Abstract
Introduction
Our aim in this study was to analyze the findings of brain magnetic resonance imaging (MRI) and magnetic resonance spectroscopy (MRS) of children with vitamin B12 deficiency.
Methods
This study included 14 cases. The findings of brain MRI and MRS in all cases were investigated. Four patients had been followed up and mean follow-up time 71.8 (59–85) day.
Results
Eight patients of the cases (57 %) had at least one abnormal MRI finding. The most commonly found MRI findings were thinning of the corpus callosum and brain atrophy, respectively. The mean ratio of NAA/Cr and Cho/Cr were measured in MRS, with values of 1.31 ± 0.17 and 1.04 ± 0.27, respectively. In two of three patients with abnormal MRI studies at presentation, subsequent MRI showed improvement while one patient remained unchanged. An increase in the ratios of metabolites were found in one case with control MRS. There was no lactate peak.
Conclusion
Brain MRI was abnormal in more than half of the cases of children with vitamin B12 deficiency. Our radiologic findings similar with literature. There was no identifiable lactate peak. B12 deficiency could be the cause of the thinning of the corpus callosum and brain atrophy in the children that were given a brain MRI.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Vitamin B12 is a vitamin that plays a role in different biological processes in humans. It contributes to myelination by playing a role in methionine synthesis from homocysteine in infants [1, 2]. It has been stated that vitamin B12 deficiency in infants is due mostly to nutritional factors [3, 4]. Honzik et al. pointed out that mothers with subclinical chronic gastrointestinal disease pose one of the highest risks due to low levels of vitamin B12 in their milk [5]. Nutritional B12 deficiency is very common in our region and it can reach upwards of 40 % in asymptomatic infants [6]. While development of damage in the brain can take months of deficiency in adults, it can cause severe disorders when it is lacking for only a few weeks in the first years of life when brain development is fast [5]. Vitamin B12 deficiency in adults may present as megaloblastic anemia, polyneuropathy, subacute combined neurodegeneration of the spinal cord, dementia or depression [5]. Vitamin B12 deficiency during childhood can cause disorders besides megaloblastic anemia such as retardation in development of physical and social neuromotor skills, apathy, and irritability [7]. After therapy, recovery can be variable, with some children remaining moderately or severely retarded [1]. Late diagnosis may result in irreversible psychomotor retardation [8]. In this area, neuroradiological findings are limited. Determined common radiological findings in the studies that are mostly case reports have been cerebral atrophy and thinning corpus callosum [3, 4].
Magnetic resonance spectroscopy (MRS) is a method that noninvasively measures metabolites such as N-acetylaspartate (NAA), choline (Cho), creatine (Cr), and lactate in selected regions of the brain [9]. The various investigators have used MRS to evaluate changes in cerebral metabolism during normal brain maturation and white matter myelination, which may be useful for assessment of developmental delay (DD); neurodegenerative, inflammatory, metabolic, and neuropsychiatric disorders; phakomatosis; hypoxic–ischemic brain injury; and epilepsy [10]. Although brain magnetic resonance imaging (MRI) findings of vitamin B12 deficiency have been identified [4], MRS studies made on this subject are very limited [11].
In this study, our aim is to present brain MRI and MRS results in patients who received an MRI because of DD and in patients who were diagnosed with B12 vitamin deficiency; also, to present changes of abnormal radiologic results in patients who were treated successfully.
Materials and Methods
Case Selection
Brain MR images of 471 consecutive cases diagnosed with DD by testing and examination made in the polyclinic of pediatric neurology between November 2009 and December 2011 were reviewed. We noticed that some of these cases had low levels of serum vitamin B12 when we were investigating the cause of DD in these cases. The cases that had MRI and MRS images were included into the study. The patients who had vitamin B12 deficiency due to intrinsic factor deficiency, gastric or distal ileal surgical interventions, and Immersland-Grasbeck syndrome were excluded by the absence of plasma intrinsic factor level and the urinary protein. Also, infants that suffered iron deficiency, congenital, metabolic, and chronic disease, were excluded from the study, respectively. MRI and MRS from a total of 14 cases who met the requirements of the study were reinvestigated. Four of these cases had control MRI and one of them had an MRS. Laboratory investigations included vitamin B12 level and the whole blood count of patients. A diagnosis of vitamin B12 deficiency was made for serum levels below 200 pg/ml [4]. The institutional review board approved this study.
MR Protocol
Brain MRI and MRS were performed on all patients with a standard head coil and 3.0-T MRI system (Achieva; Philips Medical Systems, Best, The Netherlands). In brain MRI investigations axial turbo spin-echo (TSE) T2-weighted repetition time/echo time (TR/TE, 2500/80; slice thickness, 4 mm; interslice gap, 1 mm; matrix, 400 × 255) and FLAIR images (TR/TE/TI, 10,000/125/2800 ms slice thickness, 4 mm; interslice gap, 1 mm; matrix, 352 × 198), coronal TSE T2-weighted images (TR/TE, 2624/125; slice thickness, 3 mm; interslice gap, 0 mm; matrix, 336 × 210), sagittal T1-weighted volume data set (3D Turbo Field Echo sequence TR/TE, 8.1/3.7; IT, 1006 ms; slice thickness, 1 mm; interslice gap, 0 mm; matrix, 240 × 240) were used. Single voxel MRS was performed on all patients. Three orthogonal baseline images were obtained with automated magnetic field shimming. Single voxel MRS was performed using the PRESS technique (TR 2000 ms, TE 144 ms, Flip angle 90º-180º−180º, averages 256, acquisition time 4:52). The voxels were placed in the left basal ganglia and the data set was obtained in each case using 20 × 20 × 20 mm voxels. After optimal water signal suppression with chemical shift-selective techniques, MRS data were collected. The total scanning time was about 18 min per patient. MRI and MRS were performed on all cases following sedation with oral chloral hydrate 50–100 mg/kg.
Analysis of MR Imaging and MR Spectroscopy
All examinations were evaluated by the same workstation (Philips Extended Brilliance Workspace, Philips Medical Systems, Best, The Netherlands). The brain MRI and MRS of the patients were re-evaluated by the radiologist (F. Ekici) who had 15 years of experience for general radiology and 4 years for neuroradiology. The thickness of the corpus callosum was evaluated by measuring the smallest thickness at the level of the anterior third of the body; the corpus callosum was considered atrophic when this measurement was less than 5 mm [12].
The myelination situations of the cases were evaluated by the myelination steps published by Barkovich [13]. The prevalence of MRI abnormality was investigated. Abnormal findings were determined in every case recorded.
Results
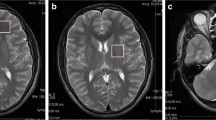
The study group consisted of 14 cases (10 boys and 4 girls; mean age: 11.8 months; range: 2–25 months). Eight of these cases (57.1 %) found at least one abnormal MRI finding. Brain MRI findings included thinning of the corpus callosum in seven (50 %; Fig. 1), atrophy in six (42.8 %; Fig. 2), delayed myelination in two (14.3 %; Fig. 3) and large sylvian fissures in two (14.3 %) patients.
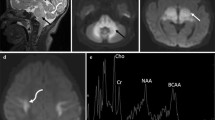
In MRS investigations, the ratios of NAA/Cr and Cho/Cr in 14 cases were found to be 1.31 ± 0.17 and 1.04 ± 0.27, respectively (Fig. 4). Lactate peak was not found in any of the cases.
At follow-up treatment, four of our patients had only brain MRI and one of the patients had brain MRS. Control MRIs were performed after an average of 72 days (59–90 days). While no changes in findings were determined in one case, a decrease in the subarachnoid distance in another case and an increase in the thickness of the corpus callosum in a separate case (Fig. 5) were found. Before and after treatment MRI of the last case were normal. After treatment, increases in the ratios of NAA/Cr and Cho/Cr were determined in the case who had control MRS investigations (ratios of before and after treatments: 1.01, 0.85, and 1.39, 0.96, respectively; Fig. 6).
Pre-treatment magnetic resonance (MR) spectroscopy performed left basal ganglia that shows brain atrophy on MR imaging and on MR-spectroscopy the NAA/Cr ratio: 1.01, the Cho/Cr ratio: 0.85 (a). Post-treatment MR spectroscopy image shows the increased NAA/Cr and Cho/Cr ratios in the same area (1.39 and 0.96, respectively) and decreased atrophy (b)
In the study group, the mean serum vitamin B12 level was 103.1 ± 47.3 (20–186) pg/ml. Mean hemoglobin and mean corpuscular volume levels were 11.1 ± 2.4 g/dL and 95.1 ± 13.8 fL, respectively. Detailed MRI, MRS findings and laboratory values for all cases are shown in Table 1.
Discussion
Vitamin B12 is a vitamin which is required for the development of the nervous system. However, the role it plays in development of the nervous system is still not fully understood [1, 14]. Vitamin B12 is known to play a role as a cofactor in the re-methylation of homocysteine and methyl malonyl CoA degradation. Vitamin B12 deficiency is thought to cause disturbance of methionine synthesis and accumulation of guanidoacetate, leading to neurotoxicity. Increased levels of homocysteine and methylmalonic acid are also a result of vitamin B12 deficiency. These may lead to demyelination, axonal degeneration, and neuronal death [2, 15]. According to Dror and Allen [16], another possible mechanism of neurologic symptoms in B12 deficiency is neurotrophic and neurotoxic cytokine imbalance, and accumulation of lactate in brain cells. Eventually, in infants, Vitamin B12 deficiency may lead to demyelination and brain atrophy [4, 5].
Even though clinical signs of vitamin B12 deficiency have been described half a century ago [5], studies on radiological findings are limited [4, 8, 11, 14]. Determined common radiological findings in the studies which are mostly case reposts has been cerebral atrophy. Taskesen et al., in their recently published study, determined abnormal MRI in 10 (67 %) of 15 infants with vitamin B12 deficiency, and the most often determined findings were thinning of the corpus callosum and brain atrophy [4]. Lövblad et al. [14] found MRI of the brain revealed severe brain atrophy with signs of retarded myelination, the frontal and temporal lobes being most severely affected in their case report. Radiological abnormality was found in 57 % of the cases in our study. Thinning of the corpus callosum, brain atrophy, and delayed myelination were the most determined MRI findings in our study. Our findings are consistent with these studies. Another study, Lam et al. [17] found small pons, partial vermian dysgenesis, abnormal signal in both basal ganglia with MRI in one patient. We did not see these findings in our patients.
Neuroradiological studies are used to determine the response to therapy in addition to investigate the changes occurred in the brain in the cases of detected vitamin B12 deficiency [14, 18]. It was reported in these studies in the form of case reports that the abnormalities detected with brain MRI disappeared after treatment. The effectiveness of the treatment response was linked to the age of the case, which was less than 10 months [8, 15]. According to Honzik et al., cumulative vitamin B12 deficiency is more likely to determine the outcome than the age of presentation alone [5]. While decline in the brain MRI findings were detected as response to the therapy in two of four cases that had control MRI in our study, no changes were detected in one of the cases. Before and after treatment brain MRI of the last case was normal. The ages of the cases who responded to therapy were 9 and 18 months while the age of the case who did not respond to therapy was 9 months. Our findings support the idea of Honzik et al. [5] for response to treatment.
MR spectroscopy is a noninvasive technique that has gained wide clinical use and has been found beneficial in the assessment of neuronal function and metabolite incompatibilities in the brain [10]. The PRESS sequence with TE performed in all cases had a maximum signal/noise ratio. We were specifically interested in assessment of NAA/Cr and Cho/Cr ratios. NAA, which is present in neuronal cells, is considered to be a marker for neuronal development. Therefore, it was identified as a neuronal determinant. Neuronal damage or loss will result in decreased NAA levels [9]. Cho, which is a membrane component, has been used to infer information about myelination and cell membrane turnover. In infants, the Cho/Cr ratio is higher than that of adults. The Cho/Cr ratio declines rapidly from age 1 month to 1 year, then declines more slowly until an age of 2 years [18]. Because choline is present in the structure of myelin-associated macromolecules, increased myelination leads to a decrease in Cho/Cr ratio [10]. The ratios of NAA/Cr and Cho/Cr were found to be 1.31 ± 0.18 and 1.05 ± 0.29 in our study. Our study is a retrospective study with no control group. The changes to be determined in Cho peak used to evaluate myelination may be helpful in clarifying the pathogenesis of abnormalities occurring in vitamin B12 deficiency. The increases in the ratio of NAA/Cr after treatment in a case with control MRS in our study group may be physiological changes or it can also support the increased number of neurons as response to therapy. Increases in the ratio of Cho/Cr may support the acceleration in myelination. Nevertheless, it is required that these results should be shown in more number of patients.
Kreis et al. [19] researched occipital gray matters’ metabolites changes with short TE. In this study, Cho peak is dominant in the neonatal period, while the NAA peak gets more prominent and becomes dominant between 0–36 months of age period. Conversely, during this time significant decrease had been found in Cho peak. In our study, the metabolites ratio changes were similar with Kreis et al. research. However, metabolite ratios can show extreme variability according to selected brain regions and the pulse sequence [19].
The MRS study is limited in cases with vitamin B12 deficiency, therefore we could only find two studies in the literature [11, 17]. In one case study of a 6-month-old infant with nutritional vitamin B12 deficiency, MRS of the white and gray matter of the brain revealed an accumulation of lactate and a sustained depletion of choline-containing compounds, reflecting a disturbance of oxidative energy metabolism in brain cells. This could be due to an increase in anaerobic glycolysis. In our recent study, differently, voxel was located at the basal ganglia in all cases. Pathological signal changes in the basal ganglia with MRI were not determined in any of our cases. Although increased diffuse signal was found around periventricular white matter in one case, abnormal lactate peak was not determined with MRS investigation in any of the cases. This finding does not support the study of Horstmann et al. [11]. In our patients, due to absence of a cystic cavity lesions, lactate peak may not be detected. Lam WWM et al. reported that decreased concentration of NAA in MRS [17].
There are some important limitations of our study. The most important one is the low number of cases due to the retrospective nature of our study and the lack of control group. Therefore, we could not show objectively whether there were changes in the ratio of metabolites obtained by MRS. The other limitation is that the single voxel spectroscopy was made in a single region (left basal ganglia). Therefore, MRS evaluation was not done for the other regions. The other limitation was to use the sequences with long TE value for all cases. Thus, changes in minor metabolites could not be investigated.
As a result, at least one abnormal brain MRI finding was determined in more than half of the cases with a deficiency of vitamin B12 which was required for the development of the central nervous system in children. Brain MRI thinning of the corpus callosum and brain atrophy should alert the radiologist for Vitamin B12 deficiency in patients with DD. This is important for accurate and effective to start treatment. Observed increased on the rate of Cho/Cr after the treatment showed that treatment may accelerate the myelination. After treatment, radiological response was usually positive. In MRS, a lactate peak was not found in the study group. Prospective studies which have more cases and have before and after therapy MRS investigations are needed in order to use MRS as a neuroimaging indicator to follow-up the response numerically for the therapy and diagnosis for the cases with vitamin B12 deficiency. And further MRS studies with large numbers of subjects and of different brain regions are needed to determine the brain metabolite ratios and to detect whether metabolite differences are present in children with vitamin B12 deficiency.
References
Black MM. Effects of vitamin B12 and folate deficiency on brain development in children. Food Nutr Bull. 2008;29(Suppl 2):S126–31.
Emadfa I, Singer I. Vitamin B-12 and homocysteine status among vegetarians: a global perspective. Am J Clin Nutr. 2009;89(Suppl):1693S–8S.
Korenke GC, Hunneman DH, Eber S, Hanefeld F. Severe encephalopathy with epilepsy in an infant caused by subclinical maternal pernicious anaemia: case report and review of the literature. Eur J Pediatr. 2007;163:196–201.
Taşkesen M, Yaramış A, Pirinçcioglu AG, Ekici F. Cranial magnetic resonance imaging findings of nutritional B(12) deficiency in 15 hypotonic infants. Eur J Paediatr Neurol. 2011;16:266–70.
Honzik T, Adamovicova M, Smolka V, Magner M, Hruba E, Zeman J. Clinical presentation and metabolic consequences in 40 breastfed infants with nutritional vitamin B12 deficiency—what have we learned? Eur J Paediatr Neurol. 2010;14:488–95.
Ertaş T, Koç A, Koçyiğit A, Baz T. Bebeklerin nöro-motor, sosyal ve fiziksel gelişmeleri üzerine B12 vitamini eksikliğinin etkileri. Çocuk Sağlığı ve Hastalıkları Derg. 2011;54:62–9.
Katar S, Nuri Ozbek M, Yaramis A, Ecer S. Nutritional megaloblastic anemia in young Turkish children is associated with Vitamin B-12 deficiency and psychomotor retardation. J Pediatr Hematol Oncol. 2006;28:559–62.
Von Schenck U, Gotze CB, Koletzko B. Persistence of neurological damage induced by dietary vitamin B12 deficiency in infancy. Arch Dis Child. 1997;77:137–9.
Hajek M, Dezortova M. Introduction to clinical in vivo MR spectroscopy. Eur J Radiol. 2008;67:185–93.
Kosucu P, Erdemli S, Sonmez M, Kul S, Aksoy A. MR spectroscopic evaluation of psychomotor delay of unknown cause in children. AJR Am J Roentgenol. 2010;94:1110–5.
Horstmann M, Neumaier-Probst E, Lukacs Z, Steinfeld R, Ullrich K, Kohlschutter A. Infantile cobalamin deficiency with cerebral lactate accumulation and sustained choline depletion. Neuropediatrics. 2003;34:261–4.
Barkovich AJ, Kjos BO. Normal postnatal development of the corpus callosum as demonstrated by MR imaging. AJNR Am J Neuroradiol. 1998;9:487–91.
Barkovich AJ. Normal development of the neonatal and infant brain, skull and spine. In:Barkovich AJ, editor. Pediatric neuroimaging, 4th ed. Philadelphia: Lippincott Williams and Wilkins; 2005. pp. 17–75.
Lövblad K, Ramelli G, Remonda L, Nirkko AC, Ozdoba C, Schroth G. Retardation of myelination due to dietary vitamin B12 deficiency: cranial MRI findings. Pediatr Radiol. 1997;27:155–8.
Graham SM, Arvela OM, Wise GA. Long-term neurological consequences of nutritional VB12 deficiency in infants. J Pediatr. 1992;121:710–4.
Dror DK, Allen LH. Effect of vitamin B12 deficiency on neurodevelopment in infants: current knowledge and possible mechanisms. Nutr Rev. 2008;66:250–5.
Lam WW, Wang ZJ, Zhao H, Berry GT, Kaplan P, Gibson J, Kaplan BS, Bilaniuk LT, Hunter JV, Haselgrove JC, Zimmermann RA. 1H MR spectroscopy of the basal ganglia in childhood: a semiquantitative analysis. Neuroradiology. 1998;40:315–23.
Filippi CG, Ulug AM, Deck MD, Zimmerman RD, Heier LA. Developmental delay in children: assessment with proton MR spectroscopy. AJNR Am J Neuroradiol. 2002;23:882–8.
Kreis R, Ernst T, Ross BD. Development of the human brain: in vivo quantification of metabolite and water content with proton magnetic resonance spectroscopy. Magn Reson Med. 1993;30:424–37.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ekici, F., Tekbas, G., Hattapoğlu, S. et al. Brain MRI and MR Spectroscopy Findings in Children with Nutritional Vitamin B12 Deficiency. Clin Neuroradiol 26, 215–220 (2016). https://doi.org/10.1007/s00062-014-0351-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00062-014-0351-1