Abstract
Background
Hydrocephalus is diagnosed when an accumulating amount of cerebrospinal fluid (CSF) fails to circulate and/or absorbed in the ventricular system. Based on its etiology, hydrocephalus can be classified into infectious and non-infectious hydrocephalus. In children, non-infectious hydrocephalus includes congenital hydrocephalus, posthemorrhagic hydrocephalus, neural tube defect–related hydrocephalus, and tumor-related hydrocephalus. Regardless of the cause, a CSF diversion device is placed to divert the excess fluid from the ventricles into peritoneal cavity. Among all, ventriculoperitoneal (VP) shunt is arguably the most commonly used CSF diversion device to date. Until now, the long-term neurodevelopmental impact of VP shunt placement in non-infectious hydrocephalus patients remained unclear.
Objective
This study aims to evaluate the neurodevelopmental outcomes in children with non-infectious hydrocephalus who had VP shunt placement.
Materials and methods
Systematic searches were performed using PubMed, Google Scholar, Scopus databases, and reference lists. Publications that fulfilled the inclusion criteria were included in the meta-analysis. Calculation of Mantel-Haezel risk ratio (RR) was applied, and heterogeneity index (I2) test was used to evaluate the existence of heterogeneity in all studies. Risk of bias was assessed based on the criteria from the Newcastle-Ottawa Scale (NOS).
Results
Of the 1929 studies identified, 12 publications were concluded to have fulfilled the inclusion criteria. Results from the meta-analysis showed that the risks of cerebral palsy, visual and hearing impairment, epilepsy, or seizures are significantly higher in children with non-infectious hydrocephalus who already had VP shunt placement (shunted non-infectious hydrocephalus, S-NIH) compared to that of the healthy control. The meta-analysis on intelligent quotient (IQ) and mental development index (MDI) showed that S-NIH children tend to score lower IQ and acquire risk of having mental development delay. On motoric development, S-NIH children scored lower motoric score and have significantly higher risk of motor development delay compared to control. Although normal children tend to have more internalizing behavior compared to S-NIH children, overall assessment on the risk of behavioral abnormalities showed that the differences between these two groups are insignificant.
Conclusion
S-NIH children have significantly higher risks of disabilities and mental and motoric development delays; thus, planning on continuous rehabilitation for children with non-infectious hydrocephalus who already had placement of VP shunt is important to acquire their optimum potentials and quality of life.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hydrocephalus is characterized by excessive accumulation of cerebrospinal fluid (CSF) in the ventricles that might be caused by CSF overproduction, obstruction of the ventricular structure(s), or impaired CSF absorption [1]. This abnormality has been regarded as one of the most common conditions presented at any neurosurgical practice. The current study estimated that annually, nearly 400,000 new pediatric hydrocephalus cases will develop globally [2]. In addition to the commonly known classifications, such as communicating/non-communicating and obstructive/non-obstructive [1, 3], based on its etiology, it is logical to classify hydrocephalus into infectious and non-infectious hydrocephalus. As the term implies, infectious hydrocephalus is caused by infection-related etiology, such as bacterial or serous meningitis and abscess. Meanwhile, hydrocephalus that is caused by non-infection origin can be classified as non-infectious hydrocephalus. Based on this classification, non-infectious hydrocephalus in children includes congenital hydrocephalus, posthemorrhagic hydrocephalus, neural tube defect–related hydrocephalus, and tumor-related hydrocephalus.
If untreated, the head circumference of the affected children will grow abnormally as the ventricles continue to enlarge. This prevents the brain cortex to grow, causing dysgenesis of corpus callosum, and eroding periventricular white matter [4, 5]. Thus, it seemed logical that the only way to treat this abnormality is by diverting the CSF. Of all CSF diversion techniques, ventriculoperitoneal (VP) shunt is arguably the most common treatment for hydrocephalus patients. In comparison to other techniques, such as ventriculoatrial (VA) shunt and endoscopic third ventriculostomy (ETV), VP shunt placement offers a relatively easier application, as it neither needs channeling to structures that are considerably more fragile like the atrium nor needs any sophisticated endoscopic instrumentation [6].
To certain extents, CSF diversion by ventriculoperitoneal shunt seemed to have worked as expected. This can be concluded when CSF accumulation in the ventricles subsides, and in the case of very young children, the growth rates of head circumference decline [7]. However, the answer to the ever-important question of whether this treatment does actually resolve the main problem of non-infectious hydrocephalus can only be addressed by evaluating the long-term outcomes in children with non-infectious hydrocephalus who already underwent shunt placement surgery. If the only problem of non-infectious hydrocephalus is the progressive accumulation of CSF, once CSF is efficiently diverted, then logically, patients could be expected to acquire optimum long-term outcomes. Outcomes that are far from ideal indicate possible existences of other problem(s) apart from CSF overaccumulation. In cases of infectious hydrocephalus, the infection characteristics, such as the types of virus or bacteria, and the general conditions of the patients could be suspected as the possible causes of undesirable outcomes. In other types of non-infectious hydrocephalus, particularly in non-syndromic cases, other confounding factors are unclear. Any efforts to broaden our understanding on the pathophysiology of non-infectious hydrocephalus and improving its current management should be based on the consensual view of the long-term neurodevelopmental outcomes in treated non-infectious hydrocephalus patients. Hence, this study aims to investigate the neurodevelopmental outcomes in patients with non-infectious hydrocephalus who had ventriculoperitoneal shunt placement surgery by meta-analysis. Since in tumor-related hydrocephalus, patients’ outcomes rely heavily on several other factors than the success of CSF diversion by the VP shunt itself, including tumor characteristics, success of tumor removal and any additional adjunct therapy(-ies), patients with tumor-related hydrocephalus were not included in this study.
Methods
Literature search and identification
This systematic review and meta-analysis were conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) reporting guidelines [8]. Searches on PubMed, Scopus, and Google Scholars’ databases were performed to collect publications up to May 31, 2020, by using the following terms: hydrocephalus OR congenital hydrocephalus OR fetal hydrocephalus OR neonatal hydrocephalus OR post-hemorrhagic hydrocephalus OR spina bifida hydrocephalus OR neural tube defect-related hydrocephalus OR non-infectious hydrocephalus AND VP shunt AND neurodevelopmental OR long term outcome.
Inclusion and exclusion criteria
We specified the criteria for inclusion of studies in meta-analysis which are as follows: (1) reported neurodevelopmental outcome in children with non-infectious hydrocephalus, (2) reported surgical treatment, (3) reported control from children without CNS anomalies with the same baseline condition (i.e., very preterm birth). Studies were excluded if (1) information were insufficient, particularly in relation to the subjects and parameters of the study; (2) it did not included original data, such as reviews, systematic reviews, comments, or editorial letters; (3) it did not include control group (e.g., case reports, case series); (4) it only included patients with infection-related hydrocephalus and/or patients with tumor-related hydrocephalus; and (5) it was not written in English.
Data collection and analysis
The titles and abstracts were independently reviewed by 3 authors (MS, DH, JKA). If they met the inclusion criteria, the full-text article was then thoroughly reviewed. Reference lists on the identified publications were checked for previously unidentified but relevant studies. The data on following items were retrieved: author, country, publication year, number of patients, control group, cause of hydrocephalus, history of VP shunt placement, age at assessment, and outcomes.
Data synthesis
The data were retrieved from a group of children aged 0–18 years with hydrocephalus that was detected during prenatal period or anytime between 0 and 18 years old by ultrasound imaging or MRI, then requiring shunt surgery, thus classified as S-NIH (shunted non-infectious hydrocephalus) group. The control group was defined as children with matching baseline characteristics (age, sex, birth term, birthweight) as S-NIH, whom (1) evaluated as normal by clinical neuroradiologist, with normal findings on MRI, no clinical or radiographic history of neurological or psychological disorders, or (2) healthy individuals recruited from general population. The data that were retrieved included neurological functions and neurobehavioral index. Neurological functions were measured as incidence of cerebral palsy as defined by Hagberg et al. (2001) [9], visual impairment, hearing impairment, epilepsy, or seizure. Visual impairment is defined as the need for corrective lenses or blindness in 1 or both eyes. Hearing impairment is defined as abnormal audiometry results or history of using hearing aid in one or both ears. Epilepsy or seizure is defined as two or more convulsive episodes, thus requiring anticonvulsant treatment. Neurobehavioral index was indicated by mental development, motoric development, and behavioral problems.
Statistical analysis
The RevMan version 5.3 software (Cochrane Collaboration) was used when performing meta-analysis. Mantel-Haezel Risk Ratio (RR) with 95% confidence interval for dichotomous data was applied. Inconsistency index (I2) test, ranges from 0 to 100%, was performed to evaluate heterogeneity across all studies. Values above 50% or p value < 0.10 indicate a significant heterogeneity. Small study effect and the risk of publication bias were evaluated using regression-based Egger’s test, while Habord’s test and Peter’s test were used to assess dichotomous outcome. p values of less than 0.05 were considered significant bias. Then, we applied the risk of bias criteria that was specified in the Newcastle-Ottawa Scale (NOS) [10].
Results
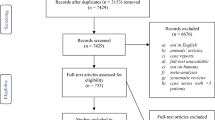
From a total of 1929 identified studies, 527 studies with relevant titles and abstracts were screened, yielded 314 studies after duplications were removed. Then, 272 full-text articles were evaluated further for eligibility. Forty-three of the articles were excluded as they were case reports, commentaries, editorials, study design registries, or reviews. From 75 studies that reported neurodevelopmental outcomes, 12 studies fulfilled all the inclusion criteria, thus included in the meta-analysis (Fig. 1). Risk of bias in all of the included studies was assessed using the 8 criteria from Newcastle-Ottawa Scale (NOS), and the results showed that only 1 study [11] was highly representative of the population, while 7 studies [12,13,14,15,16,17,18,19,20,21,22] did not show the adequacy of follow-up of cohort (Fig. 2). Nonetheless, almost all studies satisfied the other 6 NOS criteria, thus concluded to have enough qualities to be included in the meta-analysis.
Neurological functions
(A) Cerebral palsy
The prevalence of cerebral palsy in shunted non-infectious hydrocephalus children (S-NIH) compared to control was reported in 6 studies [11, 12, 14, 16, 17, 21].The risk of cerebral palsy is significantly higher in S-NIH compared to that of control (RR 8.08 (6.87, 9.50), z = 25.22, p = 0.000; I2 = 58%, p = 0.04) (Fig. 3a). No publication bias or small study effect was detected (p Habord = 0.804, p Peter = 0.206) (Supplemental Table 3).
Risk of neurological function disorders in S-NIH children compared to normal children. a Children with non-infectious hydrocephalus that acquired shunt (S-NIH) were 8.08 times at higher risk of cerebral palsy compared to the normal population. b S-NIH had 3.89 times higher risk of visual impairment. c S-NIH had 4.08 times higher risk of hearing impairment. d S-NIH had 15.75 times more risk of epilepsy or seizures
B. Visual impairment
The prevalence of visual impairment was reported in 4 studies [11, 12, 16, 21]. The risk of visual impairment is significantly higher in S-NIH in comparison to control (RR 3.89 (3.09, 4.91), z = 11.49, p = 0.000; I2 = 54%, p = 0.09) (Fig. 3b). Publication bias or small study effect was identified (p Habord = 0.772, p Peter = 0.020) (Supplemental Table 3).
C. Hearing impairment
The prevalence of hearing impairment was reported in 5 studies [11, 12, 14, 16, 21]. Risk of hearing impairment is significantly higher in S-NIH than in control (RR 4.08 (2.50, 6.67), z = 5.62, p = 0.000; I2 = 39%, p = 0.16) (Fig. 3c). Publication bias or small study effect was detected due to many of the studies which reported significantly higher results of hearing impairment in S-NIH (p Habord = 0.005, p Peter = 0.037) (Supplemental Table 3).
D. Epilepsy or seizures
The prevalence of epilepsy or neonatal seizures was reported in 4 studies [12, 15, 16, 21]. The risk of epilepsy and seizures is significantly higher in S-NIH (RR 15.75 (5.08, 48.77), z = 4.78, p = 0.000; I2 = 39%, p = 0.18) (Fig. 3d). Publication bias or small study effect was identified as many of the studies reported significantly higher results of epilepsy and seizures in S-NIH (p Habord = 0.684, p Peter = 0.002) (Supplemental Table 3).
Mental development
A. Intelligence quotient or mental development index
IQ or MDI outcomes were reported in 6 studies [11, 13, 17, 20,21,22]. S-NIH children scored lower in the Bayley-II Mental Development Index (Bayley-II MDI), Bayley-III Cognitive and Language Test (Bayley-III CLC), Weschler Preschool and Primary Scale of Intelligence-III (WPPSI-III), or Weschler Intelligence Scale for Children (WISC) (mean difference of − 16.15([− 17.98, − 14.33), z = 17.36, p = 0.000; I2 = 0%, p = 0.51) (Fig. 4a). Publication bias or small study effect was detected as many of the included studies reported significantly lower test results in S-NIH (p Egger = 0.000) (Supplemental Table 3).
Mental development in S-NIH children compared to normal children. a Children with non-infectious hydrocephalus requiring shunt surgery (S-NIH) scored significantly lower than normal children in IQ or mental development tests. b S-NIH had 2.37 times higher risk of mental development delay compared to control
B. Mental development delay
Mental development delay was reported in 5 studies [11, 17,18,19, 21]. IQ score < 70 is classified as delayed mental development/intellectually disable. S-NIH children have higher risk of mental development delay in comparison to normal children (RR2.37 (2.13, 2.64), z = 15.82, p = 0.000; I2 = 35%, p = 0.19) (Fig. 4b). No publication bias or small study effect was detected (p Habord = 0.908, p Peter = 0.350) (Supplemental Table 3).
Motoric development
A. Motoric score
The evaluation of motoric score was reported in 4 studies [11, 18, 20, 22]. S-NIH children scored lower than the control in the Bayley-II Psychomotoric Development Index (Bayley-II PDI), Bayley-III Motoric Test (Bayley-III Motor), or Vineland Adaptive Behavior motor score (Mean-diff-25.10 (− 41.53, − 8.66), z = 2.99, p = 0.003; I2 = 99%, p = 0.000) (Fig. 5a). Publication bias or small study effect was identified as many of the studies reported significantly lower test results in S-NIH (p Egger = 0.000) (Supplemental Table 3).
Motoric development in S-NIH children with compared to normal children. a Children with non-infectious hydrocephalus requiring shunt surgery (S-NIH) scored significantly lower than normal children in motoric evaluation tests. b Meta-analysis on motoric development delay shows that S-NIH children acquired 4.21 times higher risk of motoric development delay compared to control
B. Motoric development delay
Motoric development delay was reported in 5 studies [11, 17,18,19, 21]. Children were assessed using Bayley-II Motoric Development Index (Bayley-II MDI) or Bayley-III motoric score or Children Movement ABC in these studies. Children who scored below 70 were classified as having motor development delay. S-NIH children have significantly higher risk of motoric development delay compared to normal children (RR4.21 (3.78, 4.68), z = 26.37, p = 0.000; I2 = 23%, p = 0.27) (Fig. 5b). No publication bias or small study effect was detected (p Habord = 0.899, p Peter = 0.138) (Supplemental Table 3).
Behavioral abnormalities
Abnormal behavior in S-NIH children was reported in 2 studies [13, 17]. Normal children tend to develop more internalizing behavior compared to those of S-NIH (RR0.52 (0.28, 0.95), z = 2.12, p = 0.030; I2 = 0%, p = 0.88), while there was no differences in externalizing behavior (RR1.01 (0.36, 2.86), z = 0.02, p = 0.98; I2 = 0%, p = 0.67). Overall, there are no differences in the risk of behavioral abnormalities in S-NIH children compared to control population (RR0.61 (0.36, 1.04), z = 1.81, p = 0.07; I2 = 0%, p = 0.81) (Fig. 6). Publication bias and/or small study effect was detected (p Habord = 0.253, p Peter = 0.000) (Supplemental Table 3).
Risk of abnormal behavior in S-NIH children compared to normal children. Control population tends to develop internalizing behavior more than children with non-infectious hydrocephalus requiring shunt surgery (S-NIH). Meanwhile, there are no differences in the externalizing behavior. Overall, there are no differences in the risk of abnormal total behavior
Discussions
Since invented in the 1950s, shunt is arguably the treatment of choice in majority of hydrocephalus cases. The preference to apply VP shunt instead of other devices and/or techniques is based on its simplicity and safety. Despite its success in diverting the excess CSF, the long-term success of VP shunt on optimizing the functional outcomes in non-infectious hydrocephalus patients remained unclear. Publication of various studies failed to produce consensual conclusions as many were conducted using different methods, different parameters, and limited number of patients. In order to seek for consensual understanding on the success of shunt placement in non-infectious hydrocephalus patients, we performed meta-analysis on studies of neurodevelopmental outcomes in children with non-infectious hydrocephalus who already had shunt placement surgery. Due to the fact that many other confounding factors are essentials for tumor-related hydrocephalus patients’ outcomes, this study only included patients with congenital hydrocephalus, posthemorrhagic hydrocephalus, and neural tube defect–related hydrocephalus.
After rigorous screening process in accordance with PRISMA reporting guidelines, 12 studies fulfilled the inclusion criteria, thus included in the meta-analysis. The underlying diseases in all of the included studies were varied, including posthemorrhagic hydrocephalus, aqueduct stenosis, meningomyelocele, meningoencephalocele, and Dandy Walker variants. In accordance with the inclusion criteria, none of the patient was described of having any infectious or tumor etiology. Based on the characteristics of all of the evaluated area in the included studies, we grouped the meta-analysis into 4, including disabilities, mental development, motoric development, and behavioral abnormalities. The incidence of 4 major disabilities, including cerebral palsy, visual impairment, hearing impairment, and epilepsy/seizures in shunted non-infectious hydrocephalus children, was compared to their incidences in normal children. Results from meta-analysis of the incidence of cerebral palsy showed that S-NIH children acquire 8.08 times higher risk of acquiring cerebral palsy compared to normal children. The risks of visual and hearing impairment in S-NIH children are also 3.89 and 4.08 times higher than normal children, respectively. Meanwhile, the risk of acquiring seizures/epilepsy in S-NIHNIH children is 15.75 times higher than normal children. Based on these, it is safe to conclude that S-NIH children have higher risks to acquire disabilities compared to normal children, despite the intervention by VP shunt. However, when the actual number of S-NIH children with disabilities compared to the total number of the evaluated S-NIH children, the percentages of S-NIH children with cerebral palsy, visual impairment, hearing impairment, and epilepsy/seizures only constituted 9.1%, 8.7%, 1.8%, and 1.2%, respectively. Thus, majority of S-NIH children could be expected to live without any of the 4 major disabilities that were evaluated in this study. These data also highlighted the necessity to analyze individual cases, particularly when disability(-ies) occurs. It is easy to suspect that the diffuse white matter injury in the periventricular area due to the enlarged ventricles leads to any of these disabilities. This hypothesis is supported by the findings in patients with periventricular leukomalacia. This is a white matter injury that could also lead to cerebral palsy, visual impairment, hearing impairment, and epilepsy [23, 24]. However, it is also interesting to notice that not all disabled S-NIH children acquired all of the evaluated 4 disability types. Therefore, in our opinion, clinical and radiological characteristics in S-NIH children with disability(-ies) should be one of the focuses of future research to extend our knowledge on the pathophysiology of non-infectious hydrocephalus and efforts to improve the outcomes.
In regard to mental development, the intelligent quotient (IQ) score and risk of mental development delay were evaluated (Fig. 4). Either one of the 4 scoring systems, including Bayley-II MDI, Bayley-III CLC, WPPSI-III, or WISC, was used in the studies. As known earlier, Bayley-II MDI or Bayley-III CLC is applied for infant and toddler up to 42 months old, while WPPSI-III or WISC is intended for older children. Before the introduction of Bayley-III CLC in 2006, all studies were performed by using Bayley-II MDI. To homogenize the data, we converted Bayley-II MDI scores into Bayley-III CLC scores by using calculation that was previously described by Yi et al. [25]. Bayley-III has been suggested to have a strong predictive validity for WPPSI-III, while the measurements from WPPSI and WISC across revisions and versions are consistent [26, 27]. In regard to the IQ scores, 85 is defined as normal IQ. Results from meta-analysis showed that the mean difference of IQ score in S-NIH children compared to normal children is − 16.15 IQ points. When performing meta-analysis on the incidence of mental development delay, IQ score < 70 is classified as delayed mental development/intellectually disable. Results from meta-analysis showed that the risk of mental development delay in S-NIH children is 2.37 times higher than its risk in normal children. A study by Arrington et al. reported that shunt revisions might be related with the decrease in IQ scores; however, the difference is approximately only 3 IQ points [28]. Thus, we speculate that the shunt revision would not contribute significantly to the risk of mental development delay. Further investigation to reveal whether the mental development delay in S-NIH is related with brain pathology or extracranial factors is needed to confirm the precise etiology.
Similar to mental development, meta-analysis on motoric development was performed to seek the mean difference in motoric evaluation tests’ score and the risk of motoric development delay in S-NIH children in comparison to normal children (Fig. 5). Calculation model from Yi et al. (2018) was used to convert scores from Bayley-II PDI into Bayley-III motoric test score [25]. Meta-analysis results showed that S-NIH children scored significantly lower motoric evaluation test scores compared to normal children, approximately 25.10 points. Motoric test score < 70 was classified as motoric development delay. Based on the incidence of motoric development delay in S-NIH children compared to normal children, it can be concluded that S-NIH children acquired 4.21 times higher risk of motoric development delay compared to normal children. Like in any other abnormalities, the delay in motoric development in S-NIH children might be due to diffuse white matter damage resulted from enlarged ventricles. Focus on the clinical and radiological characteristics in S-NIH children with delayed motoric development could reveal the exact pathogenesis in these patients.
To investigate behavioral abnormalities among S-NIH children, meta-analysis on the levels of internalizing and externalizing behaviors was performed. Internalizing behavior is directed inward and strongly related to depression and anxiety, while externalizing behavior is directed outward and related with aggressivity and hyperactivity [29, 30]. Results from meta-analysis showed that the risk of developing internalizing behavior in S-NIH children is significantly lower compared to that of normal children, while the risk of externalizing behavior in S-NIH children is not significantly different with normal children. In regard to the lower risk of internalizing behavior in S-NIH children, we hypothesize that this might be related to the delayed mental development in S-NIH children, which might lead to insensitivity; thus, S-NIH children might be more resistant to depression and anxiety. Nonetheless, it is important to acknowledge that participants in studies on both internalizing and externalizing behaviors in S-NIH children were very limited; hence, studies in larger number of patients are needed to confirm any findings.
Lastly, based on all of the abovementioned meta-analysis’ results, it can be concluded that despite shunt application, S-NIH children remained prone to disabilities and mental and motoric development delays. In regard to disability and delayed development in S-NIH children, we hypothesize that these might be due to the pre-existing brain damage and injury related to shunt placement. Evaluation on the clinical effects of chronic brain injury due to hydrocephalus usually showed poor clinical improvement after CSF diversion, supporting the hypothesis that pre-existing hydrocephalus-related brain damage is the cause of existing risks of disability and delayed development in S-NIH children. A previous study in the animal model also showed that despite shunt placement, complete reestablishment of cortical efferent pathway was not acquired [31]. On the other hand, possible injuries due to VP shunt placement seem negligible unless the shunt becomes infected or malfunctioned. Logically, to evaluate any possible roles of shunt placement–related injuries in the risks of disability and delayed development in S-NIH children, comparison should be done between children with non-infectious hydrocephalus who are not treated with shunt and their counterparts who underwent shunt placement. However, since in most cases of non-infectious hydrocephalus, if not all, CSF diversion is truly indicated, not optional, such comparison is not doable. Thus, a study in animal model of non-infectious hydrocephalus is a visible alternative to investigate this possibility.
Overall, it is safe to say that VP shunt placement has a limited role in reversing the pre-existing neurological deficits. Despite confirmation in animal models is still required, we hypothesize that in majority of cases, the disability and delayed development in S-NIH children are pre-existing, not shunt-related. Regardless, continuous assessment, treatment, and rehabilitation in S-NIH children are keys to ensure their optimum quality of life.
Conclusion
S-NIH children have significantly higher risks of disabilities and mental and motoric development delays; thus, planning on continuous rehabilitation for children with non-infectious congenital hydrocephalus who already had placement of VP shunt is important to acquire their optimum potentials and quality of life.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Rekate HL (2009) A contemporary definition and classification of hydrocephalus. Semin Pediatr Neurol 16(1):9–15. https://doi.org/10.1016/j.spen.2009.01.002
Dewan MC, Rattani A, Mekary R, Glancz LJ, Yunusa I, Baticulon RE, Fieggen G, Wellons JC, Park KB, Warf BC (2018) Global hydrocephalus epidemiology and incidence: systematic review and meta-analysis. J Neurosurg 130:1–15. https://doi.org/10.3171/2017.10.JNS17439
Kahle KT, Kulkarni AV, Limbrick DD Jr, Warf BC (2016) Hydrocephalus in children. Lancet 387(10020):788–799. https://doi.org/10.1016/S0140-6736(15)60694-8
Al-Dosari MS, Al-Owain M, Tulbah M et al (2013) Mutation in MPDZ causes severe congenital hydrocephalus. J Med Genet 50(1):54–58. https://doi.org/10.1136/jmedgenet-2012-101294
Hommet C, Cottier JP, Billard C, Perrier D, Gillet P, De Toffol B et al (2002) MRI morphometric study and correlation with cognitive functions in young adults shunted for congenital hydrocephalus related to spina bifida. Eur Neurol 47(3):169–174. https://doi.org/10.1159/000047977
Bawa M, Dash V, Mahalik S, Rao KLN (2019) Outcome analysis of patients of congenital hydrocephalus with ventriculoperitoneal shunt at a tertiary care hospital in North India. Pediatr Neurosurg 54(4):233–236. https://doi.org/10.1159/000501018
Venkataramana NK, Mukundan CR (2011) Evaluation of functional outcomes in congenital hydrocephalus. J Pediatr Neurosci 6(1):4–12. https://doi.org/10.4103/1817-1745.84399
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D (2009) The PRISMA statement for reporting systematic reviews and meta-analysis of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 339:b2700
Hagberg B, Hagberg G, Beckung E, Uvebrant P (2001) Changing panorama of cerebral palsy in Sweden. VIII. Prevalence and origin in the birth year period 1991–94. Acta Paediatr 90:271–277
Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P (2013) The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analysis. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp 2014
Adams-Chapman I, Hansen NI, Stoll BJ, Higgins R, for the NICHD Research Network (2008) Neurodevelopmental outcome of extremely low birth weight infants with posthemorrhagic hydrocephalus requiring shunt insertion. Pediatrics 121(5):1167–1179. https://doi.org/10.1542/peds.2007-0423
Agajany N, Gigi M, Ross J, Roth J, Eshel R, Constantini S, Bassan H (2019) The impact of neonatal posthemorrhagic hydrocephalus of prematurity on family function at preschool age. Early Hum Dev 137(2019):104827. https://doi.org/10.1016/j.earlhumdev.2019.104827
Brouwer AJ, van Stam C, Venema MU, Koopman C, Groenendaal F, de Vries LS (2012) Cognitive and neurological outcome at the age of 5-8 years of preterm infants with post-hemorrhagic ventricular dilatation requiring neurosurgical intervention. Neonatology 101:210–216. https://doi.org/10.1159/000331797
Costello AM, Hamilton PA, Baudin J, Townsend J, Bradford BC, Stewart AL, Reynolds EOR (1988) Prediction of neurodevelopmental impairment at four years from brain ultrasound appearance of very preterm infants. Dev Med Child Neurol 30:711–722. https://doi.org/10.1111/j.1469-8749.1988.tb14633.x
Fletcher JM, Landry SH, Bohan TP, Davidson KC, Brookshire BL, Lachar D, Kramer LA, Francis DJ (1997) Effects of intraventricular hemorrhage and hydrocephalus on the long-term neurobehavioral development of preterm very-low-birthweight infants. Dev Med Child Neurol 39:596–606. https://doi.org/10.1111/j.469-8749.1997.tb07495.x
Gigi M, Roth J, Eshel R, Constantini S, Bassan H (2018) Health-related quality of life after post-haemorrhagic hydrocephalus in children born preterm. Dev Med Child Neurol 61(3):343–349. https://doi.org/10.1111/dmcn.14012
Holwerda JC, van Braeckel KNJA, Roze E, Hoving EW, Maathuis CGB, Brouwer OF, Martjin A, Bos AF (2016) Functional outcome at school age of neonatal post-hemorrhagic ventricular dilatation. Early Hum Dev 96(2016):15–20. https://doi.org/10.1016/.earlhumdev.2016.02.005
Lean RE, Han RH, Smyser TA, Kenley JK, Shimony JS, Rogers CE, Limbrick DD Jr, Smyser CD (2019) Altered neonatal white and gray matter microstructure is associated with neurodevelopmental impairments in very preterm infants with high-grade brain injury. Pediatr Res 86(3):365–374. https://doi.org/10.1038/s41390-019-0461-1
LeFlore JL, Broyles RS, Pritchard MA, Engle WD (2003) Value of neurosonography in predicting subsequent cognitive and motor development in extremely low birth weight neonates. J Perinatol 23:629–634. https://doi.org/10.1038/sj.jp.7211009
Mangano FT, Altaye M, McKinstry RC, Shimony JS, Powell SK, Phillips JM et al (2016) Diffusion tensor imaging study of pediatric patients with congenital hydrocephalus: 1-year postsurgical outcomes. J Neurosurg Pediatr 18(3):306–319. https://doi.org/10.3171/2016.2.PEDS15628
Roth SC, Baudin J, McCormick DC, Edwards AD, Townsend J, Stewart AL, Reynolds EOR (1993) Relation between ultrasound appearance of the brain of very preterm infants and neurodevelopmental impairment at eight years. Dev Med Child Neurol 35:755–768. https://doi.org/10.1111/j.1469-8749.1993.tb11727.x
Strahle JM, Triplett RL, Alexopoulos D, Smyser TA, Rogers CE, Limbrick DD Jr, Smyser CD (2019) Impaired hippocampal development and outcomes in very preterm infants with perinatal brain injury. NeuroImage 22(2019):101787. https://doi.org/10.1016/j.nicl.2019.101787
Shang Q, Ma CY, Lu N, Yan Y-B, Wu Z-R, Li J-J et al (2015) Clinical study of cerebral palsy in 408 children with periventricular leukomalacia. Exp Ther Med 9(4):1336–1344. https://doi.org/10.3892/etm.2015.2222
Yu B, Guo Q, Fan G, Liu N (2011) Assessment of cortical visual impairment in infants with periventricular leukomalacia: a pilot event-related FMRI study. Korean J Radiol 12(4):463–472. https://doi.org/10.3348/kjr.2011.12.4.463
Yi YG, Sung IY, Suk JS (2018) Comparison of second and third editions of the Bayley Scales in children with suspected developmental delay. Ann Rehabil Med 42(2):313–320. https://doi.org/10.5535/arm.2018.42.2.313
Bode MM, DʼEugenio DB, Mettelman BB, Gross SJ. (2014) Predictive validity of the Bayley, Third Edition at 2 years for intelligence quotient at 4 years in preterm infants. J Dev Behav Pediatr 35(9):570–575. https://doi.org/10.1097/DBP.0000000000000110
Niileksela CR, Reynolds MR (2019) Enduring the tests of age and time: Wechsler constructs across versions and revisions. Intelligence. 77:101403. https://doi.org/10.1016/j.intell.2019.101403
Arrington CN, Ware AL, Ahmed Y, Kulesz PA, Dennis M, Fletcher JM (2016) Are shunt revisions associated with IQ in congenital hydrocephalus? A meta-analysis. Neuropsychol Rev 26(4):329–339. https://doi.org/10.1007/s11065-016-9335-z
Liu J, Chen X, Lewis G (2011) Childhood internalizing behaviour: analysis and implications. J Psychiatr Ment Health Nurs 18(10):884–894. https://doi.org/10.1111/j.1365-2850.2011.01743.x
Liu J (2004) Childhood externalizing behavior: theory and implications. J Child Adolesc Psychiatr Nurs 17(3):93–103. https://doi.org/10.1111/j.1744-6171.2004.tb00003.x
Eskandari R, Mcallister JP 2nd, Miller JM et al (2004) Effects of hydrocephalus and ventriculoperitoneal shunt therapy on afferent and efferent connections in the feline sensorimotor cortex. J Neurosurg 101(2 Suppl):196–210. https://doi.org/10.3171/ped.2004.101.2.0196
Acknowledgements
We would like to extend our appreciation to Prof. Kyu-Chang Wang from the Department of Neurosurgery at the National Cancer Center in Seoul, South Korea, for revising the manuscript.
Author information
Authors and Affiliations
Contributions
Dr Sobana conceptualized and designed the study, did the literature screening, assessed study eligibility and quality, directed the discussion, and reviewed and revised the manuscript.
Dr Halim conceptualized and designed the study, did the literature screening, analyzed the data, assessed study eligibility and quality, directed the discussion, wrote, and reviewed and revised the manuscript.
Ms Aviani did the literature screening, assessed study eligibility and quality, carried out the statistical analysis, and reviewed and revised the manuscript.
Dr Gamayani conceptualized and designed the study, and wrote, reviewed, and revised the manuscript.
Dr Achmad conceptualized and designed the study, directed the discussion, wrote, and reviewed and revised the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Code availability
Not applicable
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sobana, M., Halim, D., Aviani, J.K. et al. Neurodevelopmental outcomes after ventriculoperitoneal shunt placement in children with non-infectious hydrocephalus: a meta-analysis. Childs Nerv Syst 37, 1055–1065 (2021). https://doi.org/10.1007/s00381-021-05051-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-021-05051-9