Abstract
Because ETV in infants is controversial, with strong, plausible arguments on both sides of the divide, we concluded that a randomized prospective study in this group would be ethically justified; hence, more than 4 years ago, we initiated the International Infant Hydrocephalus Study (IIHS).
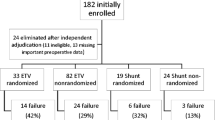
IIHS is a multicenter prospective randomized study on infants under 2 years of age with obstructive hydrocephalus due to pure aqueductal stenosis. IIHS represents a major departure from most published works on the value of neuroendoscopy in the treatment of hydrocephalus. Whereas most studies focus on the survival of the created stoma or implanted shunt and surgical complications, this study focuses primarily on the effect of treatment on the neurodevelopmental outcome at 5 years, including a comprehensive assessment of relevant risks and benefits. The study dictates patient randomization to shunt or ETV groups. Nevertheless, families who are presented with both options in a non-biased way and elect to choose one, possibly on the basis of information that they have already gathered on their own, are also included in the study. This option (termed “parental preference”) does not violate the statistical validity of the study and is built into the study design, based on a comprehensive cohort design model.
Given the complexity of the study outcomes, it is possible that there might not be a single, clear, unambiguous set of findings. With this in mind, IIHS is also analyzing other factors as secondary outcome measures, such as complication rates, hospitalization time, the need for repeat surgeries, and imaging use. This dual-level approach will ultimately provide a unique opportunity to directly compare, under controlled circumstances, the management consequences of ETV and VPS.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Endoscopic Third Ventriculostomy
- Obstructive Hydrocephalus
- Aqueductal Stenosis
- Parental Preference
- Slit Ventricle Syndrome
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
3.1 Introduction: Hydrocephalus, VPS, and ETV
Pediatric hydrocephalus is one of the most common neurosurgical conditions. It is the leading cause of brain surgery for children in the USA. The ventriculoperitoneal shunt (VPS) is the classic treatment for pediatric hydrocephalus since the early 1960s. Shunts are the “bread and butter” of pediatric neurosurgery. Shunts have modified the prognosis of hydrocephalus from a lethal disease to a curable disease with a relatively good prognosis according to etiology [1–4].
Hydrocephalus is a heterogeneous disease. Shunts are able to resolve almost all cases of hydrocephalus, whatever the etiology, with almost no contraindications. Many different types of shunts have been developed and are in use, including pressure-regulated, volume-regulated, externally regulated, shunt assistants, and dual-switch valves [5–24]. When shunts first appeared in our field, the advantages were clear and far outweighed the disadvantages; they enabled a relatively normal life with a relatively simple procedure.
It took some time to realize and acknowledge that the shunt failure rate is significant, that complications are common, and that children with shunts are dependent upon surgical maintenance throughout their lives [1, 25–34].
Shunt complication rates are unacceptably high. Children with shunts have an increased likelihood of seizures, they can develop slit ventricle syndrome, and some of them suffer from under- or over-shunting [35–53].
With all these complications in mind, the arrival of neuroendoscopy on the scene was greeted with great enthusiasm. Neuroendoscopy was seen as a means of solving the challenges of hydrocephalus without the issues of the hardware.
Endoscopic third ventriculostomy (ETV) was designed primarily for hydrocephalus cases in which there is a blockage at the level of the aqueduct of Sylvius. In these cases, the endoscope is guided to the floor of the third ventricle, and an opening is created between the third ventricle and the interpeduncular cistern. This is a straightforward diversion procedure; no hardware is usually left in place, and fluid can egress from the third ventricle to the base of skull and ultimately arrive at the normal absorption sites at the convexity of the brain.
3.2 Technical Challenges of ETV
There is no single standardized technique defined for ETV. The same basic procedure is implemented with considerable technical variability in different medical centers. Technical nuances include the use of rinsing fluid, use of navigation, scope types (rigid or flexible), techniques for creating and widening the hole in the base of the third ventricle, and even basic concepts of how to close the skin and open the bone [54–57].
Endoscopic third ventriculostomy and all other neuroendoscopic operations are advanced procedures that are heavily dependent on sophisticated technology. ETVs require a learning curve, substantial experience, and careful coaching of young neurosurgeons. Every case should be carefully discussed between the participating neurosurgeons, analyzing the indications and contraindications, following a close inspection of the specific microanatomical details on the MR. The professional discussions must be accompanied by a discussion with the family of the available alternatives, their advantages, and disadvantages.
Morbidity from endoscopic third ventriculostomy may be underreported. The nightmare of every neuroendoscopist is massive bleeding, mainly arterial, during the procedure. Perforation of the basilar artery has been reported from even the best of medical centers [58–72]. Smaller bleeds, mainly of venous origin, usually stop by themselves with either simple rinsing or a short burst of mono- or bipolar coagulation. Tissue damage during insertion and manipulation of the endoscope, subdural hematomas, endocrinological abnormalities, infections, cranial neuropathies, and other complications are also reported [60, 71, 73–82].
Although most failures from endoscopic third ventriculostomy occur in the early period after the procedure, late obstruction of the stoma may lead to increased ICP and even sudden death [83–92]. It is therefore strongly advised that patients who undergo a successful ETV should be clearly told that they are not cured from the hydrocephalus and that symptoms can reappear and may have dangerous consequences [93]. These patients should be followed on an ongoing basis, and the medical center should have an open door policy that encourages the patients to call or come back if any related symptoms are appearing. It is still not known if those patients with no flow void at the third ventricle stoma on postoperative MRI may be at a higher risk to develop a clinical syndrome and should be followed even more closely.
3.3 ETV: Meeting the Standard of Evidence-Based Medicine
Series on the results of ETV in the pediatric age group started to appear in the 1980s, developed during the 1990s, and continue to appear in the literature to this day [56, 60, 91, 94–118].
However, even with all the series that have been published to date, it is hard to extract meaningful research data or operative guidelines. There are too many inconsistencies in the basic “ground rules” used by these researchers [119]. For example, success rates of endoscopic third ventriculostomy are usually defined as one or more of the following factors: the disappearance of hydrocephalus symptoms, no signs of intracranial hypertension evident, and/or a technically successful procedure. Perhaps partially as a result of this wide range of definitions for the term “success,” large disparities are found when looking at the results of ETV in children. Success rate varies widely, ranging from a low 35 % success rate in a series from Toronto [116] up to a high of 83–89 % in other series [106, 108, 120, 121].
Analyzing the differences between successful series and series with less promising results shows that most of the differences can be traced to a gap in the early failure rate. Early ETV failures could be due to wrong technique, different selection criteria in recruiting the patients or in defining failure, and also the multifactorial etiology of the hydrocephalic process itself [122, 123]. It is essential, therefore, to define a uniform set of selection and failure criteria in order to objectively and meaningfully compare results among different centers.
Since the 1990s, ETV has been recognized as a valid alternative to shunt implants, mainly for patients with obstruction at the level of the aqueduct, the tectal plate, and the pineal region. ETV quietly developed into a mainstream, common procedure in pediatric neurosurgery without any prospective randomized trials (and certainly no multicenter trials) proving its efficacy compared to shunt procedures. Unfortunately, it seems apparent today that a classic randomized trial is no longer possible, since most of us treating these patients would not agree to expose a classic candidate for ETV to randomization between two alternatives.
As ETV technology continues to evolve and improve, and as we collectively accumulate more experience and confidence with ETV, indications for ETV have broadened, introducing more challenges in understanding the pathophysiology of hydrocephalus and in proving the efficacy of a new procedure (ETV) over the more standard alternative (shunts).
This was one of the reasons that in 2001 we established the International Study Group for Neuroendoscopy (ISGNE). The goal of this organization (more recently transformed into the International Federation for Neuroendoscopy (IFNE)) is to promote neuroendoscopy research and education.
There are many pathologies for which treatment with ETV is debatable. These include hydrocephalus in infants, patients with meningomyelocele and Chiari, Dandy-Walker malformation, fourth ventricular outlet obstruction, during tumor surgery, and patients who have had a hemorrhage or an infection in their past [39, 124–171].
Over the course of 10 years of collaboration within the IFNE, we have learned to appreciate the advantages of cooperative multicenter studies. Our first attempt was with a study on repeat ETV for those patients for whom the original ETV initially succeeded. We pooled our experiences with 20 patients recruited from four centers [114]. Another collaboration involved a multicenter study on the efficacy of ETV in patients who had previously experienced an infection and/or hemorrhage. For this study, we pooled our experiences with 101 patients from seven medical centers around the world [161]. We are currently analyzing the results of the International Neuroendoscopy Biopsy Study (INEBS) which included 293 patients from 13 medical centers (submitted for publication). In addition to these clinical series, our group has led several major multicenter epidemiological papers that have recently been published. These papers analyzed meta-results obtained by merging data from the very large number of patients recruited through a combination of other series, focusing on specific variables and how they affect success or failure in pediatric ETV [55, 93, 100, 109, 110, 172, 173].
3.4 Uncertainty Regarding ETV in Infants
For infants, the potential benefit of ETV is substantial, due to the admittedly high complication rate of shunting. Common complications include a high rate of mechanical failure, high rate of infection, slit ventricle syndrome, and seizures. Shunt complication rate (both mechanical and infectious) is age-dependent. Infants usually have more complications compared to older patients. Shunted infants generally require many surgical revisions. Twenty to forty percent of infants require revisions in the first year following insertion and, in subsequent years, generally add another 10–15 % per year [28].
There are other concerns regarding ETV in infants. Is ETV more dangerous for infants? Safety concerns fall into three areas: short term (during the surgery itself), intermediate term (e.g., postoperative leaks or infections), and long term (e.g., perhaps due to unforeseen risks to development or stoma closure leading to a sudden hydrocephalus emergency). Another unresolved concern is whether the CSF absorption mechanism in infants with aqueductal stenosis is mature enough to handle the CSF after the obstruction is bypassed.
Even if all the technical/physiological issues were resolved, another major concern is that some of the infants considered to have been successfully treated with ETV may actually have been transformed from active hydrocephalus to an arrested type. We might be paying a neurological price for such “successes” by adversely affecting their long-term development. This theory is based on the observation that children who have had their hydrocephalus treated with ETV almost always have ventricles considerably larger than children who were treated with VPS [174]. At least one study has shown a direct correlation between decreased ventricular volume and clinical improvement [175]. Unfortunately, nobody, so far, has been reviewing systematically the relevant developmental variables in children following ETV. No study has attempted to correlate the size of the ventricles to any neurodevelopmental measurement. So this belief has not been scientifically proven or refuted.
Conversely, other surgeons advocating ETV are concerned with the long-term complications of shunting on the developing brain, especially the cumulative risk of shunt infections due to multiple operations. The theory for this correlation is based on the observed link between shunt infection and reduction of IQ, as well as measured memory deficits among shunted children [94, 169, 176–186]. Advocates for ETV also claim that it is a more “physiological solution” and therefore is better for the infant brain.
Having reviewed the papers that appeared on this subject over the last 10 years, 32 papers reported an average success rate from 50 to 55 %. However, this “average” success rate does not really reflect the wide range of results found when analyzing the studies to date.
Results from around the world ranged from 25 % shunt independence [108, 126, 187–190] up to 89 % shunt independence [59, 191–197].
Two-thirds of the studies reviewed concluded that age is a significant predictor of success, suggesting that for infants up to 1 year of age, the ETV success rate is strongly age-dependent [27, 55, 79, 80, 99, 100, 106, 108, 113, 116, 149, 167, 191, 198–203]. On the other hand, one-third of the studies found no correlation between age and success rates [97, 132, 134, 150, 168, 192, 197, 204].
There is also a very wide range of failure definition after ETV in this age group. Some would shunt every post-ETV infant who still has a full fontanel, while others would wait for more overt signs of high ICP before declaring a failure [22, 55, 59, 60, 78–80, 82, 93, 94, 96, 97, 100, 102, 106–113, 116–118, 126, 132, 134, 141, 149, 150, 152, 156, 161, 166–169, 172, 173, 177, 185, 187–256].
With all these very plausible theories and beliefs, there has never been a direct controlled comparison of the two types of treatment, studying their impact on the intellectual development of children, and certainly not of infants.
3.5 The International Infant Hydrocephalus Study (IIHS)
Because ETV in infants is so controversial, with strong, plausible arguments on both sides of the divide, we concluded that a randomized prospective study in this group would be morally justified and well accepted by our community. This was why more than 4 years ago we initiated the International Infant Hydrocephalus Study (IIHS).
IIHS is a multicenter prospective randomized study on infants up to 2 years of age with no flow at the level of the aqueduct. IIHS represents a major departure from most published works on the value of neuroendoscopy in the treatment of hydrocephalus. Whereas most studies focus on the survival of the created stoma or implanted shunt and surgical complications, this study focuses primarily on the effect of treatment on the neurodevelopmental outcome at 5 years, including a comprehensive assessment of relevant risks and benefits [257].
IIHS is the first randomized study of the long-term outcome for patients with infantile hydrocephalus due to aqueductal stenosis. Due to the lack of clear superiority of either surgical technique, it became obvious that randomization to shunt or ETV groups, in the clearly defined population of infants under 2 years of age with obstructive hydrocephalus due to pure aqueductal stenosis, is not only ethical but also a duty for all medical personnel involved in the management of such patients. Nevertheless, families who are presented with both options in a non-biased way and elect to choose one, possibly on the basis of information that they have already gathered on their own, are also included in the study. This option (termed “parental preference”) does not violate the statistical validity of the study and is built into the study design, based on a comprehensive cohort design model [257, 258].
Given the complexity of the study outcomes, it is possible that there might not be a single, clear, unambiguous set of findings. For example, it may well prove that one type of treatment enjoys a considerably better neurodevelopmental outcome but possibly at the “cost” of a higher complication rate. Ultimately, we may decide that in the future, it may be up to the parents, together with the treating neurosurgeon, to choose one or the other treatment, with full awareness and understanding of the facts and details. With this in mind, IIHS is also analyzing other factors as secondary outcome measures, such as complication rates, hospitalization time, the need for repeat surgeries, and imaging use. This dual-level approach will ultimately provide a unique opportunity to directly compare, under controlled circumstances, the management consequences of ETV and VPS.
Until now, such a comparison has not been possible. Currently, when neurosurgeons counsel patients and their families before surgery, we quote complication rates from different studies. Unfortunately, the studies available to date are not even directly comparable because, at the very least, they are not based on comparable patient populations. And of course, the reality is that with all the uncertainty surrounding the question of ETV vs. VPS, we all have our own personal beliefs and biases. It is only human nature for the neurosurgeon to choose, perhaps subconsciously, the statistics most supportive of a preferred choice. Hopefully, one of the outcomes of the IIHS will be to provide a more objectively balanced set of data to discuss with the families.
3.6 How Does the IIHS Work?
Information about the IIHS administrative and organizational details, (steering committee, study coordinator, etc.), as well as the study principles, is provided on the study web site www.IIHStudy.org. IIHS principles are also presented in a paper by Sgouros, Kulkarni, and Constantini [257].
Participating medical centers must meet a stiff set of inclusion criteria. IIHS participation requires medical centers have strong neuroendoscopic orientations with at least five infant ETV operations per surgeon annually and a philosophical acceptance of the underlying principles of the study. IIHS demands a strong commitment to timely patient follow-up and data submissions, combined with the ability to follow patients for at least 5 years. Research ethics requirements are per institutional rules.
Recruited infants must meet their own set of inclusion criteria. Children must be under 2 years of age, the product of a full-term pregnancy, and newly diagnosed with untreated obstructive hydrocephalus. Ventricular enlargement and no flow at the aqueductal level must be clearly visible on the MR. Local logistics and social complexities must be considered – it must be possible for the medical center to follow the child and schedule follow-up exams for at least 5 years. Exclusion criteria include children who are either prematurely born or have other major structural neurological and brain abnormalities.
Eligible patients and their families must have a long discussion with the treating neurosurgeon. After an explanation of the study and the different arms, they may be either randomized or categorized according to “parental preference.” All children are subject to continuous follow-up until they reach the age of 5 years. The 5-year outcome measurements are based on a complete test battery, including three questionnaires completed by the parents and two questionnaires completed by professionals. These tests have being translated and validated in eight different languages. A number of secondary variables reflecting more standard secondary outcome measures will also be documented.
3.7 What Has the IIHS Achieved So Far?
IIHS patient recruitment began about 4 years ago, following a major design process. Forty-three international centers have joined the IIHS. The majority (27) are from Europe, with other participants from North America, Latin America, and other continents. Twenty-five centers have already contributed over 150 patients. Most of our patients were recruited at under 1 year of age. An interim analysis showed a similar rate of adverse surgical affects between the two arms. Our monitoring committee therefore authorized continuing the recruitment process. Outcome results will be analyzed only after study recruitment is completed. Patient recruitment will probably continue for another 3 years and follow-up for another 5 years after that.
Four years into the IIHS, we can conclude the following: We are dealing with a rare disease. Even very busy centers usually recruit no more than two to four patients yearly. So maintaining a high recruitment rate is an ongoing challenge. Randomization of a surgical procedure is a difficult challenge as well. This is a culture change and requires time and effort from the participating centers. Since our study is only modestly funded, a strong determination and certain “idealism” is part of the participation motivation.
One more point is as follows: While it is important to try to provide informative, objective, prospective data, the significance of the IIHS is much more than that of a single important study. The fact that we have the commitment of so many colleagues around the world, who are all equally passionate about treating and hydrocephalus, is very encouraging. This group of centers and investigators can be used for other collaborative hydrocephalus studies in the future. IIHS is, therefore, laying the groundwork for a future of global collaborative studies that may just change the way medical research is conducted.
3.8 Other Challenges in Infant Neuroendoscopy
ETV in infants with aqueductal stenosis is only one of the scientific and clinical dilemmas facing us today. Several other controversial indications exist.
ETV combined with Choroid Plexus Coagulation (ETV/CPC) has been rejuvenated by Benjamin Warf, who has contributed enormously to the body of research through his Uganda experience. He and others have reported on the use of CPC, mainly in post-meningitis hydrocephalus and in those with MMC [22, 141, 166, 168, 169, 200, 253, 254, 259–261]. In the coming years, the challenge will be to see if the huge African experience can be extrapolated to developed nations. It has been proposed that ETV/CPC can play an important role in post-hemorrhagic hydrocephalus, for example. This condition is rarely seen in sub-Saharan Africa, but very common in developed nations. A prospective study designed to advance our knowledge in this direction is about to start soon.
ETV for Dandy-Walker syndrome is a valid option, but only small series are available in the literature [124, 126, 146, 148, 150, 164, 262–268].
ETV for obstruction of the outlet of the fourth ventricle is another clinical front. Theoretically, ETV should work to bypass such an obstruction. Nevertheless, several obstacles in defining this entity and the role of ETV exist. First, the MR criteria to differentiate those who have a combined obstruction of the Luschka and the Magendie vs. those with “communicating” hydrocephalus are not clear. Most clinicians will not expose their patients to invasive preparatory imaging such as a dynamic ventriculography. When performing this study on several candidates, we realized the low predictive ability of MR in selecting the right candidates for this procedure. While initial results are promising [39, 53, 128, 129, 131, 133, 136, 138–140, 143, 147, 149, 153, 155], the jury is out on the indication for ETV in this situation.
ETV for hydrocephalus in dysraphic patients is also an option. Although it makes sense in selected patients, it has not yet become too popular as a first measure to control hydrocephalus in infants following closure of their MMC [127, 132, 134, 137, 141, 142, 145, 154, 163, 166–170, 269].
3.9 Conclusion
Neuroendoscopy in infants poses a special clinical research challenge. Available data is accumulating rather slowly. The IIHS offers some hope of providing more reliable data in infants with aqueductal stenosis. Other indications for endoscopy in this age group need to be better studied to expand our understanding of the indications, dangers, and benefits.
References
Aschoff A, Kremer P, Hashemi B, Kunze S (1999) The scientific history of hydrocephalus and its treatment. Neurosurg Rev 22(2–3):67–93; discussion 94–95
Beni-Adani L, Biani N, Ben-Sirah L, Constantini S (2006) The occurrence of obstructive vs absorptive hydrocephalus in newborns and infants: relevance to treatment choices. Childs Nerv Syst 22(12):1543–1563
Golden JA, Bonnemann CG (2007) Developmental structural disorders. In: Goetz CG (ed) Textbook of clinical neurology, 3rd edn. Saunders/Elsevier, Philadelphia, p ch. 28
Kinsman SL, Johnston MV (2007) Congenital anomalies of the central nervous system. In: Kliegman RM, Behrman RE, Jenson HB, Stanton BF (eds) Nelson textbook of pediatrics, 18th edn. Saunders/Elsevier, Philadelphia, p 592
Czosnyka ZH, Cieslicki K, Czosnyka M, Pickard JD (2005) Hydrocephalus shunts and waves of intracranial pressure. Med Biol Eng Comput 43(1):71–77
Davis SE, Levy ML, McComb JG, Sposto R (2000) The delta valve: how does its clinical performance compare with two other pressure differential valves without antisiphon control? Pediatr Neurosurg 33(2):58–63
Drake JM, Kestle J (1996) Rationale and methodology of the multicenter pediatric cerebrospinal fluid shunt design trial. Pediatric hydrocephalus treatment evaluation group. Childs Nerv Syst 12(8):434–447
Drake JM, Kestle J (1996) Determining the best cerebrospinal fluid shunt valve design: the pediatric valve design trial. Neurosurgery 38(3):604–607
Drake JM, Kestle JT (1998) Determining the best cerebrospinal fluid shunt valve design: the pediatric valve design trial. Neurosurgery 43(5):1259–1260
Drake JM, Kestle JR, Milner R, Cinalli G, Boop F, Piatt J Jr, Haines S, Schiff SJ, Cochrane DD, Steinbok P, MacNeil N (1998) Randomized trial of cerebrospinal fluid shunt valve design in pediatric hydrocephalus. Neurosurgery 43(2):294–303; discussion 303–305
Drake JM, Kestle JR, Tuli S (2000) CSF shunts 50 years on – past, present and future. Childs Nerv Syst 16(10–11):800–804
Hanlo PW, Cinalli G, Vandertop WP, Faber JA, Bogeskov L, Borgesen SE, Boschert J, Chumas P, Eder H, Pople IK, Serlo W, Vitzthum E (2003) Treatment of hydrocephalus determined by the European Orbis Sigma Valve II survey: a multicenter prospective 5-year shunt survival study in children and adults in whom a flow-regulating shunt was used. J Neurosurg 99(1):52–57
Jain H, Sgouros S, Walsh AR, Hockley AD (2000) The treatment of infantile hydrocephalus: “differential-pressure” or “flow-control” valves. A pilot study. Childs Nerv Syst 16(4):242–246
Jain H, Natarajan K, Sgouros S (2005) Influence of the shunt type in the difference in reduction of volume between the two lateral ventricles in shunted hydrocephalic children. Childs Nerv Syst 21(7):552–558
Lundkvist B, Eklund A, Koskinen LO, Malm J (2003) An adjustable CSF shunt: advices for clinical use. Acta Neurol Scand 108(1):38–42
Mangano FT, Menendez JA, Habrock T, Narayan P, Leonard JR, Park TS, Smyth MD (2005) Early programmable valve malfunctions in pediatric hydrocephalus. J Neurosurg 103(6 Suppl):501–507
Meier U, Zeilinger FS, Reyer T, Kintzel D (2000) Clinical experience with various shunt systems in normal pressure hydrocephalus. Zentralbl Neurochir 61(3):143–149
Meier U, Kintzel D (2002) Clinical experiences with different valve systems in patients with normal-pressure hydrocephalus: evaluation of the Miethke dual-switch valve. Childs Nerv Syst 18(6–7):288–294
Ringel F, Schramm J, Meyer B (2005) Comparison of programmable shunt valves vs standard valves for communicating hydrocephalus of adults: a retrospective analysis of 407 patients. Surg Neurol 63(1):36–41; discussion 41
Tuli S, O’Hayon B, Drake J, Clarke M, Kestle J (1999) Change in ventricular size and effect of ventricular catheter placement in pediatric patients with shunted hydrocephalus. Neurosurgery 45(6):1329–1333; discussion 1333–1335
Udayakumaran S, Roth J, Kesler A, Constantini S (2010) Miethke DualSwitch Valve in lumboperitoneal shunts. Acta Neurochir (Wien) 152(10):1793–1800
Warf BC (2005) Comparison of 1-year outcomes for the Chhabra and Codman-Hakim Micro Precision shunt systems in Uganda: a prospective study in 195 children. J Neurosurg 102(4 Suppl):358–362
Xenos C, Sgouros S, Natarajan K, Walsh AR, Hockley A (2003) Influence of shunt type on ventricular volume changes in children with hydrocephalus. J Neurosurg 98(2):277–283
Zeilinger FS, Reyer T, Meier U, Kintzel D (2000) Clinical experiences with the dual-switch valve in patients with normal pressure hydrocephalus. Acta Neurochir Suppl 76:559–562
Hoppe-Hirsch E, Laroussinie F, Brunet L, Sainte-Rose C, Renier D, Cinalli G, Zerah M, Pierre-Kahn A (1998) Late outcome of the surgical treatment of hydrocephalus. Childs Nerv Syst 14(3):97–99
Kestle J, Milner R, Drake J (1999) The shunt design trial: variation in surgical experience did not influence shunt survival. Pediatr Neurosurg 30(6):283–287
Kestle J, Milner R, Drake D (1999) An assessment of observer bias in the shunt design trial. Pediatr Neurosurg 30(2):57–61
Kestle J, Drake J, Milner R, Sainte-Rose C, Cinalli G, Boop F, Piatt J, Haines S, Schiff S, Cochrane D, Steinbok P, MacNeil N (2000) Long-term follow-up data from the Shunt Design Trial. Pediatr Neurosurg 33(5):230–236
Kestle JR (2003) Pediatric hydrocephalus: current management. Neurol Clin 21(4):883–895, vii
Kestle JR, Drake JM, Cochrane DD, Milner R, Walker ML, Abbott R 3rd, Boop FA (2003) Lack of benefit of endoscopic ventriculoperitoneal shunt insertion: a multicenter randomized trial. J Neurosurg 98(2):284–290
Renier D, Sainte-Rose C, Pierre-Kahn A, Hirsch JF (1988) Prenatal hydrocephalus: outcome and prognosis. Childs Nerv Syst 4(4):213–222
Sainte-Rose C, Piatt JH, Renier D, Pierre-Kahn A, Hirsch JF, Hoffman HJ, Humphreys RP, Hendrick EB (1991) Mechanical complications in shunts. Pediatr Neurosurg 17(1):2–9
Simon TD, Riva-Cambrin J, Srivastava R, Bratton SL, Dean JM, Kestle JR (2008) Hospital care for children with hydrocephalus in the United States: utilization, charges, comorbidities, and deaths. J Neurosurg Pediatr 1(2):131–137
Williams MA, McAllister JP, Walker ML, Kranz DA, Bergsneider M, Del Bigio MR, Fleming L, Frim DM, Gwinn K, Kestle JR, Luciano MG, Madsen JR, Oster-Granite ML, Spinella G (2007) Priorities for hydrocephalus research: report from a National Institutes of Health-sponsored workshop. J Neurosurg 107(5 Suppl):345–357
Bourgeois M, Sainte-Rose C, Cinalli G, Maixner W, Malucci C, Zerah M, Pierre-Kahn A, Renier D, Hoppe-Hirsch E, Aicardi J (1999) Epilepsy in children with shunted hydrocephalus. J Neurosurg 90(2):274–281
Bruce DA, Weprin B (2001) The slit ventricle syndrome. Neurosurg Clin N Am 12(4):709–717, viii
Bryant MJ, McEniery J, Walker DG, Campbell R, Lister B, Sargent P, Withers TK, Baker J, Guazzo E, Rossato R, Anderson D, Tomlinson F (2004) Preliminary study of shunt related death in paediatric patients. J Clin Neurosci 11(6):614–615
Buchhalter JR, Dichter MA (1990) Migraine/epilepsy syndrome mimicking shunt malfunction in a child with shunted hydrocephalus. J Child Neurol 5(1):69–71
Dollo C, Kanner A, Siomin V, Ben-Sira L, Sivan J, Constantini S (2001) Outlet fenestration for isolated fourth ventricle with and without an internal shunt. Childs Nerv Syst 17(8):483–486
Drake J (2005) Slit-ventricle syndrome. J Neurosurg 102(3 Suppl):257–258; discussion 258–259
Epstein F, Lapras C, Wisoff JH (1988) ‘Slit-ventricle syndrome’: etiology and treatment. Pediatr Neurosci 14(1):5–10
Fattal-Valevski A, Beni-Adani L, Constantini S (2005) Short-term dexamethasone treatment for symptomatic slit ventricle syndrome. Childs Nerv Syst 21(11):981–984
Klepper J, Busse M, Strassburg HM, Sorensen N (1998) Epilepsy in shunt-treated hydrocephalus. Dev Med Child Neurol 40(11):731–736
Kramer U, Kanner AA, Siomin V, Harel S, Constantini S (2001) No evidence of epilepsy following endoscopic third ventriculostomy: a short-term follow-up. Pediatr Neurosurg 34(3):121–123
Oi S, Matsumoto S (1987) Infantile hydrocephalus and the slit ventricle syndrome in early infancy. Childs Nerv Syst 3(3):145–150
Olson S (2004) The problematic slit ventricle syndrome. A review of the literature and proposed algorithm for treatment. Pediatr Neurosurg 40(6):264–269
Piatt JH Jr, Carlson CV (1996) Hydrocephalus and epilepsy: an actuarial analysis. Neurosurgery 39(4):722–727; discussion 727–728
Rekate HL (2004) The slit ventricle syndrome: advances based on technology and understanding. Pediatr Neurosurg 40(6):259–263
Roth J, Biyani N, Udayakumaran S, Xiao X, Friedman O, Beni-Adani L, Constantini S (2011) Modified bilateral subtemporal decompression for resistant slit ventricle syndrome. Childs Nerv Syst 27(1):101–110
Sato O, Yamguchi T, Kittaka M, Toyama H (2001) Hydrocephalus and epilepsy. Childs Nerv Syst 17(1–2):76–86
Saukkonen AL, Serlo W, von Wendt L (1988) Electroencephalographic findings and epilepsy in the slit ventricle syndrome of shunt-treated hydrocephalic children. Childs Nerv Syst 4(6):344–347
Saukkonen AL, Serlo W, von Wendt L (1990) Epilepsy in hydrocephalic children. Acta Paediatr Scand 79(2):212–218
Udayakumara S, Biyani N, Rosenbaum DP, Ben-Sira L, Constantini S, Beni-Adani L (2011) Posterior fossa craniotomy for trapped fourth ventricle in shunted hydrocephalic children - long term outcome. J Neurosurg Pediatr 7(1):52–63
Constantini S, Roth J (2010) Endoscopic third ventriculostomy (ETV). In: Jallo G, Kothbauer K, Padilla G (eds) Controversies in pediatric neurosurgery. Thieme Medical & Scientific Publishers, New York
Kulkarni AV, Drake JM, Mallucci CL, Sgouros S, Roth J, Constantini S (2009) Endoscopic third ventriculostomy in the treatment of childhood hydrocephalus. J Pediatr 155(2):254–259, e251
Roth J, Bo X, Beni-Adani L, Elran H, Constantini S (2007) Endoscopic third ventriculostomy – a physiological alternative to shunts as treatment for obstructive hydrocephalus in children. Harefuah 146(9):660–665, 735
Siomin VC, Constantini S (2004) Basic principles and equipment in neuroendoscopy. In: Abbott R (ed) Clinical neuroendoscopy. WB Saunders, Philadelphia
Abtin K, Thompson BG, Walker ML (1998) Basilar artery perforation as a complication of endoscopic third ventriculostomy. Pediatr Neurosurg 28(1):35–41
Baykan N, Isbir O, Gercek A, Dagcnar A, Ozek MM (2005) Ten years of experience with pediatric neuroendoscopic third ventriculostomy: features and perioperative complications of 210 cases. J Neurosurg Anesthesiol 17(1):33–37
Brockmeyer D, Abtin K, Carey L, Walker ML (1998) Endoscopic third ventriculostomy: an outcome analysis. Pediatr Neurosurg 28(5):236–240
Cartmill M, Vloeberghs M (1999) The use of transendoscopic Doppler ultrasound as a safety-enhancing measure during neuroendoscopic third ventriculostomy. Eur J Pediatr Surg 9(Suppl 1):50–51
Darbar A, Kim PD, Yusopov I, Krishnamurthy S (2008) Use of Grotenhuis perforator in endoscopic third ventriculostomy and cyst fenestration. Minim Invasive Neurosurg 51(2):126–129
Edwards RJ, Pople IK (2003) Death and third ventriculostomy. J Neurosurg 98(3):649–650; author reply 650–651
Horowitz M, Albright AL, Jungreis C, Levy EI, Stevenson K (2001) Endovascular management of a basilar artery false aneurysm secondary to endoscopic third ventriculostomy: case report. Neurosurgery 49(6):1461–1464; discussion 1464–1465
Kehler U, Gliemroth J, Knopp U, Arnold H (1998) How to perforate safely a resistant floor of the 3rd ventricle? Technical note. Minim Invasive Neurosurg 41(4):198–199
McLaughlin MR, Wahlig JB, Kaufmann AM, Albright AL (1997) Traumatic basilar aneurysm after endoscopic third ventriculostomy: case report. Neurosurgery 41(6):1400–1403; discussion 1403–1404
Peretta P, Ragazzi P, Galarza M, Genitori L, Giordano F, Mussa F, Cinalli G (2006) Complications and pitfalls of neuroendoscopic surgery in children. J Neurosurg 105(3 Suppl):187–193
Rezende MT, Spelle L, Piotin M, Mounayer C, Lucas Cde P, Abud DG, Moret J (2008) Selective endovascular treatment of a traumatic basilar aneurysm after endoscopic third ventriculostomy. Neuroradiology 50(5):443–446
Roux FE (2003) Death and third ventriculostomy. J Neurosurg 98(3):650; author reply 650–651
Schmidt RH (1999) Use of a microvascular Doppler probe to avoid basilar artery injury during endoscopic third ventriculostomy. Technical note. J Neurosurg 90(1):156–159
Schroeder HW, Niendorf WR, Gaab MR (2002) Complications of endoscopic third ventriculostomy. J Neurosurg 96(6):1032–1040
Vloeberghs M, Cartmill M (1999) Improved safety of neuroendoscopic third ventriculostomy by using an operative Doppler ultrasound probe. Technical note. Neurosurg Focus 6(4):e13
Beni-Adani L, Siomin V, Segev Y, Beni S, Constantini S (2000) Increasing chronic subdural hematoma after endoscopic III ventriculostomy. Childs Nerv Syst 16(7):402–405
Cinalli G, Spennato P, Ruggiero C, Aliberti F, Trischitta V, Buonocore MC, Cianciulli E, Maggi G (2007) Complications following endoscopic intracranial procedures in children. Childs Nerv Syst 23(6):633–644
Depreitere B, Dasi N, Rutka J, Dirks P, Drake J (2007) Endoscopic biopsy for intraventricular tumors in children. J Neurosurg 106(5 Suppl):340–346
Di Rocco C, Massimi L, Tamburrini G (2006) Shunts vs endoscopic third ventriculostomy in infants: are there different types and/or rates of complications? A review. Childs Nerv Syst 22(12):1573–1589
El-Dawlatly AA, Murshid WR, Elshimy A, Magboul MA, Samarkandi A, Takrouri MS (2000) The incidence of bradycardia during endoscopic third ventriculostomy. Anesth Analg 91(5):1142–1144
Ersahin Y, Arslan D (2008) Complications of endoscopic third ventriculostomy. Childs Nerv Syst 24(8):943–948
Hader WJ, Walker RL, Myles ST, Hamilton M (2008) Complications of endoscopic third ventriculostomy in previously shunted patients. Neurosurgery 63(1 Suppl 1):ONS168–ONS174; discussion ONS174–175
Husain M, Jha D, Vatsal DK, Thaman D, Gupta A, Husain N, Gupta RK (2003) Neuro-endoscopic surgery – experience and outcome analysis of 102 consecutive procedures in a busy neurosurgical centre of India. Acta Neurochir (Wien) 145(5):369–375; discussion 375–376
Massimi L, Di Rocco C, Tamburrini G, Caldarelli M, Iannelli A (2004) Endoscopic third ventriculostomy: complications and failures. Minerva Pediatr 56(2):167–181
Navarro R, Gil-Parra R, Reitman AJ, Olavarria G, Grant JA, Tomita T (2006) Endoscopic third ventriculostomy in children: early and late complications and their avoidance. Childs Nerv Syst 22(5):506–513
Constantini S, Siomin V (2005) Re: Death after late failure of endoscopic third ventriculostomy: a potential solution. Neurosurgery 56(3):E629
Constantini S, Siomin V (2005) Death after late failure of endoscopic third ventriculostomy: a potential solution. Neurosurgery 56(4):E873
Drake J, Chumas P, Kestle J, Pierre-Kahn A, Vinchon M, Brown J, Pollack IF, Arai H (2006) Late rapid deterioration after endoscopic third ventriculostomy: additional cases and review of the literature. J Neurosurg 105(2 Suppl):118–126
Fabiano AJ, Doyle K, Grand W (2010) Delayed stoma failure in adult communicating hydrocephalus after initial successful treatment by endoscopic third ventriculostomy: case report. Neurosurgery 66(6):E1210–E1211; discussion E1211
Hader WJ, Drake J, Cochrane D, Sparrow O, Johnson ES, Kestle J (2002) Death after late failure of third ventriculostomy in children. Report of three cases. J Neurosurg 97(1):211–215
Javadpour M, May P, Mallucci C (2003) Sudden death secondary to delayed closure of endoscopic third ventriculostomy. Br J Neurosurg 17(3):266–269
Lipina R, Palecek T, Reguli S, Kovarova M (2007) Death in consequence of late failure of endoscopic third ventriculostomy. Childs Nerv Syst 23(7):815–819
Mobbs RJ, Vonau M, Davies MA (2003) Death after late failure of endoscopic third ventriculostomy: a potential solution. Neurosurgery 53(2):384–385; discussion 385–386
Mohanty A, Vasudev MK, Sampath S, Radhesh S, Sastry Kolluri VR (2002) Failed endoscopic third ventriculostomy in children: management options. Pediatr Neurosurg 37(6):304–309
Nigri F, Telles C, Acioly MA (2010) Late obstruction of an endoscopic third ventriculostomy stoma by metastatic seeding of a recurrent medulloblastoma. J Neurosurg Pediatr 5(6):641–644
Kulkarni AV, Shams I, Cochrane DD, McNeely PD (2010) Does treatment with endoscopic third ventriculostomy result in less concern among parents of children with hydrocephalus? Childs Nerv Syst 26(11):1529–1534
Bullivant KJ, Hader W, Hamilton M (2009) A pediatric experience with endoscopic third ventriculostomy for hydrocephalus. Can J Neurosci Nurs 31(2):16–19
Cinalli G, Salazar C, Mallucci C, Yada JZ, Zerah M, Sainte-Rose C (1998) The role of endoscopic third ventriculostomy in the management of shunt malfunction. Neurosurgery 43(6):1323–1327; discussion 1327–1329
Cinalli G (1999) Alternatives to shunting. Childs Nerv Syst 15(11–12):718–731
Cinalli G, Sainte-Rose C, Chumas P, Zerah M, Brunelle F, Lot G, Pierre-Kahn A, Renier D (1999) Failure of third ventriculostomy in the treatment of aqueductal stenosis in children. Neurosurg Focus 6(4):e3
Di Rocco C, Cinalli G, Massimi L, Spennato P, Cianciulli E, Tamburrini G (2006) Endoscopic third ventriculostomy in the treatment of hydrocephalus in pediatric patients. Adv Tech Stand Neurosurg 31:119–219
Drake JM (2007) Endoscopic third ventriculostomy in pediatric patients: the Canadian experience. Neurosurgery 60(5):881–886; discussion 881–886
Drake JM, Kulkarni AV, Kestle J (2009) Endoscopic third ventriculostomy versus ventriculoperitoneal shunt in pediatric patients: a decision analysis. Childs Nerv Syst 25(4):467–472
Elbabaa SK, Steinmetz M, Ross J, Moon D, Luciano MG (2001) Endoscopic third ventriculostomy for obstructive hydrocephalus in the pediatric population: evaluation of outcome. Eur J Pediatr Surg 11(Suppl 1):S52–S54
Fukuhara T, Vorster SJ, Luciano MG (2000) Risk factors for failure of endoscopic third ventriculostomy for obstructive hydrocephalus. Neurosurgery 46(5):1100–1109; discussion 1109–1111
Gangemi M, Donati P, Maiuri F, Longatti P, Godano U, Mascari C (1999) Endoscopic third ventriculostomy for hydrocephalus. Minim Invasive Neurosurg 42(3):128–132
Gangemi M, Mascari C, Maiuri F, Godano U, Donati P, Longatti PL (2007) Long-term outcome of endoscopic third ventriculostomy in obstructive hydrocephalus. Minim Invasive Neurosurg 50(5):265–269
Goumnerova LC, Frim DM (1997) Treatment of hydrocephalus with third ventriculocisternostomy: outcome and CSF flow patterns. Pediatr Neurosurg 27(3):149–152
Hopf NJ, Grunert P, Fries G, Resch KD, Perneczky A (1999) Endoscopic third ventriculostomy: outcome analysis of 100 consecutive procedures. Neurosurgery 44(4):795–804; discussion 804–806
Jones RF, Stening WA, Brydon M (1990) Endoscopic third ventriculostomy. Neurosurgery 26(1):86–91; discussion 91–92
Kadrian D, van Gelder J, Florida D, Jones R, Vonau M, Teo C, Stening W, Kwok B (2005) Long-term reliability of endoscopic third ventriculostomy. Neurosurgery 56(6):1271–1278; discussion 1278
Kulkarni AV, Drake JM, Kestle JR, Mallucci CL, Sgouros S, Constantini S (2010) Predicting who will benefit from endoscopic third ventriculostomy compared with shunt insertion in childhood hydrocephalus using the ETV Success Score. J Neurosurg Pediatr 6(4):310–315
Kulkarni AV, Drake JM, Kestle JR, Mallucci CL, Sgouros S, Constantini S (2010) Endoscopic third ventriculostomy vs cerebrospinal fluid shunt in the treatment of hydrocephalus in children: a propensity score-adjusted analysis. Neurosurgery 67(3):588–593
Kulkarni AV, Hui S, Shams I, Donnelly R (2010) Quality of life in obstructive hydrocephalus: endoscopic third ventriculostomy compared to cerebrospinal fluid shunt. Childs Nerv Syst 26(1):75–79
Murshid WR (2000) Endoscopic third ventriculostomy: towards more indications for the treatment of non-communicating hydrocephalus. Minim Invasive Neurosurg 43(2):75–82
Singh D, Gupta V, Goyal A, Singh H, Sinha S, Singh AK, Kumar S (2003) Endoscopic third ventriculostomy in obstructed hydrocephalus. Neurol India 51(1):39–42
Siomin V, Weiner H, Wisoff J, Cinalli G, Pierre-Kahn A, Saint-Rose C, Abbott R, Elran H, Beni-Adani L, Ouaknine G, Constantini S (2001) Repeat endoscopic third ventriculostomy: is it worth trying? Childs Nerv Syst 17(9):551–555
Tisell M, Almstrom O, Stephensen H, Tullberg M, Wikkelso C (2000) How effective is endoscopic third ventriculostomy in treating adult hydrocephalus caused by primary aqueductal stenosis? Neurosurgery 46(1):104–110; discussion 101–110
Tuli S, Alshail E, Drake J (1999) Third ventriculostomy versus cerebrospinal fluid shunt as a first procedure in pediatric hydrocephalus. Pediatr Neurosurg 30(1):11–15
Warf BC, Mugamba J, Kulkarni AV (2010) Endoscopic third ventriculostomy in the treatment of childhood hydrocephalus in Uganda: report of a scoring system that predicts success. J Neurosurg Pediatr 5(2):143–148
Zong XY, Zhang YZ (2006) Endoscopic third ventriculostomy in treatment of obstructive hydrocephalus. Zhonghua Yi Xue Za Zhi 86(41):2905–2907
Klimo P Jr, Kestle JR (2005) Potentially useful outcome measures for clinical research in pediatric neurosurgery. J Neurosurg 103(3 Suppl):207–212
Dalrymple SJ, Kelly PJ (1992) Computer-assisted stereotactic third ventriculostomy in the management of noncommunicating hydrocephalus. Stereotact Funct Neurosurg 59(1–4):105–110
Hopf NJ, Perneczky A (1998) Endoscopic neurosurgery and endoscope-assisted microneurosurgery for the treatment of intracranial cysts. Neurosurgery 43(6):1330–1336; discussion 1336–1337
Cochrane DD, Kestle J (2002) Ventricular shunting for hydrocephalus in children: patients, procedures, surgeons and institutions in English Canada, 1989–2001. Eur J Pediatr Surg 12(Suppl 1):S6–S11
Cochrane DD, Kestle JR (2003) The influence of surgical operative experience on the duration of first ventriculoperitoneal shunt function and infection. Pediatr Neurosurg 38(6):295–301
Asai A, Hoffman HJ, Hendrick EB, Humphreys RP (1989) Dandy-Walker syndrome: experience at the Hospital for Sick Children, Toronto. Pediatr Neurosci 15(2):66–73
Beni-Adani L, Ben-Sira L, Constantini S (2010) Surgical treatment of post hemorrhagic hydrocephalus. In: Berger I, Schimmel MS (eds) Hot topics in neonatal neurology. Nova, New York, p Ch 9
Buxton N, Macarthur D, Mallucci C, Punt J, Vloeberghs M (1998) Neuroendoscopic third ventriculostomy in patients less than 1 year old. Pediatr Neurosurg 29(2):73–76
Buxton N, Jaspan T, Punt J (2002) Treatment of Chiari malformation, syringomyelia and hydrocephalus by neuroendoscopic third ventriculostomy. Minim Invasive Neurosurg 45(4):231–234
Chai WX (1995) Long-term results of fourth ventriculo-cisternostomy in complex versus simplex atresias of the fourth ventricle outlets. Acta Neurochir (Wien) 134(1–2):27–34
Cinalli G, Spennato P, Savarese L, Ruggiero C, Aliberti F, Cuomo L, Cianciulli E, Maggi G (2006) Endoscopic aqueductoplasty and placement of a stent in the cerebral aqueduct in the management of isolated fourth ventricle in children. J Neurosurg 104(1 Suppl):21–27
Decq P, Le Guerinel C, Sol JC, Brugieres P, Djindjian M, Nguyen JP (2001) Chiari I malformation: a rare cause of noncommunicating hydrocephalus treated by third ventriculostomy. J Neurosurg 95(5):783–790
Ersahin Y (2007) Endoscopic aqueductoplasty. Childs Nerv Syst 23(2):143–150
Faggin R, Bernardo A, Stieg P, Perilongo G, d’Avella D (2009) Hydrocephalus in infants less than six months of age: effectiveness of endoscopic third ventriculostomy. Eur J Pediatr Surg 19(4):216–219
Fritsch MJ, Kienke S, Manwaring KH, Mehdorn HM (2004) Endoscopic aqueductoplasty and interventriculostomy for the treatment of isolated fourth ventricle in children. Neurosurgery 55(2):372–377; discussion 377–379
Fritsch MJ, Kienke S, Ankermann T, Padoin M, Mehdorn HM (2005) Endoscopic third ventriculostomy in infants. J Neurosurg 103(1 Suppl):50–53
Fritsch MJ, Doerner L, Kienke S, Mehdorn HM (2005) Hydrocephalus in children with posterior fossa tumors: role of endoscopic third ventriculostomy. J Neurosurg 103(1 Suppl):40–42
Hamada H, Hayashi N, Endo S, Kurimoto M, Hirashima Y, Takaku A (1999) Endoscopic aqueductal plasty via the fourth ventricle through the cerebellar hemisphere under navigating system guidance–technical note. Neurol Med Chir (Tokyo) 39(13):950–952; discussion 952–954
Hayashi N, Hamada H, Hirashima Y, Kurimoto M, Takaku A, Endo S (2000) Clinical features in patients requiring reoperation after failed endoscopic procedures for hydrocephalus. Minim Invasive Neurosurg 43(4):181–186
Hayhurst C, Osman-Farah J, Das K, Mallucci C (2008) Initial management of hydrocephalus associated with Chiari malformation type I-syringomyelia complex via endoscopic third ventriculostomy: an outcome analysis. J Neurosurg 108(6):1211–1214
Hu ZQ, Zhu GT, Huang H, Dai B, Xiao ZY, Guan F, Zhang Y (2009) Endoscopic therapy for obstructive hydrocephalus caused by cysts in the pathway of CSF circulation. Zhonghua Yi Xue Za Zhi 89(17):1179–1184
Inamura T, Morioka T, Nishio S, Ikezaki K, Nonaka H, Yoshiura T (2002) Diverticular enlargement of the foramina of Luschka and congenital hydrocephalus. Childs Nerv Syst 18(11):652–655
Jimenez-Leon JC, Jimenez CS, Betancourt YM (2007) Neuroendoscopy. Its usefulness in the hydrocephalus management of children in developing countries. Medicina (B Aires) 67(6 Pt 1):665–673
Kadri H, Mawla AA (2004) Variations of endoscopic ventricular anatomy in children suffering from hydrocephalus associated with myelomeningocele. Minim Invasive Neurosurg 47(6):339–341
Longatti P, Fiorindi A, Feletti A, Baratto V (2006) Endoscopic opening of the foramen of Magendie using transaqueductal navigation for membrane obstruction of the fourth ventricle outlets. Technical note. J Neurosurg 105(6):924–927
Macarthur DC, Buxton N, Vloeberghs M, Punt J (2001) The effectiveness of neuroendoscopic interventions in children with brain tumours. Childs Nerv Syst 17(10):589–594
Marlin AE (2004) Management of hydrocephalus in the patient with myelomeningocele: an argument against third ventriculostomy. Neurosurg Focus 16(2):E4
Mohanty A (2003) Endoscopic third ventriculostomy with cystoventricular stent placement in the management of dandy-walker malformation: technical case report of three patients. Neurosurgery 53(5):1223–1228; discussion 1228–1229
Mohanty A (2005) Endoscopic options in the management of isolated fourth ventricles. Case report. J Neurosurg 103(1 Suppl):73–78
Mohanty A, Biswas A, Satish S, Praharaj SS, Sastry KV (2006) Treatment options for Dandy-Walker malformation. J Neurosurg 105(5 Suppl):348–356
Mohanty A, Biswas A, Satish S, Vollmer DG (2008) Efficacy of endoscopic third ventriculostomy in fourth ventricular outlet obstruction. Neurosurgery 63(5):905–913; discussion 913–914
O’Brien DF, Seghedoni A, Collins DR, Hayhurst C, Mallucci CL (2006) Is there an indication for ETV in young infants in aetiologies other than isolated aqueduct stenosis? Childs Nerv Syst 22(12):1565–1572
O’Brien DF, Hayhurst C, Pizer B, Mallucci CL (2006) Outcomes in patients undergoing single-trajectory endoscopic third ventriculostomy and endoscopic biopsy for midline tumors presenting with obstructive hydrocephalus. J Neurosurg 105(3 Suppl):219–226
Oertel JM, Mondorf Y, Baldauf J, Schroeder HW, Gaab MR (2009) Endoscopic third ventriculostomy for obstructive hydrocephalus due to intracranial hemorrhage with intraventricular extension. J Neurosurg 111(6):1119–1126
Oertel JM, Mondorf Y, Schroeder HW, Gaab MR (2010) Endoscopic diagnosis and treatment of far distal obstructive hydrocephalus. Acta Neurochir (Wien) 152(2):229–240
Pavez A, Salazar C, Rivera R, Contreras J, Orellana A, Guzman C, Iribarren O, Hernandez H, Elzo J, Moraga D (2006) Description of endoscopic ventricular anatomy in myelomeningocele. Minim Invasive Neurosurg 49(3):161–167
Psarros TG, Coimbra C (2004) Endoscopic third ventriculostomy for patients with hydrocephalus and fourth ventricular cysticercosis: a review of five cases. Minim Invasive Neurosurg 47(6):346–349
Ray P, Jallo GI, Kim RY, Kim BS, Wilson S, Kothbauer K, Abbott R (2005) Endoscopic third ventriculostomy for tumor-related hydrocephalus in a pediatric population. Neurosurg Focus 19(6):E8
Ruggiero C, Cinalli G, Spennato P, Aliberti F, Cianciulli E, Trischitta V, Maggi G (2004) Endoscopic third ventriculostomy in the treatment of hydrocephalus in posterior fossa tumors in children. Childs Nerv Syst 20(11–12):828–833
Sainte-Rose C, Cinalli G, Roux FE, Maixner R, Chumas PD, Mansour M, Carpentier A, Bourgeois M, Zerah M, Pierre-Kahn A, Renier D (2001) Management of hydrocephalus in pediatric patients with posterior fossa tumors: the role of endoscopic third ventriculostomy. J Neurosurg 95(5):791–797
Schijman E, Peter JC, Rekate HL, Sgouros S, Wong TT (2004) Management of hydrocephalus in posterior fossa tumors: how, what, when? Childs Nerv Syst 20(3):192–194
Singh D, Sachdev V, Singh AK, Sinha S (2005) Endoscopic third ventriculostomy in post-tubercular meningitic hydrocephalus: a preliminary report. Minim Invasive Neurosurg 48(1):47–52
Siomin V, Cinalli G, Grotenhuis A, Golash A, Oi S, Kothbauer K, Weiner H, Roth J, Beni-Adani L, Pierre-Kahn A, Takahashi Y, Mallucci C, Abbott R, Wisoff J, Constantini S (2002) Endoscopic third ventriculostomy in patients with cerebrospinal fluid infection and/or hemorrhage. J Neurosurg 97(3):519–524
Tamburrini G, Pettorini BL, Massimi L, Caldarelli M, Di Rocco C (2008) Endoscopic third ventriculostomy: the best option in the treatment of persistent hydrocephalus after posterior cranial fossa tumour removal? Childs Nerv Syst 24(12):1405–1412
Teo C, Jones R (1996) Management of hydrocephalus by endoscopic third ventriculostomy in patients with myelomeningocele. Pediatr Neurosurg 25(2):57–63; discussion 63
Villavicencio AT, Wellons JC 3rd, George TM (1998) Avoiding complicated shunt systems by open fenestration of symptomatic fourth ventricular cysts associated with hydrocephalus. Pediatr Neurosurg 29(6):314–319
Vindigni M, Tuniz F, Ius T, Cramaro A, Skrap M (2010) Endoscopic third ventriculostomy in patients with secondary triventricular hydrocephalus from a haemorrhage or ischaemia in the posterior cranial fossa. Minim Invasive Neurosurg 53(3):106–111
Warf BC (2005) Comparison of endoscopic third ventriculostomy alone and combined with choroid plexus cauterization in infants younger than 1 year of age: a prospective study in 550 African children. J Neurosurg 103(6 Suppl):475–481
Warf BC (2005) Hydrocephalus in Uganda: the predominance of infectious origin and primary management with endoscopic third ventriculostomy. J Neurosurg 102(1 Suppl):1–15
Warf BC, Campbell JW (2008) Combined endoscopic third ventriculostomy and choroid plexus cauterization as primary treatment of hydrocephalus for infants with myelomeningocele: long-term results of a prospective intent-to-treat study in 115 East African infants. J Neurosurg Pediatr 2(5):310–316
Warf B, Ondoma S, Kulkarni A, Donnelly R, Ampeire M, Akona J, Kabachelor CR, Mulondo R, Nsubuga BK (2009) Neurocognitive outcome and ventricular volume in children with myelomeningocele treated for hydrocephalus in Uganda. J Neurosurg Pediatr 4(6):564–570
Yamada H, Nakamura S, Tanaka Y, Tajima M, Kageyama N (1982) Ventriculography and cisternography with water-soluble contrast media in infants with myelomeningocele. Radiology 143(1):75–83
Yamini B, Refai D, Rubin CM, Frim DM (2004) Initial endoscopic management of pineal region tumors and associated hydrocephalus: clinical series and literature review. J Neurosurg 100(5 Suppl Pediatrics):437–441
Kulkarni AV, Shams I, Cochrane DD, McNeely PD (2010) Quality of life after endoscopic third ventriculostomy and cerebrospinal fluid shunting: an adjusted multivariable analysis in a large cohort. J Neurosurg Pediatr 6(1):11–16
Kulkarni AV, Warf BC, Drake JM, Mallucci CL, Sgouros S, Constantini S (2010) Surgery for hydrocephalus in sub-Saharan Africa versus developed nations: a risk-adjusted comparison of outcome. Childs Nerv Syst 26(12):1711–1717
St George E, Natarajan K, Sgouros S (2004) Changes in ventricular volume in hydrocephalic children following successful endoscopic third ventriculostomy. Childs Nerv Syst 20(11–12):834–838
Hiraoka K, Yamasaki H, Takagi M, Saito M, Nishio Y, Iizuka O, Kanno S, Kikuchi H, Kondo T, Mori E (2010) Changes in the volumes of the brain and cerebrospinal fluid spaces after shunt surgery in idiopathic normal-pressure hydrocephalus. J Neurol Sci 296(1–2):7–12
Ammirati M, Raimondi AJ (1987) Cerebrospinal fluid shunt infections in children. A study on the relationship between the etiology of hydrocephalus, age at the time of shunt placement, and infection rate. Childs Nerv Syst 3(2):106–109
Bakar EE, Bakar B (2010) Neuropsychological assessment of adult patients with shunted hydrocephalus. J Korean Neurosurg Soc 47(3):191–198
Kestle JR, Hoffman HJ, Soloniuk D, Humphreys RP, Drake JM, Hendrick EB (1993) A concerted effort to prevent shunt infection. Childs Nerv Syst 9(3):163–165
Kestle JR, Garton HJ, Whitehead WE, Drake JM, Kulkarni AV, Cochrane DD, Muszynski C, Walker ML (2006) Management of shunt infections: a multicenter pilot study. J Neurosurg 105(3 Suppl):177–181
Lacy M, Oliveira M, Austria E, Frim MD (2009) Neurocognitive outcome after endoscopic third ventriculocisterostomy in patients with obstructive hydrocephalus. J Int Neuropsychol Soc 15(3):394–398
Lindquist B, Persson EK, Fernell E, Uvebrant P (2011) Very long-term follow-up of cognitive function in adults treated in infancy for hydrocephalus. Childs Nerv Syst 27(4):597–601
Pople IK, Bayston R, Hayward RD (1992) Infection of cerebrospinal fluid shunts in infants: a study of etiological factors. J Neurosurg 77(1):29–36
Renier D, Lacombe J, Pierre-Kahn A, Sainte-Rose C, Hirsch JF (1984) Factors causing acute shunt infection. Computer analysis of 1174 operations. J Neurosurg 61(6):1072–1078
Simon TD, Hall M, Riva-Cambrin J, Albert JE, Jeffries HE, Lafleur B, Dean JM, Kestle JR (2009) Infection rates following initial cerebrospinal fluid shunt placement across pediatric hospitals in the United States. Clinical article. J Neurosurg Pediatr 4(2):156–165
Takahashi Y (2006) Long-term outcome and neurologic development after endoscopic third ventriculostomy versus shunting during infancy. Childs Nerv Syst 22(12):1591–1602
Walters BC, Hoffman HJ, Hendrick EB, Humphreys RP (1984) Cerebrospinal fluid shunt infection. Influences on initial management and subsequent outcome. J Neurosurg 60(5):1014–1021
Buxton N, Macarthur D, Mallucci C, Punt J, Vloeberghs M (1998) Neuroendoscopy in the premature population. Childs Nerv Syst 14(11):649–652
Koch D, Wagner W (2004) Endoscopic third ventriculostomy in infants of less than 1 year of age: which factors influence the outcome? Childs Nerv Syst 20(6):405–411
Mori H, Nishiyama K, Tanaka R (2003) Endoscopic third ventriculostomy for pediatric hydrocephalic patients: discussion of the indication and strategy for shunt removal. Childs Nerv Syst 19:618
Wagner W, Koch D (2005) Mechanisms of failure after endoscopic third ventriculostomy in young infants. J Neurosurg 103(1 Suppl):43–49
Abdullah J, Ariff AR, Ghazaime G, Naing NN (2001) Stereotactic neuroendoscopic management of hydrocephalus: a three-year follow-up and analysis of Malaysian children with aqueduct stenosis. Stereotact Funct Neurosurg 76(3–4):175–180
Beems T, Grotenhuis JA (2002) Is the success rate of endoscopic third ventriculostomy age-dependent? An analysis of the results of endoscopic third ventriculostomy in young children. Childs Nerv Syst 18(11):605–608
Bilginer B, Oguz KK, Akalan N (2009) Endoscopic third ventriculostomy for malfunction in previously shunted infants. Childs Nerv Syst 25(6):683–688
Garg A, Suri A, Chandra PS, Kumar R, Sharma BS, Mahapatra AK (2009) Endoscopic third ventriculostomy: 5 years’ experience at the All India Institute of Medical Sciences. Pediatr Neurosurg 45(1):1–5
Idowu O, Doherty A, Tiamiyu O (2008) Initial experience with endoscopic third ventriculostomy in Nigeria, West Africa. Childs Nerv Syst 24(2):253–255; discussion 257
Sufianov A, Sufianova G, Iakimov I (2008) Neuroendoscopic procedures in achievement of shunt independence: outcome analysis of 28 patients with shunt malfunction. Minim Invasive Neurosurg 51(3):158–164
Yadav YR, Jaiswal S, Adam N, Basoor A, Jain G (2006) Endoscopic third ventriculostomy in infants. Neurol India 54(2):161–163
Baldauf J, Oertel J, Gaab MR, Schroeder HW (2007) Endoscopic third ventriculostomy in children younger than 2 years of age. Childs Nerv Syst 23(6):623–626
Helseth E, Due-Tonnessen B, Egge A, Eide PK, Meling T, Lundar T, Froslie KF (2002) Treatment of hydrocephalus with endoscopic third ventriculocisternostomy. Tidsskr Nor Laegeforen 122(10):994–998
Kamikawa S, Inui A, Kobayashi N, Kuwamura K, Kasuga M, Yamadori T, Tamaki N (2001) Endoscopic treatment of hydrocephalus in children: a controlled study using newly developed Yamadori-type ventriculoscopes. Minim Invasive Neurosurg 44(1):25–30
Koch-Wiewrodt D, Wagner W (2006) Success and failure of endoscopic third ventriculostomy in young infants: are there different age distributions? Childs Nerv Syst 22(12):1537–1541
Oertel JM, Baldauf J, Schroeder HW, Gaab MR (2009) Endoscopic options in children: experience with 134 procedures. J Neurosurg Pediatr 3(2):81–89
Sacko O, Boetto S, Lauwers-Cances V, Dupuy M, Roux FE (2010) Endoscopic third ventriculostomy: outcome analysis in 368 procedures. J Neurosurg Pediatr 5(1):68–74
Lipina R, Reguli S, Dolezilova V, Kuncikova M, Podesvova H (2008) Endoscopic third ventriculostomy for obstructive hydrocephalus in children younger than 6 months of age: is it a first-choice method? Childs Nerv Syst 24(9):1021–1027
Algin O (2010) Role of complex hydrocephalus in unsuccessful endoscopic third ventriculostomy. Childs Nerv Syst 26(1):3–4; author reply 5–6
Balthasar AJ, Kort H, Cornips EM, Beuls EA, Weber JW, Vles JS (2007) Analysis of the success and failure of endoscopic third ventriculostomy in infants less than 1 year of age. Childs Nerv Syst 23(2):151–155
Burn SC, Saxena A, Tyagi A, Chumas PD (2003) Endoscopic third ventriculostomy in children during the first year of life. Childs Nerv Syst 19:618–619
Costa Val JA (2009) Minicraniotomy for endoscopic third ventriculostomy in babies: technical note with a 7-year-segment analysis. Childs Nerv Syst 25(3):357–359
Cota Val JA, Lara A, Herval LM, Serrano P (2003) Endoscopic third ventriculostomy in children under 2 years old: experience of a neuropediatric service in Brazil. Childs Nerv Syst 19:688
de Ribaupierre S, Rilliet B, Vernet O, Regli L, Villemure JG (2007) Third ventriculostomy vs ventriculoperitoneal shunt in pediatric obstructive hydrocephalus: results from a Swiss series and literature review. Childs Nerv Syst 23(5):527–533
Egger D, Balmer B, Altermatt S, Meuli M (2010) Third ventriculostomy in a single pediatric surgical unit. Childs Nerv Syst 26(1):93–99
El-Ghandour NM (2011) Endoscopic third ventriculostomy versus ventriculoperitoneal shunt in the treatment of obstructive hydrocephalus due to posterior fossa tumors in children. Childs Nerv Syst 27(1):117–126
Elgamal EA (2010) Continuous monitoring of intracranial pressure after endoscopic third ventriculostomy in the management of CSF shunt failure. Minim Invasive Neurosurg 53(2):49–54
Elgamal EA, El-Dawlatly AA, Murshid WR, El-Watidy SM, Jamjoom ZA (2011) Endoscopic third ventriculostomy for hydrocephalus in children younger than 1 year of age. Childs Nerv Syst 27(1):111–116
Etus V, Ceylan S (2005) Success of endoscopic third ventriculostomy in children less than 2 years of age. Neurosurg Rev 28(4):284–288
Fritsch MJ (2003) Indication and controversies for endoscopic third ventriculostomy in children. Childs Nerv Syst 19:706–707
Fritsch MJ, Mehdorn M (2002) Endoscopic intraventricular surgery for treatment of hydrocephalus and loculated CSF space in children less than one year of age. Pediatr Neurosurg 36(4):183–188
Galstian AG, Melikian AG, Korshunov AE, Arutoinov NV (2008) Endoscopic ventriculostomy of the third ventricle in infants of less than 2 years of age. Zh Vopr Neirokhir Im N N Burdenko 1:11–16; discussion 16–17
Gangemi M, Maiuri F, Colella G, Magro F, Seneca V, de Divitiis E (2007) Is endoscopic third ventriculostomy an internal shunt alone? Minim Invasive Neurosurg 50(1):47–50
Garg AK, Suri A, Sharma BS, Shamim SA, Bal CS (2009) Changes in cerebral perfusion hormone profile and cerebrospinal fluid flow across the third ventriculostomy after endoscopic third ventriculostomy in patients with aqueductal stenosis: a prospective study. Clinical article. J Neurosurg Pediatr 3(1):29–36
Garton HJ, Kestle JR, Cochrane DD, Steinbok P (2002) A cost-effectiveness analysis of endoscopic third ventriculostomy. Neurosurgery 51(1):69–77; discussion 77–78
Gathura E, Poenaru D, Bransford R, Albright AL (2010) Outcomes of ventriculoperitoneal shunt insertion in Sub-Saharan Africa. J Neurosurg Pediatr 6(4):329–335
Genitori L, Peretta P, Mussa F, Giordano F (2003) Endoscopic third ventriculostomy in children: are age and etiology of hydrocephalus predictive factors influencing the outcome in primary and secondary treated patients? A series of 254 patients and 276 procedures. Childs Nerv Syst 19:619
Gorayeb RP, Cavalheiro S, Zymberg ST (2004) Endoscopic third ventriculostomy in children younger than 1 year of age. J Neurosurg 100(5 Suppl Pediatrics):427–429
Greenfield JP, Hoffman C, Kuo E, Christos PJ, Souweidane MM (2008) Intraoperative assessment of endoscopic third ventriculostomy success. J Neurosurg Pediatr 2(5):298–303
Javadpour M, Mallucci C, Brodbelt A, Golash A, May P (2001) The impact of endoscopic third ventriculostomy on the management of newly diagnosed hydrocephalus in infants. Pediatr Neurosurg 35(3):131–135
Jones RF, Kwok BC, Stening WA, Vonau M (1994) Neuroendoscopic third ventriculostomy. A practical alternative to extracranial shunts in non-communicating hydrocephalus. Acta Neurochir Suppl 61:79–83
Kandasamy J, Dwan K, Hartley JC, Jenkinson MD, Hayhurst C, Gatscher S, Thompson D, Crimmins D, Mallucci C (2011) Antibiotic-impregnated ventriculoperitoneal shunts-a multi-centre British paediatric neurosurgery group (BPNG) study using historical controls. Childs Nerv Syst 27(4):575–581
Kim SK, Wang KC, Cho BK (2000) Surgical outcome of pediatric hydrocephalus treated by endoscopic III ventriculostomy: prognostic factors and interpretation of postoperative neuroimaging. Childs Nerv Syst 16(3):161–168; discussion 169
King JA, Auguste KI, Halliday W, Drake JM, Kulkarni AV (2010) Ventriculocystostomy and endoscopic third ventriculostomy/shunt placement in the management of hydrocephalus secondary to giant retrocerebellar cysts in infancy. J Neurosurg Pediatr 5(4):403–407
Koksal V, Oktem S (2010) Ventriculosubgaleal shunt procedure and its long-term outcomes in premature infants with post-hemorrhagic hydrocephalus. Childs Nerv Syst 26(11):1505–1515
Kombogiorgas D, Sgouros S (2006) Assessment of the influence of operative factors in the success of endoscopic third ventriculostomy in children. Childs Nerv Syst 22(10):1256–1262
Komolafe EO, Adeolu AA, Komolafe MA (2008) Treatment of cerebrospinal fluid shunting complications in a Nigerian neurosurgery programme. Case illustrations and review. Pediatr Neurosurg 44(1):36–42
Kulkarni AV (2010) Quality of life in childhood hydrocephalus: a review. Childs Nerv Syst 26(6):737–743
Lipina R, Reguli S, Novackova L, Podesvova H, Brichtova E (2010) Relation between TGF-beta 1 levels in cerebrospinal fluid and ETV outcome in premature newborns with posthemorrhagic hydrocephalus. Childs Nerv Syst 26(3):333–341
Marton E, Feletti A, Basaldella L, Longatti P (2010) Endoscopic third ventriculostomy in previously shunted children: a retrospective study. Childs Nerv Syst 26(7):937–943
Meling TR, Tiller C, Due-Tonnessen BJ, Egge A, Eide PK, Froslie KF, Lundar T, Helseth E (2007) Audits can improve neurosurgical practice–illustrated by endoscopic third ventriculostomy. Pediatr Neurosurg 43(6):482–487
Ogiwara H, Dipatri AJ Jr, Alden TD, Bowman RM, Tomita T (2010) Endoscopic third ventriculostomy for obstructive hydrocephalus in children younger than 6 months of age. Childs Nerv Syst 26(3):343–347
Osman-Farah JJM, Buxton N, May P, Mallucci C (2003) Endoscopic ventricular fenestration and ETV for the treatment of multicompartmental neonatal hydrocephalus. Childs Nerv Syst 19:702–703
Peretta P, Cinalli G, Spennato P, Ragazzi P, Ruggiero C, Aliberti F, Carlino C, Cianciulli E (2009) Long-term results of a second endoscopic third ventriculostomy in children: retrospective analysis of 40 cases. Neurosurgery 65(3):539–547; discussion 547
Peretta P, Ragazzi P, Carlino CF, Gaglini P, Cinalli G (2007) The role of Ommaya reservoir and endoscopic third ventriculostomy in the management of post-hemorrhagic hydrocephalus of prematurity. Childs Nerv Syst 23(7):765–771
Rahme R, Rahme RJ, Hourani R, Moussa R, Nohra G, Okais N, Samaha E, Rizk T (2009) Endoscopic third ventriculostomy: the Lebanese experience. Pediatr Neurosurg 45(5):361–367
Ros-Lopez B, Jaramillo-Dallimonti AM, De Miguel-Pueyo LS, Rodriguez-Barcelo S, Dominguez-Paez M, Ibanez-Botella G, Marquez-Marquez B, Arraez-Sanchez MA (2009) Ventricular haemorrhage in preterm neonates and posthemorrhagic hydrocephalus. Proposal of a management protocol based on early ventriculo-peritoneal shunt. Neurocirugia (Astur) 20(1):15–24
Scarrow AM, Levy EI, Pascucci L, Albright AL (2000) Outcome analysis of endoscopic III ventriculostomy. Childs Nerv Syst 16(7):442–444; discussion 445
Scavarda D, Bednarek N, Litre F, Koch C, Lena G, Morville P, Rousseaux P (2003) Acquired aqueductal stenosis in preterm infants: an indication for neuroendoscopic third ventriculostomy. Childs Nerv Syst 19(10–11):756–759
Sharman A (2000) Endoscopic third ventriculocisternostomies in the infant: pre- & post-operative magnetic resonance imaging evaluation elective project. Clin Radiol 55(12):989
Shim KW, Kim DS, Choi JU (2008) Simultaneous endoscopic third ventriculostomy and ventriculoperitoneal shunt for infantile hydrocephalus. Childs Nerv Syst 24(4):443–451
Souweidane MM, Morgenstern PF, Kang S, Tsiouris AJ, Roth J (2010) Endoscopic third ventriculostomy in patients with a diminished prepontine interval. J Neurosurg Pediatr 5(3):250–254
Sufianov AA, Sufianova GZ, Iakimov IA (2010) Endoscopic third ventriculostomy in patients younger than 2 years: outcome analysis of 41 hydrocephalus cases. J Neurosurg Pediatr 5(4):392–401
van Beijnum J, Hanlo PW, Fischer K, Majidpour MM, Kortekaas MF, Verdaasdonk RM, Vandertop WP (2008) Laser-assisted endoscopic third ventriculostomy: long-term results in a series of 202 patients. Neurosurgery 62(2):437–443; discussion 443–444
Wagner W (2006) Why a focus session on endoscopic third ventriculostomy in infants? Childs Nerv Syst 22(12):1527
Wagner W, Koch D (2003) Success or failure of endoscopic third ventriculostomy in young infants: is there a correlation with age in patients up to 12 months old? Childs Nerv Syst 19:618
Warf BC (2010) Pediatric hydrocephalus in East Africa: prevalence, causes, treatments, and strategies for the future. World Neurosurg 73(4):296–300
Warf BC, Kulkarni AV (2010) Intraoperative assessment of cerebral aqueduct patency and cisternal scarring: impact on success of endoscopic third ventriculostomy in 403 African children. J Neurosurg Pediatr 5(2):204–209
Warf BC (2007) Endoscopic third ventriculostomy and choroid plexus cauterization for pediatric hydrocephalus. Clin Neurosurg 54:78–82
Wiewrodt D, Schumacher R, Wagner W (2008) Hygromas after endoscopic third ventriculostomy in the first year of life: incidence, management and outcome in a series of 34 patients. Childs Nerv Syst 24(1):57–63
Sgouros S, Kulkharni AV, Constantini S (2006) The international infant hydrocephalus study: concept and rational. Childs Nerv Syst 22(4):338–345
Olschewski M, Schumacher M, Davis KB (1992) Analysis of randomized and nonrandomized patients in clinical trials using the comprehensive cohort follow-up study design. Control Clin Trials 13(3):226–239
Edwards RJ, Witchell C, Pople IK (2003) Chronic headaches in adults with spina bifida and associated hydrocephalus. Eur J Pediatr Surg 13(Suppl 1):S13–S17
Enchev Y, Oi S (2008) Historical trends of neuroendoscopic surgical techniques in the treatment of hydrocephalus. Neurosurg Rev 31(3):249–262
Pople IK, Edwards RJ, Aquilina K (2001) Endoscopic methods of hydrocephalus treatment. Neurosurg Clin N Am 12(4):719–735, viii
Carmel PW, Antunes JL, Hilal SK, Gold AP (1977) Dandy-Walker syndrome: clinico-pathological features and re-evaluation of modes of treatment. Surg Neurol 8(2):132–138
Cinalli G, Vinikoff L, Zerah M, Renier D, Pierre-Kahn A (1997) Dandy-Walker malformation associated with syringomyelia. Case illustration. J Neurosurg 86(3):571
Hirsch JF, Pierre-Kahn A, Renier D, Sainte-Rose C, Hoppe-Hirsch E (1984) The Dandy-Walker malformation. A review of 40 cases. J Neurosurg 61(3):515–522
Klein O, Pierre-Kahn A (2006) Focus on Dandy-Walker malformation. Neurochirurgie 52(4):347–356
Klein O, Pierre-Kahn A, Boddaert N, Parisot D, Brunelle F (2003) Dandy-Walker malformation: prenatal diagnosis and prognosis. Childs Nerv Syst 19(7–8):484–489
Kumar R, Jain MK, Chhabra DK (2001) Dandy-Walker syndrome: different modalities of treatment and outcome in 42 cases. Childs Nerv Syst 17(6):348–352
Sikorski CW, Curry DJ (2005) Endoscopic, single-catheter treatment of Dandy-Walker syndrome hydrocephalus: technical case report and review of treatment options. Pediatr Neurosurg 41(5):264–268
Jones RF, Kwok BC, Stening WA, Vonau M (1996) Third ventriculostomy for hydrocephalus associated with spinal dysraphism: indications and contraindications. Eur J Pediatr Surg 6(Suppl 1):5–6
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Constantini, S., Sgouros, S., Kulkarni, A.V. (2014). Neuroendoscopy in Infants and the International Infant Hydrocephalus Study (IIHS). In: Sgouros, S. (eds) Neuroendoscopy. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-39085-2_3
Download citation
DOI: https://doi.org/10.1007/978-3-642-39085-2_3
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-39084-5
Online ISBN: 978-3-642-39085-2
eBook Packages: MedicineMedicine (R0)