Abstract
Background
Only a few cases have been previously published about clear cell meningiomas in children, the majority of them in the location of the spine. We describe an unusual case of clear cell meningioma occurring at the petro-clival region in a 5-year-old child. We further seek to determine the impact of several growth factors as well as the AKT1 mutation on the tumor growth pattern.
Case presentation
A five-year-old girl was presented with a one-week history of cephalgia, ataxia, and left sided torticollis. Magnetic resonance imaging (MRI) revealed a dumbbell-shaped homogeneously petro-clival gadolinium-enhancing mass. A staged operative approach was chosen, and a complete removal of the tumor was achieved. Due to recurrent tumor progression, the child underwent several tumor surgeries and two cranial radiations. None of the treatments were able to stop tumor progression. Consequently, the child died at the age of 14 after further extensive intracranial and extracranial tumor progression. The initial histological examination revealed a clear cell meningioma WHO grade II with an MIB-1 labeling index of <1 %, which gradually increased with every recurrence up to 10 % by the last progression at the age of 13 years. Analogically, an increasing overexpression of epidermal growth factor receptor (EGFR), the platelet-derived growth factor receptor (PDGFR), and the vascular endothelial growth factor receptor (VEGFR) was observed with each recurrence. The AKT1 (E17K) mutation in the tumor was not detectable in all investigated specimens.
Conclusion
Pediatric clear cell meningiomas WHO grade II are very rare. Our data demonstrate the progressive overexpression of EGF-, PDGF-, and VEGF-receptors in each recurrence, providing one of these receptors as targeted therapy in such cases. Further evaluation of these growth factors in clear cell meningioma is required to establish the optimal treatment of these aggressive tumors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Meningiomas are common intracranial tumors in the adult population but occur only seldom in children, with an incidence of 3.7 % [1]. Clear cell meningiomas are a very rare variant and represent only 0.2 % of all meningiomas [2]. Although established as grade II neoplasia according to its bland histological features, a higher rate of local recurrence and even of cerebrospinal fluid metastases has been previously reported [3]. At present, there exist a limited number of cases reporting on clear cell meningiomas in children, the majority of them in the location of the spine [1, 4, 5]. Here, we report an unusual case of a clear cell meningioma occurring at the petro-clival region in a five-year-old child as well as correlate the radiological and clinical course of the disease with newly described relevant genetic and molecular alterations in meningiomas.
Case report
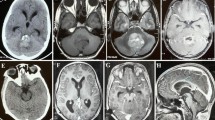
The five-year-old girl was presented with a one-week history of cephalgia, ataxia, and left sided torticollis. In the time prior to admission, she was healthy and normally developed without any pathological signs. Physical examination revealed a truncal ataxia and an abnormal gait. Magnetic resonance imaging (MRI) revealed a dumbbell-shaped homogenously gadolinium enhancing mass in the right cerebello-pontine angle and the right temporal fossa adjacent to the cavernous sinus and the major vessels of the anterior and posterior circulation (Fig. 1a, b). There was a massive displacement of the brain stem to the left with consecutive compression of the aqueduct and the fourth ventricle.
a, b Preoperative sagittal and coronar T1-weighted gadolinium-enhanced MR images showing a brightly enhancing intrinsic tumor of the brain stem upon admission. c–e One year after the initial surgery, a suspicious right parasellar recurrence was shown in the sagittal and coronar T1-weighted gadolinium-enhanced MR images. f–h Three months after termination (at age of 14 years), the girl developed an extensive intracranial and extracranial tumor progression which massively displaced the brain stem and the caudal cranial nerves. At this stage, no further treatment was achieved
Operation and postoperative course
A staged operative approach was chosen. First, a suboccipital craniotomy was performed, and the tumor was resected completely within the posterior fossa. All cranial nerves were pushed apart without a clear neural or dural matrix. A second operation via a modified frontotemporal craniotomy was performed three months later. Upon repeated MRI upfront to the second operation, further tumor growth was observed. However, a gross total resection of the tumor was achieved. The postoperative course was uneventful, and the patient was discharged two and a half weeks later from our hospital without focal neurological deficits.
Follow-up course and tumor progression
One year after the initial surgery, a suspicious right parasellar recurrence was shown in the MRI. The recurrence was confirmed after repeated MRI control (Fig. 1c, d). Therefore, 18 months after the second operation—at the age of six years—irradiation with 55 Gy to the recurrent mass at the cerebellopontine angle was applied. During the next three years, no tumor progression was detected in the follow-up MRIs which were performed at six-month intervals. The girl exhibited mild cognitive impairment and behavioral problems. After four years—at the age of 10—a new tumor recurrence was seen in the petrosal bone, foramen magnum, and tentorium. In an interdisciplinary operation, subtotal resection was achieved; postoperatively, a newly developed paresis of the right sided facial nerves as well as deafness in the right ear was observed. During the next three years, the child underwent two further surgeries—at the age of 11 and 13—to remove local recurrences in the petrosal bone and foramen magnum followed by tumor re-irradiation at the middle cranial fossa and carotic canal with 50 Gy. Three months after termination of radiotherapy, the girl developed an extensive intracranial and extracranial tumor progression which massively displaced the brain stem and the caudal cranial nerves (Fig. 1f–h). As a consequence, the patient developed a paresis of the left sided facial nerves as well as of hypoglossal and glossopharyngeal nerves with progressive dysphagia. A percutaneous endoscopic gastrostomy for feeding was placed at age of 14 years. The patient deteriorated rapidly over the next four months. She became comatose and was cared for in a home setting by a pediatric palliative care team until she died at the age of 14, eight years after the first tumor diagnosis.
Pathology
The submitted specimen from the first surgery—at the age of five years—revealed a mostly patternless, moderately cellular tumor composed of large polygonal cells with clear glycogen-rich cytoplasm (Fig. 2a). In some areas, the tumor cells formed small whorls around patches of dense collagen; reticulin fibers were rarely found outside of blood vessels (Fig. 2b). The nuclei were rounded with a few nucleoli and some intranuclear vacuoles. The tumor showed weak but consistently positive immunoreaction to the epithelial membrane antigen (Fig. 2c), and only a few proliferating cells were positive for the nuclear proliferation marker MIB-1 (<1 %, Fig. 2d). Mitotic activity was notably absent. The tumor was classified as clear cell meningiomas WHO grade II.
Histopathology of the presented clear cell meningioma at first appearance. a H&E staining showing a moderately cellular tumor with monomorphous nuclear appearance and large tumor cells with clear cytoplasm (H&E, original magnification ×20). b Elastica van Gieson trichrome. Collagen between tumor cells appears red; sparse perivascular reticulin fibers are black (EvG, original magnification ×20). c Immunochemistry for epithelial membrane antigen (EMA, Dako), which is present here similar to most other menigiomas. Indirect immunoperoxidase technique (Dako, LSAB) with diaminobenzidine (DAB, Sigma) as a chromogen, producing a brown positive reaction product located on cellular membranes. d Immunochemistry using the proliferation marker MIB-1 (indirect peroxidase technique (LSABII, DAKO, diaminobenzidine (DAB) as a chromogen, brown; original magnification ×20; counterstaining with hematoxylin). Note that only a few nuclei are positive (<1 %)
Histological examinations obtained from the recurrent tumors showed a gradual increase of the MIB-1 labeling index to 5 % then 6 % and finally to 10 % at the age of 6, 11, and 13 years, respectively. The EMA immunoreaction also increased with each recurrence.
Moreover, the expression level of the epidermal growth factor receptor (EGFR), platelet-derived growth factor receptor 1alpha (PDGFR), and the vascular endothelial growth factor receptor (VEGFR) was detected by immunohistochemistry (IHC) using the indirect peroxidase technique on all of the paraffin-embedded specimens from the patient. Staining was evaluated using a semiquantitative scoring system. The recurrent meningiomas showed an increased expression of all three receptors with each recurrence, whereas no overexpression was seen in the first tumor specimen (Fig. 3).
Representative images of overexpression of epidermal growth factor receptor (EGFR) (upper row) and the platelet-derived growth factor receptor (PDGFR) (lower row) at the point of last tumor recurrence. Indirect HRP-peroxidase technique (Medac) with diaminobenzidine (DAB) as a chromogen (brown); counter-staining with hematoxylin (Mayer) (blue) (original magnification ×40)
We further sought to determine the AKT1 (E17K) mutation status in this case using the PCR technique, since it has been newly described in medial skull base meningiomas [6]. However, the AKT1 mutation was absent in all tumor samples in our patient.
Discussion
In this case, we report on the progressive clinical course in a rare case of a pediatric clear cell meningioma WHO grade II in petro-clival location with several recurrences after surgical resection. The primary focus is on the assessment of the histopathological findings using immunohistochemistry to detect the expression level of many relevant growth factors during tumor progression.
The current clinical understanding is that clear cell meningiomas are very rare but equally very aggressive tumors of meningiothelial origin as recognized by the WHO [7]. They represent a therapeutic challenge given their rates of high recurrence and greater mortality. Interestingly, meningiomas with at least a 10 % clear cell component tend to have an increased risk of recurrence [8], especially in an intracranial rather than in spinal location. Fortunately, most of the pediatric cases are at spinal origin, and there are only few reports (n = 10) of intracranial clear cell meningiomas cases in childhood found in the literature [3, 4, 9–11] (Table 1). Most of these cases (9 of 10) are from pediatric patients older than eight years, with a median age of 11 years [2]. In our case, the child was five years old at the time of first tumor diagnosis. In general, young patients with a mean age of 29 years seem to be more affected by intracranial clear cell meningiomas than children [2, 3].
The treatment of clear cell meningiomas is challenging. Surgical resection is considered the gold standard treatment, with gross total resection resulting in complete long-term remission [2]. In addition, radiosurgery and radiotherapy have been generally considered as a treatment option for residual and recurrent cases following surgery and for surgically hazardous locations [2]. In the present case, the effect of the first applied irradiation to the recurrent mass resulted in a relatively long-term progression-free survival (four years). However, the second radiation was not able to stop the tumor progression for more than three months. Given their high rates of recurrence, clear cell meningiomas require close clinical follow-up and an individualized treatment strategy. Chemotherapy as a treatment option in this meningioma subtype has, however, not been investigated in prospective studies due to their low frequency. In general, there is most likely no place for chemotherapy or targeted therapy for meningiomas regardless of WHO grade, since several trials of chemotherapeutic agents did not reveal significant tumor control or regression [12]. This can be explained by the fact that meningiomas are heterogeneous tumors with distinct histopathological and cytogentic features. However, in recent years, important advances have been achieved in the identification of the genetic/molecular alterations of meningiomas and the signaling pathways involved there-with [6]. Thus, early mutations involving—among others—the genes AKT1, SMO, and TRAF7 have recently emerged as being oncogenic pathways in meningiomas, distinguishing them in subgroups with variant gene expression and distinct potential for malignant transformation, anatomical location, and histological appearance [6]. For instance, the AKT1 (E17K) mutation was detected in midline meningiomas of the posterior skull base [6]. In this case, we aimed to investigate the AKT1 mutation status, since none of the investigated tumors by Clark et al. was a clear cell meningioma and since the location of the tumor was in the posterior skull base [6]. We did not, however, find a mutant AKT1 gene, indicating a distinct etiology of these tumors in comparison with other midline posterior skull base meningiomas. Thus, using an AKT1-inhibitor as systemic treatment in this case might be ineffective.
In order to characterize the phenotype of the tumor further, we performed several immunohistological stainings. Immunohistochemsitry is helpful to diagnose clear cell meningiomas and to differentiate them from other meningioma subtypes, as well as from other tumor entities, such as ependymoma or metastatic renal cell carcinoma [3, 8]. In our case, the MIB-1 labeling index from the first tumor tissue was <1 %. This is markedly lower than the reported values in the literature, varying between 2 and 12 % [5]. It has been shown that MIB-1 labeling index is appreciably higher in tumors that recur than in those that do not [2]. However, despite the low MIB-1 labeling index initially, the child in our case developed several recurrences over the next years. Furthermore, an interesting aspect is the increasing MIB-1 labeling index in each recurrence (<1 % in the first and >10 % in the last manifestation), revealed in the consecutive histopathological investigation. This issue was reflected clinically by the increasing aggressiveness of the tumor in course.
Finally, we tested the expression level of several growth factor receptors (including EGF-, PDGF-, and VEGF-receptors) which have been described in the literature to be enhanced in meningiomas, although their clinical impact is still largely unknown. The EGFR has been found to be overexpressed in more than 60 % of meningiomas, enhancing meningioma cell proliferation and tumor progression [13]. Similarly, the overexpression of the PDGF-receptor has been associated with meningioma cell growth via an autocrine loop [14]. Furthermore, the VEGF receptor was reported to be overexpressed in meningioma, playing a central role in meningioma angiogenesis—which are highly vascular tumors—and in the formation of peritumoral edema [12]. The expression of all these receptors was observed to be more enhanced in atypical and malignant meningioma than in benign meningiomas. Further, an overexpression was reported to correlate with a more highly aggressive tumor nature [14, 15]. In concordance with the latter fact, the recurrent meningiomas in our patient showed an increased overexpression of all three receptors with each recurrence, associated with a decreased progression-free survival time after every surgery and a pronounced infiltration of the skull base with each recurrence.
Due to the fact that the child in our case developed several recurrences despite salvage surgeries and radiations, a recommendation for a treatment with a tyrosine kinase inhibitor (Imatinib)—a potent inhibitor of the BCL-Abl, PDGFR-ɑ, and PDGF-ß receptors—was made due to proven tumor overexpression of PDGFR. It is worth mentioning that a previous phase II trial with Imatinib showed a minimal clinical and radiological response in 32 meningioma patients [16]. However, this therapy was not implemented because of the rapid neurological deterioration of our patient.
Although there is at present no defined role for chemotherapy or targeted therapy in meningiomas, it might be recommended to start this treatment modality at an earlier tumor stage in such cases. Recent recommendation for chemotherapy is based upon a comparatively small amount of literature treating patients with surgery and radiation refractory meningioma; thus, we would have to decide which therapy to initiate—EGF-, PDGF-, or VEGF-receptor inhibitors. This decision would be made on the basis of a number of factors, including the highest density of receptor overexpression in the immunohistochemistry, tumor burden, and rate of disease progression. According to the published data, in such a patient as ours, with rapidly progressive, symptomatic disease following salvage surgery, and with VEGFR-overexpression, an adjuvant treatment with bevazicumab (Avastin®)—as an anti-VEGF antibody—might be beneficial. The decision would be based on the results of two newly published studies in patients treated for recurrent and malignant meningiomas, who showed a high rate of improved clinical response as well as a good tolerability and safety profile of the agent [17, 18]. However, the response to this treatment was reported to be of limited duration, and prospective studies are required to define the safety and efficacy of bevacizumab and other targeted therapies in this tumor entity [18]. Categorization of clear cell meningiomas by histology including immunohistochemistry, location, and genetic mutation will help in the improvement of treatment options.
References
Ravindranath K, Vasudevan MC, Pande A, Symss N (2013) Management of pediatric intracranial meningiomas: an analysis of 31 cases and review of literature. Childs Nerv Syst 29:573–582
Chen H, Li XM, Chen YC, Wu JS, Dou YF, Wang Y, Xu J, Zhong P, Jiang CC, Wang XQ (2011) Intracranial clear cell meningioma: a clinicopathologic study of 15 cases. Acta Neurochir (Wien) 153:1769–1780
Zorludemir S, Scheithauer BW, Hirose T, Van Houten C, Miller G, Meyer FB (1995) Clear cell meningioma. A clinicopathologic study of a potentially aggressive variant of meningioma. Am J Surg Pathol 19:493–505
Yu KB, Lim MK, Kim HJ, Suh CH, Park HC, Kim EY, Han HS (2002) Clear-cell meningioma: CT and MR imaging findings in two cases involving the spinal canal and cerebellopontine angle. Korean J Radiol 3:125–129
Jain D, Sharma MC, Sarkar C, Suri V, Garg A, Singh M, Sharma BS, Mahapatra AK (2007) Clear cell meningioma, an uncommon variant of meningioma: a clinicopathologic study of nine cases. J Neuro-Oncol 81:315–321
Clark VE, Erson-Omay EZ, Serin A, Yin J, Cotney J, Ozduman K, Avşar T, Li J, Murray PB, Henegariu O, Yilmaz S, Günel JM, Carrión-Grant G, Yilmaz B, Grady C, Tanrikulu B, Bakircioğlu M, Kaymakçalan H, Caglayan AO, Sencar L, Ceyhun E, Atik AF, Bayri Y, Bai H, Kolb LE, Hebert RM, Omay SB, Mishra-Gorur K, Choi M, Overton JD, Holland EC, Mane S, State MW, Bilgüvar K, Baehring JM, Gutin PH, Piepmeier JM, Vortmeyer A, Brennan CW, Pamir MN, Kiliç T, Lifton RP, Noonan JP, Yasuno K, Günel M (2013) Genomic analysis of non-NF2 meningiomas reveals mutations in TRAF7, KLF4, AKT1, and SMO. Science 339:1077–1080
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114:97–109
Prayson RA, Chamberlain WA, Angelov L (2010) Clear cell meningioma: a clinicopathologic study of 18 tumors and examination of the use of CD10, CA9, and RCC antibodies to distinguish between clear cell meningioma and metastatic clear cell renal cell carcinoma. Appl Immunohistochem Mol Morphol 18:422–428
Shih DF, Wang JS, Pan RG, Tseng HH (1996) Clear cell meningioma: a case report. Zhonghua Yi Xue Za Zhi (Taipei) 57:452–456
Teo JG, Goh KY, Rosenblum MK, Muszynski CA, Epstein FJ (1998) Intraparenchymal clear cell meningioma of the brainstem in a 2-year-old child. Case report and literature review. Pediatr Neurosurg 28:27–30
Lee W, Chang KH, Choe G, Chi JG, Chung CK, Kim IH, Han MH, Park SW, Shin SJ, Koh YH (2000) MR imaging features of clear-cell meningioma with diffuse leptomeningeal seeding. AJNR Am J Neuroradiol 21:130–132
Chamberlain MC (2012) The role of chemotherapy and targeted therapy in the treatment of intracranial meningioma. Curr Opin Oncol 24:666–671
Wrobel G, Roerig P, Kokocinski F, Neben K, Hahn M, Reifenberger G, Lichter P (2005) Microarray-based gene expression profiling of benign, atypical and anaplastic meningiomas identifies novel genes associated with meningioma progression. Int J Cancer 114:249–256
Simon M, Boström JP, Hartmann C (2007) Molecular genetics of meningiomas: from basic research to potential clinical applications. Neurosurgery 60:787–798, discussion 787–798
Lamszus K, Lengler U, Schmidt NO, Stavrou D, Ergün S, Westphal M (2000) Vascular endothelial growth factor, hepatocyte growth factor/scatter factor, basic fibroblast growth factor, and placenta growth factor in human meningiomas and their relation to angiogenesis and malignancy. Neurosurgery 46:938–947, discussion 947–938
Wen PY, Yung WK, Lamborn KR, Norden AD, Cloughesy TF, Abrey LE, Fine HA, Chang SM, Robins HI, Fink K, Deangelis LM, Mehta M, Di Tomaso E, Drappatz J, Kesari S, Ligon KL, Aldape K, Jain RK, Stiles CD, Egorin MJ, Prados MD (2009) Phase II study of Imatinib mesylate for recurrent meningiomas (North American brain tumor consortium study 01–08). Neuro Oncol 11:853–860
Nayak L, Iwamoto FM, Rudnick JD, Norden AD, Lee EQ, Drappatz J, Omuro A, Kaley TJ (2012) Atypical and anaplastic meningiomas treated with Bevacizumab. J Neuro-Oncol 109:187–193
Lou E, Sumrall AL, Turner S, Peters KB, Desjardins A, Vredenburgh JJ, McLendon RE, Herndon JE, McSherry F, Norfleet J, Friedman HS, Reardon DA (2012) Bevacizumab therapy for adults with recurrent/progressive meningioma: a retrospective series. J Neuro-Oncol 109:63–70
Conflict of interest
The authors have no financial interests to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Juratli, T.A., Geiger, K.D., Weigel, P. et al. A five year-old child with clear cell petro-clival meningioma: case report with clinical and histopathological long-term follow-up. Childs Nerv Syst 31, 2193–2198 (2015). https://doi.org/10.1007/s00381-015-2782-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-015-2782-7