Abstract
Purpose
Neuroepithelial cyst is considered an unusual differential diagnosis for cysts in the posterior fossa. Here, we present a paediatric case with such a pathology and review the pertinent literature.
Methods
A 12-year old girl with headache, vertigo and disturbed gait was diagnosed with a cystic lesion in the fourth ventricle after brain MRI study. She was operated with the pre-operative diagnosis of arachnoid cyst.
Results
A transparent, colourless cyst was observed intra-operatively. As frozen sections were consistent with endodermal cyst, total removal of the cyst was attempted. Definite histopathological studies and immunohistochemistry stains were in favour of neuroepithelial cyst. No regrowth of the cyst or recurrence of the symptoms was observed in her 2-year follow-up.
Conclusions
As neuroepithelial cyst is rarely encountered in the posterior fossa, the clinical, radiological and pathological characteristics of our case along with similar cases in the literature were reviewed and discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neuroepithelial cyst is a rare pathological diagnosis for posterior fossa lesions, and due its scarcity in the literature, there are still some ambiguities about its classification, diagnosis and treatment [1]. Among the reported cases in the posterior fossa, those arising from the fourth ventricle are even less frequent than those found at the cerebello-pontine angle. Also, different pathologies have been described under the same terminology of “neuroepithelial cyst” which should not be considered the same lesions when reviewing and comparing these reports. Actually, any cystic lesion with ependymal or epithelial lining is designated as neuroepithelial cyst, but specification of the pathology is necessary. Our experience in this case showed that it is sometimes very difficult to come up with a definite pathological diagnosis even after extensively performing immunohistochemistry (IHC) studies. Although it has been described in some case reports, more cases are still required to better clarify the clinical presentations, pathological differentiation, imaging findings and long-term follow-up of different surgical strategies. Here, we present a paediatric case with epithelial pathology and review the pertinent literature.
Case report
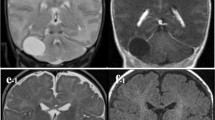
A 12-year-old girl complaining of morning headache in the previous weeks added by vertigo and disturbed gait was referred to our outpatient clinic. On her physical examination, she showed unstable walking with disturbed tandem gait. Finger to nose test was normal. Nystagmus existed with the fast phase towards the right. Cranial nerves were normal on examination except for slight right abducent nerve palsy. Her recent brain MRI showed a cystic lesion in the fourth ventricle dilating the right foramen of Luschka and reaching into the right cerebello-pontine angle (Fig. 1). The MRI characteristics of the cyst content were consistent with cerebrospinal fluid (CSF): hypo-intense in T1, hyper-intense in T2, signal eliminated in FLAIR and no restriction in diffusion-weighted images. No enhancement happened after gadolinium injection. A primary diagnosis of arachnoid cyst was made, and considering the symptoms, she was selected for open surgery with the aim of intra-operative pathological diagnosis and restoration of CSF flow.
MRI sequences of the patient before surgery. a A hypo-intense lesion in the fourth ventricle dilating the right foramen of Luschka on axial non-enhanced T1 MRI. b MRI T2 sequence shows the caudocranial extension of the lesion with intensity similar to CSF. c No enhancement is observed in T1 enhanced sequence. d The cyst content does not restrict in diffusion-weighted imaging
The patient was placed in prone position, and after a midline linear incision, suboccipital craniotomy was performed. After opening the dura, a cystic lesion protruding through the fourth ventricle outlet into the cervico-medullary junction was observed (Fig. 2a). The cyst wall was transparent, and the content was colourless and clear. An intra-operative frozen section was in favour of an endodermal rather than arachnoid cyst. So, we decided to proceed with total cyst removal if possible. The cyst wall was lifted with one hand and, little by little, dissected from the attached structures with the other hand (Fig. 2b). First, it was dissected apart from the fourth ventricle floor (Fig. 2c) and then through the already dilated foramen of Luschka into the right cerebello-pontine angle (Fig. 2d). The cyst was firmly attached only to the right rhomboid lip where we believe to be the origin of the lesion. After meticulous haemostasis, the dura and the incision were closed in layers. The post-operative course was uneventful, and the patient recovered from pre-operative symptoms gradually. Histopathological studies showed a single-layer columnar epithelium which was mostly ciliated and interspersed with goblet cells (Fig. 3a). This was different from arachnoid cells (usually flattened cells without cilia and villi). Complementary IHC stains were used to further clarify the origin and subtype of the cyst with the specimen being positive for epithelial membrane antigen (EMA), cytokeratin CK-7 (Fig. 3c), CAM5.2, vimentin and glial fibrillary acidic protein (GFAP) (Fig. 3d) while negative for cytokeratin CK20, TTF-1, carcinoembryonic antigen (CEA), CDX-2, p63 and pre-albumin. Though, the pathology was inconsistent with defined features of a certain neuroepithelial lesion, it was closer to the enterogenous cysts. In her 2-year follow-up examination, she was completely normal with no evidence of recurrence on MRI studies (Fig. 3d).
a Intra-operative view of the cyst protruding through the fourth ventricle outlet. b Cyst dissection from the surrounding structures and its removal were started out of the ventricle, c continued towards the fourth ventricle floor and d finished at the right cerebello-pontine angle. e Schematic view of d showing vertebral artery (VA), posterior inferior cerebellar artery (PICA) and lower cranial nerves. f Final view of the cervico-medullary junction after total cyst removal
a Photomicrograph of the cyst wall stained with haematoxylin and eosin showing a stroma of connective tissue and a single layer of columnar ciliated epithelial cells which turn pseudo-stratified on occasion. Immunohistochemistry stains for glial fibrillary acidic protein (b) and cytokeratin 7 (c) had highly positive results in epithelial cells. d Two years after the surgery, midsagittal T1 MRI depicted no sign of recurrence with resolution of the pre-operative mass effects on cerebellum and brain stem
Discussion
Definition
Neuroepithelial cyst is a terminology used interchangeably to describe a diverse group of lesions with different pathological features and origins causing some confusion. Some of these terms are epithelial cyst, enterogenous cyst, neuroenteric cyst, colloid cyst and choroidal epithelial cyst [11]. So, when reading under the general term of neuroepithelial cysts, one should be aware of the specific pathology, as their origin and long-term course may differ. One group includes cysts of true neuroepithelial origin whose pathologies resemble ependymal or choroid plexus cells. Another group includes respiratory or enterogenous cysts whose epithelial layers are similar to those of respiratory or gastrointestinal systems, respectively. A pathology that used to be named neuroepithelial cyst is colloid cyst whose origin was a matter of controversy throughout the last century, but endodermal origin seems more likely than the originally proposed paraphysial origin [9, 12]. These entities should be addressed separately, as their origin, pathology, location and radiology differ. This fact becomes more important when reviewing earlier literature as in some of them, pathological distinction has not been made.
Differential diagnosis
Other cystic lesions of the posterior fossa are possible diagnoses when reviewing pre-operative images [6]. Epidermoid cyst, arachnoid cyst, cystic schwannoma and different neuroepithelial cysts should be considered. These lesions, except for various neuroepithelial cysts, are easily differentiated pathologically, and their radiological characteristics are discussed later.
Pathology and origin
Unlike the true neuroepithelial cysts (i.e. choroid plexus or ependymal cysts) which have neuroectodermal origin, other mentioned epithelial cysts including enterogenous, respiratory and colloid cysts are of endodermal origin. In light microscopy, all these lesions share common features: columnar, mostly ciliated epithelium which is pseudostriated and turns single-layer in some portions. These cells may be replaced by cuboidal non-ciliated cells in some parts of the cyst [2, 13]. Epithelium interspersed with goblet cells is usually observed in enterogenous or colloid cysts. It is almost difficult to distinguish these various entities under light microscope, and electron microscopy or IHC staining is useful adjunct in most cases [9]. Earlier publications which did not use these modalities may not be accurate in diagnosing the correct pathology. Choroid plexus and ependymal cysts originate from neuroepithelium and lack both basement membrane and cellular interdigitations which are common features for endodermal cysts [5]. IHC reactivity for endodermal and ectodermal markers helps with diagnosis of these lesions (Table 1). However, the results are not always convincing, as in our case, the mixed characteristics of both endodermal (e.g. EMA and cytokeratin) and ectodermal (e.g. GFAP and vimentin) were positive, making a definite diagnosis challenging. In this case, GFAP positivity is in favour of ependymal origin, and cytokeratin-positive reactions indicate choroid plexus origin. This dual characteristic may be explained by the genesis of the cyst during the development, but as this phenomenon has not been discussed in the literature, further cases are required to address this theory. Overall, it seems that the term “neuroepithelial cyst” including both ependymal and choroid plexus epithelial cysts is the most appropriate term to name our case. According to Inoue et al., pre-albumin and S-100 protein IHC studies can help with diagnosis of choroidal epithelial cysts and CEA with neuroenteric cyst [7, 8]. Although both CEA and pre-albumin were negative, we cannot exclude these two pathologies, as other markers were consistent with their diagnosis. It more emphasizes on the fact that no single marker should be relied on for definite diagnosis of these cysts, and our knowledge of their differentiation is still limited. Maybe more cases with extensive IHC and genetic studies help with differentiation of these rare lesions in future.
Clinical features
Though the subject in our case was a 12-year-old girl, these pathologies can be found at any age [13]. This might be explained by lack of pericystic oedema and gradual increase in size of the cyst due to accumulation of breakdown products or secretions by the epithelial cells [3]. If the embryologic connection with the environment outside the nervous system exists, they may present as recurrent bouts of meningitis [10]. Otherwise, clinical symptoms are usually determined by the location of the cyst. Those present at cerebello-pontine angle present most commonly with vertigo, gait disturbances and hearing complaints [13]. Symptoms of increased intra-cranial pressure (i.e. headache, blurred vision, nausea and vomiting) due to obstructive hydrocephaly are observed more frequently with fourth ventricle cystic lesions, although ataxia and vertigo are also common features [1, 14, 15]. Symptoms of increased intra-cranial pressure usually exist for a few months when overt symptoms of gait disturbances bring the patient to the physician. These were the same findings in our patient at her presentation. Usually, headache is neglected by the patient, but its persistence and aggravation or additional new symptoms make her seek medical care. Rarely, fourth ventricle cysts can be totally asymptomatic only to be found at autopsy after an unrelated death [2].
Imaging
Most common location of neuroenterogenous cysts is cervico-thoracic spine, and intra-cranial lesions are mostly located in the pre-pontine cistern. Colloid cysts are often found in third ventricle, and their infratentorial fourth ventricle location is an exception. Choroid plexus cysts are mostly found in atrium of the lateral ventricle. However, they can be found anywhere along the choroid plexus. Similarly, ependymal cysts are often observed in lateral ventricle atrium and rarely found in the fourth ventricle [15]. As mentioned, the localization of a cyst to the fourth ventricle does not narrow differential list per se, as all these lesions are encountered rarely at clinic. Additionally, all these lesions come in radiological differential diagnosis to some other pathologies such as arachnoid and inclusion cysts. Radiological features of a cystic lesion confined to the fourth ventricle are summarized in Table 2. However, these are just the most common findings, and other MRI features may also be observed. Neuroepithelial cysts may have variable T1 and T2 appearances based on their mucinous, cholesterol and calcium content, but hypo-T1 or hyper-T2 is the classical finding. Usually, it is very difficult if not impossible to have a definite pre-operative diagnosis, but these lesions should be taken into consideration when facing with a cyst with stereotypical features. In our case, the cyst content was similar to CSF intensity in different MRI sequences. The unusual location for an arachnoid cyst may bring some other pathologies including various types of neuroepithelial cysts higher into differential diagnosis list, but still no pre-operative imaging clue exists to diagnose these lesions efficiently.
Treatment
In asymptomatic patients, the main concern is differentiation of a benign cyst from a malignant process. For this purpose, minimally invasive biopsy or follow-up with imaging has been recommended. In symptomatic cases, surgical removal of the fourth ventricle cysts through a midline suboccipital craniotomy is suggested [11, 15]. Partial resection of these lesions is showed to be associated with high recurrence rate after variable time intervals [13]. So, the goal of surgery should be total removal although not always possible. If the location of the cyst makes cyst wall removal impossible or recurrence occurs, an external drainage device, cysto-ventricular or cysto-subarachnoid shunt is recommended [4]. Whenever the diagnosis is in question, an intra-operative pathological confirmation can affect the goal of surgery, as for some of the pathologies (e.g. arachnoid cyst), a total cyst resection is neither necessary nor recommended. Adjuvant chemoradiotherapy has not been proposed for the treatment of these lesions considering their benign nature.
Conclusion
Neuroepithelial cysts should be considered a potential diagnosis for posterior fossa cysts either midline or at cerebello-pontine angle. We believe that the surgeon should intend to remove these cysts completely, although intra-operative judgement is mandatory to retreat to partial resection if releasing the cyst wall adhesions is considered to be harmful. Due to the slowly progressive nature of these lesions, long-term follow-up with imaging and clinical examination is warranted.
References
Afshar F, Scholtz CL (1981) Enterogenous cyst of the fourth ventricle. J Neurosurg 54:836–838
Challa VR, Markesbery WR (1978) Infratentorial neuroepithelial cyst (colloid cyst). J Neurosurg 49:457–459
Ciricillo SF, Davis RL, Wilson CB (1990) Neuroepithelial cysts of the posterior fossa. J Neurosurg 72:302–305
Heran NS, Berk C, Constantoyannis C, Honey CR (2003) Neuroepithelial cysts presenting with movement disorders: two cases. Can J Neurol Sci 30:393–396
Hirano A, Ghatak NR (1974) The fine structure of colloid cysts of the third ventricle. J Neuropathol Exp Neurol 33:333–341
Holman MA, Schmitt WR, Carlson ML, Driscoll CLW, Beatty CW, Link MJ (2013) Pediatric cerebellopontine angle and internal auditory canal tumors. J Neurosurg Pediatr 12:317–324
Inoue T, Kuromatsu C, Iwata Y, Matsushima T (1985) Symptomatic choroidal epithelial cyst in the fourth ventricle. Surg Neurol 4:57–62
Inoue T, Matsushima T, Fukui M, Iwaki T, Takeshita I, Kuromatsu C (1988) Immunohistochemical study of intracranial cysts. Neurosurgery 23(5):576–581
Kondziolka D, Bilbao JM (1989) An immunohistochemical study of neuroepithelial (colloid) cysts. J Neurosurg 71:91–97
Muller J, Voelker JL, Campbell RL (1989) Respiratory epithelial cyst of the cerebellopontine angle. Neurosurgery 24:936–939
Okabe S, Kamata K, Kohno T, Harada Y (1995) Enterogenous cyst in the fourth ventricle. Neurol Med Chir (Tokyo) 35:40–44
Palacios E, Azar-kia B, Shannon M, Messina AV (1976) Neuroepithelial (colloid) cysts: pathogenesis and unusual features. Am J Roentgenol 126(1):56–62
Perrini P, Rutherford SA, King AT, du Plessis D, Di Lorenzo N (2008) Enterogenous cysts of the cerebellopontine angle: short review illustrated by two new patients. Acta Neurochir (Wien) 150:177–184
Sharpe JA, Deck JHN (1977) Neuroepithelial cyst of the fourth ventricle. J Neurosurg 46:820–824
Tillich M, Ranner G, Trummer M, Kleinert R (1999) Symptomatic neuroepithelial (ependymal) cyst of the fourth ventricle: MR appearance. Am J Roentgenol 172(2):553–554
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hasegawa, M., Nouri, M., Nagahisa, S. et al. Neuroepithelial cyst of the fourth ventricle. Childs Nerv Syst 31, 155–159 (2015). https://doi.org/10.1007/s00381-014-2478-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-014-2478-4