Abstract
Recent reports attribute neurological and cerebral disorders to the accumulation of manganese (Mn) in the brain in patients receiving home parenteral nutrition (HPN). It is desirable to control the amount of Mn delivered to these patients, but a suitable method for monitoring an individual's Mn status and assessing Mn accumulation remains debatable. The aim of this study was to evaluate whether whole-blood manganese levels (WB-Mn) correlate with the accumulation of Mn in the brains of children who receive long-term HPN, using magnetic resonance imaging (MRI) of the brain. Six patients who had received HPN (duration of HPN, 18–137 months) were included in this study. The daily parenteral doses of Mn were calculated while on HPN. WB-Mn was measured and T1-weighted MRI of the brain was obtained for each patient with a 1.5-T MR imager. Twelve months after the withdrawal of Mn from HPN, measurements of WB-Mn and brain MRI were repeated in all patients except for one who was lost after initial examination. The same examinations were performed on an additional patient who had been successfully weaned off a 179 month course of HPN 20 months prior to the initial examination. The parenteral dose of Mn while receiving HPN ranged from 15.7 to 91.5 μg/kg/day. Initially, MRI showed hyperintensity in the globus pallidus in all patients and in the anterior pituitary in one patient. WB-Mn was elevated in four patients, but was in the normal range in the remaining three. Following subsequent measurements 12 months later, WB-Mn was normal in all patients and MRI hyperintensity remained in the globus pallidus in one patient. One patient was lost after the initial examinations. WB-Mn does not necessarily correlate with the accumulation of Mn in the brain. Periodic MRI should be performed in patients receiving long-term NPN to monitor for excessive Mn accumulation in the brain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Manganese (Mn) is an essential trace element and is routinely added as a supplement to parenteral preparations to meet nutritional requirements. Recently, some reports describe an occasional association between long-term parenteral nutrition increased whole-blood Mn levels (WB-Mn; references 0.8–2.5 μg/dl), and excessive Mn accumulation in the basal ganglia of the brain, which can be detected by cerebral T1-weighted magnetic resonance imaging (MRI). Some patients receiving long-term home parenteral nutrition (HPN) developed neurological abnormalities attributed to excessive Mn accumulation in the brain [5, 6, 7,10, 14, 15, 16, 17, 19, 22]. In clinical settings WB-Mn is measured; however, routine cerebral MRI studies are not really feasible. Although the WB-Mn is a popular indicator of Mn status in adult patients [21], it's accuracy is questionable, and a robust correlation between WB-Mn and brain MRI findings in children receiving long-term HPN is yet to be shown [3]. The purpose of this study was to evaluate whether WB-Mn measurements in children receiving long-term HPN are a clinically useful indicator of Mn accumulation in the brain.
Patients and methods
Between May 1979 and December 2000, three male and four female patients from our hospital received HPN. The ages of the patients ranged from 41 to 249 months (average 125 months), with HPN duration of 18–179 months (mean 93 months). The indication for HPN was intestinal failure due to massive bowel resection in five patients, functional, intestinal failure due to ileocecal volvulus in one, and Crohn's disease in one (Table 1). One of the seven patients had been successfully weaned off HPN 20 months prior to this study after 179 months of HPN (case 1). Five of the seven patients, including the one who had been weaned off HPN, were able to receive partial to full enteral nutrition and received intermittent HPN only at night. The other two patients could not take any enteral nutrition and were dependent on whole day HPN. All of them received standard doses of glucose, amino acids and lipids. Commercially available trace element products (Elemenmic; Ajinomoto pharma, Tokyo, Japan) were added from 1992; one ampule of 2 ml contains 1099 μg of Mn, 3932 μg of zinc, 317 μg of copper, 1269 μg of iodine and 1995 μg of iron. These elements were added to HPN according to the patient's weight: 0.5 ml per day if the weight was less than 10 kg, 1.0 ml per day if the weight was more than 10 kg and less than 20 kg, 1.5 ml per day if the weight was more than 20 kg and less than 30 kg, and 2.0 ml per day if the weight was more than 30 kg. The daily parenteral doses of Mn for age were calculated while on HPN. Neurological evaluation, cerebral T1-weighted MRI and measurements of WB-Mn were performed initially in the six patients still receiving HPN (first study). Whole-blood Mn assays were performed by SRL (Special Reference Laboratories, Niigata, Japan) using graphite furnace atomic absorption spectroscopy. T1-weighted MRI was obtained in axial planes in all patients with a 1.5-T MR imager: slice thickness was 6 mm with an interslice gap of 1 mm, TR 490 ms, TE 9 ms, number of excitations (NEX) 2.0. Sagittal T1-weighted images with 3 mm thickness (TR 360 ms, TE 9 ms, NEX 2.0) were also acquired in six patients. After the initial examination, Mn was withdrawn from the HPN formulation. Twelve months later, brain MRI and the measurements of WB-Mn were repeated in five patients (second study). One patient was lost because of unrelated disease (Case 3) after the initial examination. The same examinations were performed at 20 months and 32 months after cessation of HPN (duration 179 months) in one patient (Case 1). Cholestasis was defined as a total plasma bilirubin (references 0.3–1.1 mg/dl) greater than 1.2 mg/dl at any time while on HPN. This study was reviewed by the appropriate ethics committee and was performed in accordance with the ethical standards laid down in the appropriate version of the 1964 Declaration of Helsinki. All persons gave their informed consent prior to their inclusion in this study.
Results
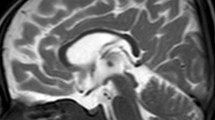
The results are summarized in Table 2. Calculated parenteral doses of Mn during HPN treatment ranged from 15.7 to 91.5 μg/kg/day. The initial MRI study showed symmetrical hyperintensity in the globus pallidus in all patients and in the anterior pituitary in one. (Figs. 1A and 2A,B). WB-Mn was elevated in four patients (3.1–5.1 μg/dl), while still within the normal range in the other three (1.8–2.4 μg/dl). The latter group contained one patient who had been successfully weaned off HPN (case 1) and two patients who were totally dependent on whole day HPN (cases 2 and 3). The 12 month repeat examinations showed that hyperintensity of the globus pallidus had disappeared in five patients, and remained in one case (case 1, Figs. 1B and 2C,D), in which hyperintensity of the anterior pituitary had disappeared. WB-Mn was normal in the surviving six patients. None of the patients had clinical neurological abnormalities even though initially they all had MRI abnormalities. Dermatitis that was strongly suspected to be due to zinc deficiency was observed in one patient (case 5) after withdrawal of Mn from HPN, although the plasma zinc level was normal. The dermatitis improved after administration of zinc. No patients displayed severe cholestasis.
T1 weighted MRI findings of the first MRI study in case 1. A, B High intensity signal was observed in the globus pallidus and anterior pituitary at the beginning of this study. C, D The abnormal signal intensity in the anterior pituitary had disappeared 12 months after parenteral manganese supplementation was withdrawn whereas that of globus pallidus remained
Discussion
Mn is an essential trace element for humans [1]. It functions as a component of the enzymes pyruvate carboxylase and the mitochondrial form of superoxide dismutase. Mn supports the mucopolysaccharide production and the formation of normal bone, cartilage, and connective tissue [8]. When Mn is orally ingested regulatory mechanisms usually prevent excessive absorption or retention [8]. When administered parenterally, these regulatory mechanisms are bypassed, allowing for excessive absorption and tissue deposition of Mn.
Recently, neurological and cerebral disorders related to Mn accumulation have been reported in patients receiving HPN [5, 6, 7, 10, 14, 15, 16, 17, 19, 22]. MRI demonstrates specific patterns of intensity associated with Mn accumulation in exposed individuals [14]. As routine MRI study is not clinically feasible, Mn status is usually monitored in patients receiving long-term HPN by analyzing blood samples. However, the appropriate methods for assessing Mn stores in humans are still debated [3]. Although WB-Mn is supposed to increase with excessive accumulation of Mn, three of the seven patients showed normal WB-Mn despite evidence of Mn accumulation on brain MRI. We speculated that this inconsistency might be due to the characteristics of WB-Mn [3] and individual variations in Mn metabolism [5, 18, 22]. Mn is incorporated in erythrocytes with porphyrin and its clearance relates to the lifespan of the erythrocyte [22]. WB-Mn does not correlate with the degree of cholestasis, amount of parenteral Mn intake, or duration of HPN [22]. In addition, WB-Mn is not a homogenous entity and it is affected by intravenous delivery of Mn [9]. Accordingly, the pattern of HPN (i.e. intermittent HPN vs. continuous whole-day HPN) may have affected WB-Mn in our series. Meanwhile, changes in the distribution of Mn between tissues and blood can occur in patients receiving HPN and result in individual variation in response to parenterally administered Mn. Though tissue Mn levels were not measured, we speculate that such changes occurred in our three patients who showed normal WB-Mn, and that the body distribution of Mn was modified while on long-term HPN [18, 22]. Recent studies suggest that the Mn content in red blood cells [20] and mononuclear blood cells [12], as well as the activity of Mn superoxide dismutase and a mitochondrial antioxidant enzyme [4, 11], are better indicators of Mn nutritional status than WB-Mn. These indicators are stable and not directly influenced by current intravenous supplementation. However, these indicators are not always clinically available and sometimes require access to expensive instrumentation. It was interesting that the two patients who showed normal WB-Mn received whole-day HPN and could not take any enteral nutrition. It is therefore possible that the patients with elevated WB-Mn were receiving a higher amount of Mn, via their combined oral and parenteral route, than the two patients with normal WB-Mn. The major route of execretion of Mn from the body is via bile, and the cholestasis caused by HPN promotes Mn accumulation. In our series, no patients showed severe cholestasis. In the two patients who could not take any enteral nutrition and who showed normal WB-Mn, the enterohepatic circulation was expected to be exhausted. The effect of this exhausted enterohepatic circulation on Mn status is uncertain.
The T1-weighted MRI findings of our series resembled those found in cases reported by Ejima and Imamura [5] and Mirowitz and Westrich [14]. The site of the earliest and most pronounced deposition of Mn is the globus pallidus. With prolonged parenteral nutrition, subsequent deposition will be observed in the caudate nucleus and putamen [14]. The deposition in the anterior pituitary is less constantly observed. For case 1 of our series, hyperintensity in the globus pallidus and anterior pituitary remained 20 months after cessation of HPN that had lasted 179 months. In the brain, Mn accumulation disappears very slowly from the mitochondria even after discontinuation of parenteral Mn supplements [14].
Mn accumulation does not necessarily correlate with degree of clinically apparent neurological abnormality [13] and fortunately no neurological abnormalities were detected in our patients despite obvious MRI changes. Periodic brain MRI examination will be necessary to monitor for excess Mn accumulation in the brain of children receiving HPN.
Appropriate parenteral doses of Mn were recommended by the ASPN [2] in 1997. Parenteral doses of Mn in our patients exceeded this recommendation. The AMA recommended against the fixed use of commercially available trace element products [1] because of the risk of over-dosage of some trace elements when the demand for other elements is increased [8]. On the other hand, relative zinc deficiency can occur after discontinuition of parenteral Mn supplementation, as happend in our case. The required daily parenteral dose of Mn is still controversial and needs further research. Further commercial product development for children is also required.
In conclusion, WB-Mn does not accurately reflect the accumulation of Mn in the brain of children receiving long-term HPN. Occasional MRI studies are recommended to monitor for excessive Mn accumulation in the brain. Further studies are necessary to evaluate the correlation between Mn accumulation in the brain and the Mn content in the red blood cells and mononuclear blood cells, as well as the activity of Mn superoxide dismutase and mitochondrial antioxidant enzymes.
References
American Medical Association, Department of Foods and Nutrition (1979) Guidelines for essential trace element preparations for parenteral use. JAMA 241:2051–2054
A.S.P.N. Board of Directors (1998) Safe practices for parenteral nutrition formulations. JPEN J Parenter Enteral Nutr 22:49–66
Bertinet DB, Tinivella M (2000) Brain manganese deposition and blood levels in patients undergoing home parenteral nutrition. JPEN J Parenter Enteral Nutr 24:223–227
Davis CD, Greger JL (1992) Longitudinal changes in women of manganese superoxide dismutase and other indices of manganese and iron status with diet. Am J Clin Nutr 55:747–752
Ejima A, Imamura T (1992) Manganese intoxication during TPN. Lancet 339:426
Fell JME, Reynolds AP (1996) Manganese toxicity in children receiving long-term parenteral nutrition. Lancet 347:1218–1221
Fitzgerald K, Mikalunas V (1999) Hypermanganesemia receiving total parenteral nutrition. JPEN J Parenter Enteral Nutr 23:333–336
Greene HL, Hambidge KM (1998) Guidelines for the use of vitamins, trace elements, calcium, magnesium, and phosphorus in infants and children receiving TPN: report of the subcommittee on clinical Pediatric Parenteral Nutrient requirements from the Committee on Clinical Practice Issues of The American Society for Clinical Nutrition. Am J Clin Nutr 48:1324–1342
Keen CL, Clegg MS (1983) Whole blood manganese as an indicator of blood manganese. N Engl J Med 308:1230
Kouhichirou O, Ito J (1998) Reversible hyperintensity of the anterior pituitary gland on T1-weighted MR images in patients receiving temporary parenteral nutirition. AJNR Am J Neuroradiol 19:1287–1289
Malecki EA, Lo H-C (1995) Tissue manganese concentrations and antioxidant enzyme activities in rat given total parenteral nutrition with and without supplemented manganese. JPEN J Parenter Enteral Nutr 19:222–226
Masuda A, Mimura M (1994) Changes in manganese content of mononuclear blood cells in patients receiving total parenteral nutrition. Clin Chem 40:829–832
Masumoto K, Suita S (2001) Manganese intoxication during intermittent parenteral nutrition: report of two cases. JPEN J Parenter Enteral Nutr 25:95–99
Mirowitz SA, Westrich TJ (1991) Hyperintense basal ganglia on T1-weighted MR imagings in patients receiving long term parenteral nutrition. Radiology 181:117–120
Misselwitz B, Muhler A (1995) A toxicologic risk for using manganese complexes? A literature survey of existing data through several medical specialities. Invest Radiol 30:611–620
Ono J, Harada K (1995) Manganese deposition in the brain during long-term total parenteral nutrition. JPEN J Parenter Enteral Nutr 19:310–312
Quaghebeur G, Taylor WJ (1996) MRI in children receiving total parenteral nutirition. Neuroradiology 38:680–683
Reimund JM, Dietemann JL (2000) Factors associated with hypermanganesemia in patients receiving home parenteral nutrition. Clin Nutr 19:343–348
Reynolds AP, Kiely E (1994) Manganese deposition in long term parenteral nutrition. Arch Dis Child 71:527–528
Reynolds N, Blumsohn A (1998) Manganese requirement and toxicity in patients on home parenteral nutrition. Clin Nutr 17:227–230
Takagi Y, Okada A (2001) On-off study of manganese administration to adult patients undergoing home parenteral nutrition: new indicates of in vivo manganese level. JPEN J Parenter Enteral Nutr 25:87–92
Wardle CA, Forbes A(1999) Hypermanganesemia in long-term intravenous nutrition and chronic liver disease. JPEN J Parenter Enteral Nutr 23:350–355
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Iinuma, Y., Kubota, M., Uchiyama, M. et al. Whole-blood manganese levels and brain manganese accumulation in children receiving long-term home parenteral nutrition. Ped Surgery Int 19, 268–272 (2003). https://doi.org/10.1007/s00383-002-0929-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00383-002-0929-6