus, we chose to study the period between E12.5 and E13.5 when the ENCC migrate from proximal colon to distal colon.
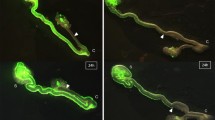
At 0 h, Venus positive ENCC were observed in the cecum and in the region of the proximal half of the colon in both control and laminin groups (Fig. 1). At 12 h, the distal ends of strands of Venus positive ENCC (wavefront of ENCC) advanced toward the anal end of the hindgut in both groups. However, the wavefront of migrating ENCC was more advanced in the laminin group compared to control. The red line and count in Fig. 1 show the distances from the cecum to the wavefront of Venus positive ENCC along the mesenteric and the anti-mesenteric side of the colon. The average distance of the mesenteric and the anti-mesenteric side was defined as ‘the length of ENCC migration’. The extension rate of ENCC migration after 12 h was significantly higher in laminin group (n = 5) compared to controls (n = 5), 22.84 ± 4.36 and 13.96 ± 3.84 %, respectively (p < 0.01) (Fig. 2a). The blue line and count in Fig. 1 indicate the distance from the cecum to the distal end of the gut, which was defined as ‘the length of colorectum’. The extension rate of ENCC migration per colorectum was also significantly higher in the laminin group (18.18 ± 5.97 %) compared to controls (10.25 ± 4.27 % p < 0.05) (Fig. 2b). The extension rate of the colorectum was not significantly different between controls (3.40 ± 2.39 %) and laminin group (4.27 ± 5.86 %). These results indicate that laminin-1 influences the development of the ENS but not the development of gut.
Discussion
We have investigated the role of laminin-1 in ENS development using SOX10-Venus transgenic mice to visualize ENCC migration. We identified that laminin-1 advanced the wavefront of migrating ENCC compared to the control experiments. The development of the ENS requires the normal processes of differentiation, proliferation and migration of ENCC. Although a functional role for laminin in each step is not clarified in our experiments, the development of a mesh-like nervous system was accelerated in the laminin group compared to controls.
Much of our information on the etiology and pathogenesis of HD have been obtained from analyses of ENCC development in genetically mutant mice. These experiments suggest that normal ENS formation depends on a critical balance of ENCC proliferation, differentiation and migration during all stages of ENS development. The ENCC microenvironment also plays a critical role in regulating ENS development. Several studies have found changes in the gut environment associated with aganglionosis. Laminin-1 is one of the candidate ECM molecules suspected to play a role in aganglionosis. Accumulated laminin-1 has been found in the presumptive aganglionic tissue of Edn3 ls/ls mice [7]. Some in vitro experiments have shown that laminin-1 increased the number of neurons and promoted the neurite outgrowth obtained from immunoselected ENCC, suggesting laminin-1 promotes differentiation of ENCC [13, 14]. This evidence has led to the hypothesis that laminin-1, by promoting differentiation, causes ENCC to cease migration before they complete colonization of the gut, resulting in an aganglionic colon [7, 15, 16]. Our results are consistent with the in vitro experiments showing the effect of laminin-1 promoting ENCC differentiation. However, in contrast to the hypothesis that an excess of laminin-1 ceases ENCC migration, our results showed that laminin-1 advanced the wavefront of ENCC migration. This may imply that the excess of laminin-1 in aganglionic segment is not a direct cause of aganglionosis. In fact, Druckenbrod et al. have demonstrated that laminin was increased at both the ENCC wavefront of control and mutant guts from E13.5 to E14.5 and there were no differences in laminin expression between them. However, whereas the control ENCC had reached the terminal colon, the mutant had not, suggesting that increased laminin might be a normal phenomenon from E13.5 to E14.5 [17]. We have found that laminin-1 accelerates ENCC migration during development in normal mice for 12 h from E12.5. Timing of increased laminin and its distribution and/or concentration gradient may also influence ENCC migration. It is also reported that the migratory behavior of ENCC was influenced by cell number and cell–cell contact and that the speed of ENCC migration was reduced when the population of ENCC was reduced [5]. Although the number of ENCC was not examined in our study, we speculate that laminin increased the number of ENCC, causing the increased speed of migration. Further studies are required to clarify the effects of laminin on ENCC migration.
Our results suggest that laminin promotes ENS development in mice. This technique allowed us to visualize the effects of extracellular molecules in ENS development and it potentially provides us with an insight into the pathophysiology of developmental disorder of the ENS, such as HD.
References
Sasaki T, Fässler R, Hohenester E (2004) Laminin: the crux of basement membrane assembly. J Cell Biol 29:959–963
Ichikawa N, Iwabuchi K, Kurihara H et al (2009) Binding of laminin-1 to monosialoganglioside GM1 in lipid rafts is crucial for neurite outgrowth. J Cell Sci 15:289–299
Le Douarin NM, Teillet MA (1973) The migration of neural crest cells to the wall of the digestive tract in avian embryo. J Embryol Exp Morphol 30:31–48
Okamoto E, Satani M, Kuwata K (1982) Histologic and embry-ologic studies on the innervation of the pelvic viscera in patients with Hirschsprung’s disease. Surg Gynecol Obstet 155:823–828
Young HM, Bergner AJ, Anderson RB et al (2004) Dynamics of neural crest-derived cell migration in the embryonic mouse gut. Dev Biol 270:455–473
Burns AJ, Le Douarin NM (1998) The sacral neural crest contributes neurons and glia to the post-umbilical gut: spatiotemporal analysis of the development of the enteric nervous system. Development 125:4335–4347
Payette RF, Tennyson VM, Pomeranz HD et al (1988) Accumulation of components of basal laminae: association with the failure of neural crest cells to colonize the presumptive aganglionic bowel of ls/ls mutant mice. Dev Biol 125:341–360
Parikh DH, Tam PK, Lloyd DA et al (1992) Quantitative and qualitative analysis of the extracellular matrix protein, laminin, in Hirschsprung’s disease. J Pediatr Surg 27:991–996
Li AW, Zhang WT, Wang R et al (2008) The relationship between expressions of the laminin gene and RET gene in Hirschsprung’s disease. World J Pediatr 4:135–139
Heanue TA, Pachnis V (2007) Enteric nervous system development and Hirschsprung’s disease: advances in genetic and stem cell studies. Nat Rev Neurosci 8:466–479
Shibata S, Yasuda A, Renault-Mihara F et al (2010) SOX10-Venus mice: a new tool for real-time labeling of neural crest lineage cells and oligodendrocytes. Mol Brain 3:31
Miyahara K, Kato Y, Koga H et al (2011) Visualization of enteric neural crest cell migration in SOX10 transgenic mouse gut using time-lapse fluorescence imaging. J Pediatr Surg 46:2305–2308
Pomeranz HD, Rothman TP, Chalazonitis A et al (1993) Neural crest-derived cells isolated from the gut by immunoselection develop neuronal and glial phenotypes when cultured on laminin. Dev Biol 156:341–361
Chalazonitis A, Tennyson VM, Kibbey MC et al (1997) The alpha1 subunit of laminin-1 promotes the development of neurons by interacting with LBP110 expressed by neural crest-derived cells immunoselected from the fetal mouse gut. J Neurobiol 33:118–138
Wu JJ, Chen JX, Rothman TP et al (1999) Inhibition of in vitro enteric neuronal development by endothelin-3: mediation by endothelin B receptors. Development 126:1161–1173
Jacobs-Cohen RJ, Payette RF et al (1987) Inability of neural crest cells to colonize the presumptive aganglionic bowel of ls/ls mutant mice: requirement for a permissive microenvironment. J Comp Neurol 255:425–438
Druckenbrod NR, Epstein ML (2009) Age-dependent changes in the gut environment restrict the invasion of the hindgut by enteric neural progenitors. Development 136:3195–3203
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nakazawa, N., Miyahara, K., Okawada, M. et al. Laminin-1 promotes enteric nervous system development in mouse embryo. Pediatr Surg Int 29, 1205–1208 (2013). https://doi.org/10.1007/s00383-013-3388-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00383-013-3388-3