Abstract
Purpose
The purpose of this study is to investigate the incidence of cystic malacia in long-term survivors of pediatric brain tumors treated with high-dose cranial irradiation.
Materials and methods
Between 1997 and 2015, we treated 41 pediatric patients (26 males, 15 females; age ranging from 3.3 to 15.7 years, median 9-year-old) of pediatric brain tumors [17 medulloblastomas, 7 primitive neuroectodermal tumors (PNET), 3 pineoblastomas, 6 non-germinomatous germ cell tumors (NGGCT), 8 gliomas (including 4 ependymomas, 1 anaplastic astrocytoma, 1 oligodendroglioma, 1 pilocytic astrocytoma, 1 astroblastoma)] with high-dose craniospinal irradiation. Follow-up ranged from 14.0 to 189.2 months (median 86.0 months, mean 81.5 months), the irradiation dose to the whole neural axis ranged from 18 to 41.4 Gy, and the total local dose from 43.2 to 60.4 Gy. All patients underwent follow-up magnetic resonance imaging (MRI) studies at least once a year. Diagnosis of cystic malacia was based solely on MRI findings. Of the 41 patients, 31 were censored during their follow-up due to recurrence of the primary disease (n = 5), detection of secondary leukemia after development of cystic malacia (n = 1), or the absence of cystic malacia on the last follow-up MRI study (n = 25). We also evaluated the development of post-irradiation cavernous angioma and white matter changes.
Results
Following irradiation treatment, 11 patients developed 19 cystic malacia during a median course of 30.8 months (range 14.9 to 59.3 months). The site of predilection for cystic malacia was white matter around trigone of lateral ventricles with an incidence of 47.4% (9 of 19 lesions, 7 in 11 patients). Patients with supratentorial tumors developed cystic malacia statistically earlier than the patients with infratentorial tumors (P = 0.0178, log-rank test). Among the same patient group, incidence of post-irradiation cavernous angioma increased progressively, while the incidence of post-irradiation cystic malacia did not increase after 5 years. White matter degeneration developed earlier than cystic malacia or cavernous angioma, and these three clinical entities developed mutually exclusive of each other.
Conclusion
We attribute the higher incidence of post-irradiation cystic malacia, in our long-term follow-up study, to the cranial irradiation for pediatric brain tumors, particularly supratentorial brain tumors, and recommend a regular, long-term follow-up of brain tumor patients treated with cranial irradiation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Irradiation to central nervous system (CNS) plays an irreplaceable role in the treatment of some malignant brain tumors. The goal of therapeutic radiation is to provide a sufficient dose to treat malignant CNS tumors without affecting adjacent normal brain. As more therapeutic regimens are being implemented, long-term late effects of neurotoxicity in the surviving patients are being recognized [1–4]. Identification of radiation damage as a distinct ailment apart from the effects of the surgery, chemotherapy, tumor recurrence, or incidental degenerative disease is crucial to devise appropriate therapy [5]. A spectrum of CNS changes on MR imaging from atrophy, decreased attenuation of the deep cerebral white matter to radiation vasculopathy including moyamoya phenomenon and/or brain infarction, and rarely focal, enhancing radionecrosis has been noted [6]. Recent development in radiation therapy including three-dimensional conformal radiation therapy (3D-CRT), intensity-modulated radiotherapy, and volumetric arc therapy has reduced the development of delayed radiation injury [7]. However, on long-term follow-up, development of delayed radiation injury including cognitive impairment, hormonal deficiency, and secondary tumor including meningioma, glioma, and cavernous angioma is still clinically relevant [8, 9].
Cystic malacia (white matter necrosis) is one of the delayed radiation injury. Considered as final form of radiation-induced white matter injury, it is characterized by the necrotic changes of white matter [10–12]. However, relationship between cystic malacia and white matter changes including leukoencephalopathy is still (baffling) unsolved and that between cystic malacia and vasculopathy including cavernous angioma is obscure. In this study, we focused on the delayed radiation injury in long-term follow-up of pediatric brain tumor patients after treatment with high-dose irradiation. Our study revealed that incidence of post-radiotherapy cavernous angioma increased progressively, while the incidence of post-radiotherapy cystic malacia did not increase after 5 years. We further affirmed that lateral periventricular zone of trigone at lateral ventricle is the site of predilection for radiation induced cystic malacia implying that this region is susceptible to radiation injury.
Materials and methods
This retrospective study was approved by our institutional review board and waived the need of written patient consent. On behalf of all authors, the corresponding author states that there is no conflict of interest. To protect patient privacy, all identifiers were removed from our records upon completion of our analyses. Between 1997 and 2015, we treated 41 pediatric patients (26 males, 15 females; age ranging from 3.3 to 15.7 years, median 9-year-old) of brain tumors [17 medulloblastomas, 7 primitive neuroectodermal tumors (PNET), 3 pineoblastomas, 6 non-germinomatous germ cell tumors (NGGCT), 8 gliomas (including 4 ependymomas, 1 anaplastic astrocytoma, 1 oligodendroglioma, 1 pilocytic astrocytoma, 1 astroblastoma)] with high-dose craniospinal irradiation by 3D-CRT. The patients with medulloblastoma, PNET, and pineoblastoma received cerebrospinal irradiation (CSI) with local boost irradiation, while patients with pediatric glioma and ependymoma received local irradiation alone. Four of seven patients with NGGCT received CSI and local boost irradiation, and the remaining three patients received whole ventricle irradiation in addition to local boost irradiation. All patients with medulloblastoma, PNET, pineoblastoma, pediatric glioma, and ependymoma and two patients with NGGCT received adjuvant radiotherapy or chemoradiotherapy after removal of tumor. Rest four of six patients with NGGCT underwent biopsy followed by chemoradiotherapy and surgical removal of residual mature/immature teratoma component after chemoradiotherapy.
Follow-up ranged from 14.0 to 189.2 months (median 86.0 months, mean 81.5 months), the irradiation dose in CSI ranged from 18 to 41.4 Gy and the total local dose from 43.2 to 60.4 Gy. All patients underwent follow-up magnetic resonance imaging (MRI) studies at least once a year. Diagnosis of cystic malacia was based solely on MRI findings. Preoperative MR studies denied the presence of cystic malacia and cavernous angioma prior to treatment in all patients. No patient had a history of previous surgery, chemotherapy, or RT.
Of the 41 patients, 31 were censored during their follow-up due to recurrence of the primary disease (n = 5), detection of secondary leukemia after development of cystic malacia (n = 1), or the absence of cystic malacia on the last follow-up MRI study (n = 25).
Evaluation of cystic malacia and white matter degeneration
The diagnosis of cystic malacia was based solely on post-irradiation MRI findings. The lesion was classified as positive if the size was more than 3 mm and could be identified in both T1-weighted and T2-weighted imaging. The lesions developing in close proximity within 1 cm were considered as a single lesion.
White matter degeneration was graded according to the neuroimaging leukoencephalopathy toxicity grading criteria defined by the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE), version 3.0, briefly, grade 0, no change; grade 1, involving less than one third of white matter T2 hyperintensities of cerebrum; grade 2, involving one third to two thirds of white matter T2 hyperintensities of cerebrum; grade 3, two thirds or more of white matter T2 hyperintensities of cerebrum; and grade 4, near total white matter T2 hyperintensities. We also evaluated the white matter degeneration based on Fazekas rating scales [13]. The scale divides the white matter into periventricular and deep white matter region, and each is given a grade depending on the size and confluence of the lesions on T2-weighted and/or fluid attenuated inversion recovery images. Briefly, the periventricular white matter (PVWM) was graded from 0 to 3 (0 = absent; 1 = “caps” or pencil-thin lining; 2 = smooth “halo”; 3 = irregular periventricular signal extending into the deep white matter), and deep white matter (DWM) was also graded from 0 to 3 (0 = absent; 1 = punctate foci; 2 = beginning confluence; 3 = large confluent areas).
Evaluation of cavernous angioma
The development of cavernous angioma was also evaluated, and the cavernous angiomas were classified into four types based on the Zabramski classification [14]. Briefly, type of cavernous angioma was classified based on MR imaging: type I, hyperintense on T1- and T2-weighted images; type II, reticulated core of mixed signal intensity with a surrounding hemosiderin ring on T1- and T2-weighted images; type III, iso- to hypointense on T1- and T2-weighted images; and type IV, poorly visualized, except on gradient-echo images. None of the patient underwent surgery for cavernous angioma, and none of the lesions were confirmed histologically.
Statistical analysis
Statistical analyses were done with PRISM version 7.0 (GraphPad Software Inc., La Jolla, CA, USA). The interval between the delivery of craniospinal irradiation and the subsequent detection of cystic malacia was the time between the first irradiation session and the post-irradiation diagnosis of cystic malacia. To evaluate the diagnostic value, we performed Kaplan-Meier analysis (log-rank test) incorporating the diagnosis of cystic malacia based on MRI findings. The time to the development of the white matter change and post-irradiation cavernous angioma was also analyzed in the same way.
Results
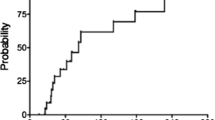
At the time of this writing, 34 were alive and free of the primary disease, recurrence, or the development of secondary neoplasms other than cavernous angioma; 5 died of recurrence, 1 died of secondary leukemia, and 1 was alive with recurrence of the primary disease. Following irradiation treatment, 11 patients developed 19 cystic malacia during a median course of 30.8 months with a range from 14.9 to 59.3 months (Fig. 1). The site of predilection for cystic malacia was white matter around trigone of lateral ventricles with the incidence of 47.4% (9 of 19 lesions, 7 in 11 patients). Even two of our patients with posterior fossa tumors developed cystic malacia around trigone of lateral ventricle. Other sites for development of cystic malacia were as follows: five in frontal, two in parietal, two in temporal, and one in cerebellum. Children treated at an age younger than 4.5 years developed cystic malacia significantly earlier than those who were at least 4.5 years old (Fig. 2; median 32.2 months vs not reached, P = 0.0169, log-rank test).
Patients with supratentorial tumors (n = 20) developed cystic malacia more frequently than the patients with infratentorial tumors (n = 21) (Fig. 3a; P = 0.0178, log-rank test). Similarly, patients with supratentorial tumors developed Zabramski type 1 and 2 cavernous angiomas significantly earlier than the patients with infratentorial tumors (Fig. 3b; P = 0.0157, log-rank test). There was no difference in the development of Zabramski type 3 and type 4 cavernous angiomas between supratentorial and infratentorial tumors (P = 0.8754, log-rank test). In our study, patients with supratentorial tumors developed white matter changes significantly earlier than did the patients with infratentorial tumors (Fig. 3c; P = 0.0007, log-rank test). Nineteen of 41 patients developed grade 1 leukoencephalopathy, while no patients developed grades 2–4 leukoencephalopathy (CTCAE version 3.0).
a Probability of developing cystic malacia in 41 pediatric patients who were followed for more than 12 months after high-dose craniospinal irradiation for brain tumors. Comparison was done between patients with supratentorial and infratentorial tumors. b Probability of developing Zabramski type I and II cavernous angiomas in 41 pediatric patients who were followed for more than 12 months after high-dose craniospinal irradiation for brain tumors. Comparison was done between patients with supratentorial and infratentorial tumors. c Probability of developing white matter change in 41 pediatric patients who were followed for more than 12 months after high-dose craniospinal irradiation for brain tumors. Comparison was done between patients with supratentorial and infratentorial tumors
When evaluating white matter changes based on Fazekas rating scales in pediatric brain tumors, patients were treated with high-dose irradiation, 17 patients developed grade 1 DWM hyperintensity, and 2 developed grade 2 DWM hyperintensity. Other patients did not develop DWM hyperintensity (Fazekas grade 0), and none of them developed Fazekas grade 3 DWM hyperintensity. PVWM hyperintensity, on the other hand, was less common, and only seven patients showed grade 1 PVWM hyperintensity. No patients showed Fazekas grade 2/3 PVWM hyperintensity.
Comparison of cystic malacia with cavernous angioma and white matter degeneration in the same patient group (Fig. 4) revealed that the incidence of cavernous angioma after high-dose cranial irradiation increased progressively, while that of cystic malacia did not increase after 5 years. White matter degeneration developed earlier than cystic malacia or cavernous angioma. Development of these three entities was mutually exclusive, and there was no association between the site of occurrence of cystic malacia and that of white matter degeneration or cavernous angioma. Representative cases are shown in Fig. 5a–c.
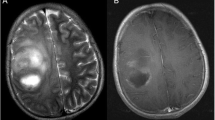
a A boy with a left temporal primitive neuroectodermal tumor who underwent chemoradiotherapy at the age of 10.9 years. MR examination 28 months later revealed cystic malacia on axial T2-weighted images (left side panel). He also had multiple secondary cavernous angiomas (right side panel). b A pediatric patient with left temporal anaplastic astrocytoma who underwent radiotherapy at the age of 4.2 years. MR examination 24.5 months later revealed cystic malacia on axial T2-weighted images. Subsequently, he developed seizure and needed anticonvulsants for seizure control. He did not have secondary cavernous angioma at the follow-up period of 56.5 months. c A pediatric patient with pineoblastoma who underwent radiotherapy at the age of 7.6 years. MR examination 42.0 months later revealed cystic malacia at right cerebellum and right parietal deep white matter on axial T2-weighted images (left side panel). He also had multiple secondary cavernous angiomas (right side panel)
In our patient series, none of the patients developed any symptom except for one who developed seizure requiring anticonvulsant therapy for seizure control. We did not perform cognitive examination, visual field examination, and electroencephalography routinely. We could not find any association between primary tumor site and site of cystic malacia or cavernous angioma. White matter change, on the other hand, developed mainly around primary tumor site in pediatric glioma patients.
Discussion
Before 1970, the human brain was thought to be radioresistant; the acute central nervous system syndrome occurs after single doses of ≥30 Gy, and cystic malacia can occur at fractionated doses of ≥60 Gy [15]. Although cystic malacia has been considered to be uncommon with modern radiation therapy techniques, irradiation to pediatric brain tumors could be a risk factor for cystic malacia. In this study, we revealed that the risk factors for cystic malacia after high-dose cranial irradiation by 3D-CRT are younger age at irradiation and supratentorial location of tumors. We also revealed that about half of cystic malacia developed around trigone of lateral ventricle. This implies that white matter around trigone of lateral ventricles is susceptible to irradiation injuries. Despite different etiologies, the common site for cystic malacia is the same as for periventricular leukomalacia suggesting that a common underlying pathophysiological mechanism may be associated between these two entities.
Periventricular leukomalacia is a form of white-matter brain injury, characterized by the necrosis of white matter near the lateral ventricles [16]. It can affect newborns, and premature infants are particularly at greatest risk of this disease. Full-term infants with congenital heart diseases may also exhibit periventricular leukomalacia [17]. Two major factors are reported to be involved in the development of this disease: decreased blood or oxygen supply to the periventricular region and damage to glial cells [18]. Some affected patients exhibit relatively minor deficits, while others have significant deficits and disabilities including delayed motor development, cerebral palsy, and/or epilepsy [18–20]. One study estimated that 47% of children with periventricular leukomalacia have epilepsy [21], whereas cystic malacia may not be a strong causative factor for epilepsy as substantiated by our study wherein only 1 of 12 patients developed epilepsy after development of cystic malacia. Other possible effects arising due to cystic malacia may be the visual field defect, motor disturbance, sensory disturbance, and cognitive decline. Future studies are necessary to confirm the diverse effects of cystic malacia.
Radiation-induced brain injury has been classified as acute, early delayed, or late delayed. Acute injury, occurring within days to weeks after irradiation, is rarely seen with current radiotherapy techniques. Early delayed injury occurs within 1–6 months of irradiation and often involves a transient somnolence. Late delayed effects develop around 6–12 months post-irradiation. They appear to be progressive and irreversible and include severe cognitive impairment. The pathological correlates at autopsy include vascular abnormalities including hyalinization and thickening of arteriolar walls, gliosis, demyelination, leukoencephalopathy, and ultimately, white matter necrosis.
Recent development in radiation therapy techniques has eliminated the occurrence of early and delayed radiation injuries. However, improvement in the survival of pediatric brain tumors revealed that radiotherapy of brain tumors can lead to devastating brain impairments even after many years of treatment [22]. In fact, the incidence of post-irradiation cavernous angioma increased progressively and this result is consistent with previous studies [24–27]. Our study also revealed that the incidence of post-irradiation cystic malacia did not increase after 5 years in patients of pediatric brain tumors. It may suggest that the mechanism of development of cystic malacia and cavernous angioma is totally different. Cavernous angioma may develop as a result of damage to vascular structures, while cystic malacia may develop due to damage to the glial cells and/or the white matter tract itself.
Focal and diffuse high signal intensities in the white matter are generally accepted findings on T2-weighted/FLAIR images after radiation therapy [3, 27]. The incidence of these white matter changes increases with the dose of irradiation and the duration after therapy [28]. White matter hyperintensity changes on T2-weighted/FLAIR images after irradiation are termed leukoencephalopathy. In our study, 19 of 41 patients developed cerebral white matter hyperintensities. Among them, all except two showed small white matter changes. Two patients showed confluence of hyperintense areas, but still categorized as CTCAE grade 1. CTCAE grade of leukoencephalopathy may not be suitable for the evaluation of “pediatric” brain tumor patients. In our evaluation with Fazekas rating scales, DWM hyperintensity was observed in 17 patients at grade 1, 2 patients at grade 2, and were more common than PVWM hyperintensity. More importantly, the etiology of PVWM and DWM changes reportedly differ in leukoaraiosis and may also differ in post-irradiation leukoencephalopathy. In leukoaraiosis patients, DWM change is considered to result from chronic small vessel ischemia, whereas PVWM change is not ischemic in nature, but a result of the combination of demyelination, ependymitis granularis, and subependymal gliosis [29]. Future studies are necessary to confirm the etiology and distribution of white matter changes after cranial irradiation in pediatric brain tumor patients.
Recently, functional deficits, including progressive impairments in memory, attention, and executive functions, having profound effects on quality of life, have become increasingly daunting [30]. The pathogenesis of the cognitive decline is not fully understood, but some evidence suggests that it could be a type of vascular dementia [31]. In our study, irradiation to supratentorial pediatric brain tumors developed cystic malacia, white matter changes, and Zabramski type I/II cavernous angiomas earlier than infratentorial tumors. These results imply that irradiation to supratentorial tumors will cause considerable damage to brain development compared to infratentorial tumors in pediatric patients. Development of cerebellum precedes the development of cerebrum during childhood, and we speculated that the developing brain may be more susceptible to radiation during childhood. Moreover, irradiation dose to cerebrum is higher in supratentorial tumors, which may be the reason for the development of cystic malacia at a higher incidence in the supratentorial tumors. However, we could not evaluate the contribution and pathogenesis of each entity for functional deficits. It could be a very complex mechanism, and future larger studies are necessary to elucidate the effect of these pathologies, underlying pathogenesis, and correlations with cognitive function.
Our study has some limitations. There are no previous reports regarding the methods of evaluating cystic malacia after cranial irradiation, and future studies are necessary to evaluate the radiation induced damage burden to the brain utilizing the technique of image standardization. The patient group was heterogeneous and received different chemotherapies, and we did not consider the effects of chemotherapy. Although the effect of radiotherapy on brain development is reportedly much higher than chemotherapy, future studies are necessary to confirm the contribution of chemotherapy as an etiology of cystic malacia, cavernous angioma, and white matter changes. The irradiation fields were heterogeneous because of the difference in diseases and variation in location of tumors. Small number of long-term survivors of each pediatric brain tumors precluded us from evaluating the effect of radiation dose, and we did not evaluate any of the pathologies histologically. Some Zabramski type 4 CVAs may have included areas of calcification or microbleeds due to etiologies such as senescence, and the clinical implications of detecting Zabramski type 4 CVAs remain to be determined. Diffusion-tensor imaging may be a promising non-invasive technique able to detect early changes in white matter integrity prior to radiographic evidence of radiation-induced demyelination or white matter necrosis [32, 33]. Future studies are necessary to confirm the etiology of cystic malacia.
Conclusion
We attribute the high incidence of post-irradiation cystic malacia in our long-term follow-up study to the cranial irradiation for pediatric brain tumors. Cystic malacia did not increase 5 years after irradiation, whereas cavernous angioma increased continuously with the passage of time. The incidence of cystic malacia, Zabramski type 1 or 2 CVA, and white matter changes were higher in patients with supratentorial brain tumors. Based on our findings, we recommend that survivors of pediatric brain tumors be followed regularly for a long-term as secondary changes may be detected long after the delivery of radiation therapy.
References
Rottenberg DA, Horten B, Kim JH, Posner JB (1980) Progressive white matter destruction following irradiation of an extracranial neoplasm. Ann Neurol 8:76–78
Wang AM, Skias DD, Rumbaugh CL, Schoene WC, Zamani A (1983) Central nervous system changes after radiation therapy and/or chemotherapy: correlation of CT and autopsy findings. AJNR Am J Neuroradiol 4:466–471
Tsuruda JS, Kortman KE, Bradley WG, Wheeler DC, Van Dalsem W, Bradley TP (1987) Radiation effects on cerebral white matter: MR evaluation. AJR Am J Roentgenol 149:165–171
Constine LS, Konski A, Ekholm S, McDonald S, Rubin P (1988) Adverse effects of brain irradiation correlated with MR and CT imaging. Int J Radiat Oncol Biol Phys 15:319–330
Walker AJ, Ruzevick J, Malayeri AA, Rigamonti D, Lim M, Redmond KJ, Kleinberg L (2014) Postradiation imaging changes in the CNS: how can we differentiate between treatment effect and disease progression? Future Oncol 10:1277–1297
Yamasaki F, Takayasu T, Nosaka R, Kenjo M, Akiyama Y, Tominaga A, Sugiyama K, Kobayashi M, Kurisu K (2015) The postirradiation incidence of cavernous angioma is higher in patients with childhood pineoblastoma or primitive neuroectodermal tumors than medulloblastoma. Childs Nerv Syst 31:901–907
Greene-Schloesser D, Robbins ME, Peiffer AM, Shaw EG, Wheeler KT, Chan MD (2012) Radiation-induced brain injury: a review. Front Oncol 2:73
Amirjamshidi A, Abbassioun K (2000) Radiation-induced tumors of the central nervous system occurring in childhood and adolescence. Four unusual lesions in three patients and a review of the literature. Childs Nerv Syst 16:390–397
Inskip PD, Sigurdson AJ, Veiga L, Bhatti P, Ronckers C, Rajaraman P, Boukheris H, Stovall M, Smith S, Hammond S, Henderson TO, Watt TC, Mertens AC, Leisenring W, Stratton K, Whitton J, Donaldson SS, Armstrong GT, Robison LL, Neglia JP (2016) Radiation-related new primary solid cancers in the childhood cancer survivor study: comparative radiation dose response and modification of treatment effects. Int J Radiat Oncol Biol Phys 94:800–807
Lee AW, Ng SH, Ho JH, Tse VK, Poon YF, Tse CC, Au GK, O SK, Lau WH, Foo WW (1988) Clinical diagnosis of late temporal lobe necrosis following radiation therapy for nasopharyngeal carcinoma. Cancer 61:1535–1542
Tarvonen-Schroder S, Roytta M, Raiha I, Kurki T, Rajala T, Sourander L (1996) Clinical features of leuko-araiosis. J Neurol Neurosurg Psychiatry 60:431–436
King AD, Ahuja AT, Yeung DK, Wong JK, Lee YY, Lam WW, Ho SS, Yu SC, Leung SF (2007) Delayed complications of radiotherapy treatment for nasopharyngeal carcinoma: imaging findings. Clin Radiol 62:195–203
Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA (1987) MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am J Roentgenol 149:351–356
Zabramski JM, Wascher TM, Spetzler RF, Johnson B, Golfinos J, Drayer BP, Brown B, Rigamonti D, Brown G (1994) The natural history of familial cavernous malformations: results of an ongoing study. J Neurosurg 80:422–432
Greene-Schloesser D, Robbins ME (2012) Radiation-induced cognitive impairment—from bench to bedside. Neuro Oncol 14(Suppl 4):iv37–iv44
Rezaie P, Dean A (2002) Periventricular leukomalacia, inflammation and white matter lesions within the developing nervous system. Neuropathology 22:106–132
Miller SP, McQuillen PS, Hamrick S, Xu D, Glidden DV, Charlton N, Karl T, Azakie A, Ferriero DM, Barkovich AJ, Vigneron DB (2007) Abnormal brain development in newborns with congenital heart disease. N Engl J Med 357:1928–1938
Kohelet D, Shochat R, Lusky A, Reichman B (2006) Risk factors for seizures in very low birthweight infants with periventricular leukomalacia. J Child Neurol 21:965–970
Glass HC, Fujimoto S, Ceppi-Cozzio C, Bartha AI, Vigneron DB, Barkovich AJ, Glidden DV, Ferriero DM, Miller SP (2008) White-matter injury is associated with impaired gaze in premature infants. Pediatr Neurol 38:10–15
Fetters L, Huang HH (2007) Motor development and sleep, play, and feeding positions in very-low-birthweight infants with and without white matter disease. Dev Med Child Neurol 49:807–813
Gurses C, Gross DW, Andermann F, Bastos A, Dubeau F, Calay M, Eraksoy M, Bezci S, Andermann E, Melanson D (1999) Periventricular leukomalacia and epilepsy: incidence and seizure pattern. Neurology 52:341–345
Laprie A, Hu Y, Alapetite C, Carrie C, Habrand JL, Bolle S, Bondiau PY, Ducassou A, Huchet A, Bertozzi AI, Perel Y, Moyal E, Balosso J (2015) Paediatric brain tumours: a review of radiotherapy, state of the art and challenges for the future regarding protontherapy and carbontherapy. Cancer Radiother 19:775–789
Lew SM, Morgan JN, Psaty E, Lefton DR, Allen JC, Abbott R (2006) Cumulative incidence of radiation-induced cavernomas in long-term survivors of medulloblastoma. J Neurosurg 104:103–107
Burn S, Gunny R, Phipps K, Gaze M, Hayward R (2007) Incidence of cavernoma development in children after radiotherapy for brain tumors. J Neurosurg 106:379–383
Vinchon M, Leblond P, Caron S, Delestret I, Baroncini M, Coche B (2011) Radiation-induced tumors in children irradiated for brain tumor: a longitudinal study. Childs Nerv Syst 27:445–453
von Hoff K, Hinkes B, Gerber NU, Deinlein F, Mittler U, Urban C, Benesch M, Warmuth-Metz M, Soerensen N, Zwiener I, Goette H, Schlegel PG, Pietsch T, Kortmann RD, Kuehl J, Rutkowski S (2009) Long-term outcome and clinical prognostic factors in children with medulloblastoma treated in the prospective randomised multicentre trial HIT’91. Eur J Cancer 45:1209–1217
Curran WJ, Hecht-Leavitt C, Schut L, Zimmerman RA, Nelson DF (1987) Magnetic resonance imaging of cranial radiation lesions. Int J Radiat Oncol Biol Phys 13:1093–1098
Nishimura R, Takahashi M, Morishita S, Sumi M, Uozumi H, Sakamoto Y (1992) MR imaging of late radiation brain injury. Radiat Med 10:101–108
Kim KW, MacFall JR, Payne ME (2008) Classification of white matter lesions on magnetic resonance imaging in elderly persons. Biol Psychiatry 64:273–280
Krull KR, Brinkman TM, Li C, Armstrong GT, Ness KK, Srivastava DK, Gurney JG, Kimberg C, Krasin MJ, Pui CH, Robison LL, Hudson MM (2013) Neurocognitive outcomes decades after treatment for childhood acute lymphoblastic leukemia: a report from the St Jude lifetime cohort study. J Clin Oncol 31:4407–4415
Brown WR, Blair RM, Moody DM, Thore CR, Ahmed S, Robbins ME, Wheeler KT (2007) Capillary loss precedes the cognitive impairment induced by fractionated whole-brain irradiation: a potential rat model of vascular dementia. J Neurol Sci 257:67–71
Nagesh V, Tsien CI, Chenevert TL, Ross BD, Lawrence TS, Junick L, Cao Y (2008) Radiation-induced changes in normal-appearing white matter in patients with cerebral tumors: a diffusion tensor imaging study. Int J Radiat Oncol Biol Phys 70:1002–1010
Connor M, Karunamuni R, McDonald C, White N, Pettersson N, Moiseenko V, Seibert T, Marshall D, Cervino L, Bartsch H, Kuperman J, Murzin V, Krishnan A, Farid N, Dale A, Hattangadi-Gluth J (2016) Dose-dependent white matter damage after brain radiotherapy. Radiother Oncol 121:209–216
Acknowledgement
This study was partially supported by Japan Society for the promotion of Science Grant-in-Aid for Scientific Research (C) (Grant-in-aid nos. 16K10757) and financial support for research project related to childhood cancer by Children’s Cancer Association of Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This retrospective study was approved by our institutional review board (No. E-454) and waived the need of written patient consent.
Conflict of interest
All authors, none.
Rights and permissions
About this article
Cite this article
Yamasaki, F., Takayasu, T., Nosaka, R. et al. Development of cystic malacia after high-dose cranial irradiation of pediatric CNS tumors in long-term follow-up. Childs Nerv Syst 33, 957–964 (2017). https://doi.org/10.1007/s00381-017-3400-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-017-3400-7