Abstract
Objects
Carotid–vertebrobasilar anastomoses—the trigeminal, otic, hypoglossal, and proatlantal intersegmental arteries—serve as transitory channels between primitive internal carotid arteries and bilateral longitudinal neural arterial plexus, which is the precursor of future basilar artery, when the human embryo reaches about 4-mm length.
Material and methods
Normal and/or abnormal morphofunctional aspects of the prenatal and postnatal forms of the trigeminal artery are described according to personal and literature data. Many arteries of similar origin and course are also noted in the differential diagnosis of the trigeminal artery.
Conclusions
The persistent primitive trigeminal artery, as the most commonly carotid–vertebrobasilar anastomosis, has a reported incidence of 0.03–2.2% in the literature. There is female sex predilection, and it may be discovered in patients of any age, on either side, and in association with many vascular variants. Although the significance of persistent primitive trigeminal artery regarding the development of an aneurysm or association with another pathological condition may not be clear, its (ab)normal morphology is the inspiration for anatomists, especially for neurosurgeons, before planning diagnostic and therapeutic procedures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Development
The internal carotid, basilar, and vertebral arteries are formed during the first three embryonic vascular stages, respectively, which belong to the Congdon branchial period (approximately 4–12 mm). The primary, or branchial, stage appears at about 22 days of gestation and involves the appearance of a vascular apparatus destined to become the precursor of the posterior arteries. In the second, or post-branchial, stage, the vascular apparatus is replaced by the adult arterial system during a period that lasts about 18 days [90].
During embryonic development, six pairs of aortic arches are formed, coursing around the five branchial arches by the 32nd day. The internal carotid artery (ICA) originates from the dorsal aorta and the third aortic arch at the 4- to 5-mm embryonic stage. The ICA receives contributions from the upper intersegmental and presegmental arteries, connecting the longitudinal neural artery (LNA) on the ventral side of the hindbrain and forming carotid–vertebrobasilar (trigeminal, otic, hypoglossal, and proatlantal intersegmental) anastomoses. The proatlantal intersegmental artery (PIA) supplies the caudal part of the LNAs until this embryonic stage, when the developing vertebral arteries (VAs) take over this function. The basilar artery (BA) becomes evident in the 7- to 12-mm stage through the union of the LNAs. The caudal end of every LNA reaches the cervical region and anastomoses with the primitive VAs ascending from the longitudinal anastomotic vessels of cervical intersegmental arteries which are branches of the dorsal aorta (Fig. 1). At the 11.5-mm stage (34 days), the BA and VA are completely formed. At approximately 15- to 17-mm crown rump length of the embryo, the superior cerebellar artery (SCA) and anterior inferior cerebellar artery (AICA) on each side become prominent [107].
Modified drawing of carotid–vertebrobasilar anastomoses at the 4- to 5-mm embryonic stage (http://www.ajnr.org/cgi/content/full/25/9/1622/F5). DAo dorsal aorta, ECA external carotid artery, ICA internal carotid artery, VAo ventral aorta, CIAs cervical intersegmental arteries, PTA primitive trigeminal artery, POA primitive otic artery, PHA primitive hypoglossal artery, PIA primitive proatlantal intersegmental artery
The establishment of a primitive trigeminal artery (PTA) according to Streeter [109], as quoted by Schmid [102], is thought to be related to the development of the trigeminal ganglion. The PTA regression is usually complete at the 45-mm stage because of the subsequent development of the posterior cerebral artery (PCA) which takes over blood supply to the BA, the increased distance between the ICA and BA, and the increased angulation between ICA and PTA. The PTA usually regresses at its transdural segment [136]. The persistence of the PTA in adults mirrors the 11- to 14-mm embryonic stage (41–43 days).
The carotid–vertebrobasilar anastomoses are patent for a period of 7 to 10 days. Why one of the carotid–vertebrobasilar anastomoses persists into later fetal and adult stage or why a vessel cannot be resorbed once it reaches a critical size is not completely clear. It is thought that no rigid genetic programming exists in the development of the cerebral blood vessels and that the momentary needs in the dynamic process of the developing brain continually reshape the vessels by formation, regression, and anastomosis [110].
Developmental mirrors
Chambers and Lukin [18] pointed out embryologic data about trigeminal variants. They noted that the ICA may provide the entire supply to any of the three main cerebellar arteries through a persistent primitive trigeminal artery (PPTA) that may furnish the dominant supply to an undifferentiated group of vascular patches along the paired LNAs. The dorsal neural vascular patches differentiate to form the SCA or AICA or PICA, and the PTA retains its primitive connection. The remaining LNAs fuse in the midline as a BA with little or no connection with the cerebellar artery fed from the ICA.
A common origin of the embryonic vessels may lead to a common origin of their remnants [136]. Their coexistence could be related to some underlying embryologic dysgenesis or some common insult resulting in vascular maldevelopment during the first several weeks of gestation [60].
Persistent primitive trigeminal artery
First literature data
An anomalous carotid–basilar anastomosis which passes alongside the trigeminal root was, according to Lie [58], first described by Quain [93]. Sutton [111], according to Lie [58], was the first who showed PPTA on the arteriogram.
Incidence
Since the first angiogram in 1950, more than 100 cases until 1967 have been described [76]. The incidences in the recent study of O'uchi and O'uchi [87], including 103 cases of PTA and so-called primitive trigeminal artery variants (PTAVs), are as follows: 0.29% of PPTAs, 0.34% of PTAVs, and 0.03% of the unclassified type of PPTAs. However, a case described by Samra et al. [100] was the only instance of PPTA in a series of 1,500 angiograms (0.06%), while De Bondt et al. [22] revealed PPTAs in 2.2% of cases.
The PPTA is by far the most common of the three types of persistent carotid–basilar anastomosis; the reason for its relatively high incidence is related to the fact that it is the major anastomotic channel of the presegmental arteries and also the last to disappear [107].
Gender
Many decades ago, Wise and Palubinskas [25] noted six female out of seven PPTA cases. However, in the recent study of O'uchi and O'uchi [87], the male/female ratio was 17:31.
Aging
Khodadad [51] described three cases of the PPTA in fetuses. Generally, the PPTA was documented as well in a female newborn [123], in an 83-year-old female case [91], in a 6-month-old male [84], as well as in an 83-year-old male case [79].
Left–right side appearance
There were different findings in many papers. Wise and Palubinskas [137] discovered six left PPTAs in series of seven cases, while Piotin et al. [92] observed PPTA on the right side in five cases and on the left in three. The citations from older literature regarding this anomaly, performed by Abe and Suzuki [1], presented that the PPTA originated from the right ICA in 15 and from the left ICA in 15 cases. The laterality of the origin of all PPTAs in a recent study was as follows: PPTA consisted of 16 right- and 32 left-sided cases [87]. Bilateral PPTA was also described [6, 13, 82].
Origin
The PPTA usually originates from the cavernous portion of the ICA [1, 2, 16, 27, 30, 38, 104, 108, 121, 125, 137], near the posterior genu [10, 20, 83, 91, 129], or from superomedial portion of the horizontal segment of cavernous ICA [110]. The vessel might originate from the medial [92, 102, 110], posteromedial [27], lateral [49, 92], posterolateral [91, 139], or posterior [36] aspect of the cavernous ICA. It was also documented that PPTA arose from the aneurysm sac of the right cavernous ICA [141]. The PPTA is located superior to the oculomotor, trochlear, and abducens nerves and medial to the ophthalmic branch of the trigeminal nerve and trigeminal ganglion. The abducens nerve was seen coursing just superior and contacting with PPTA, taking a ~90° course over the ICA–PPTA junction [125]. The trigeminal and abducens nerves enveloped in a thick membrane were attached to the PPTA [66].
Some authors described that the PPTA might arise from precavernous ICA [58, 89, 105] or about 1.5 cm below the carotid siphon [40] or from preselar portion of the ICA [59, 60]. Other authors [131, 137, 138] described the PPTA originating from supraclinoid (cerebral) ICA (Fig. 2). A portion of the BA between the SCA and AICA [4, 102, 136] or part near the top of BA [97] gives off a PPTA.
Origin of the persistent primitive trigeminal artery (PPTA) from the cerebral part of internal carotid artery (ICA) at cadaveric specimen [131]. ACoA anterior communicating artery, A1 pre-communicating part of the anterior cerebral artery, PCoA posterior communicating artery, P1 pre-communicating part of the posterior cerebral artery, P2 post-communicating part of the posterior cerebral artery, BA basilar artery, SCAs superior cerebellar arteries, AICA anterior inferior cerebellar artery
Differentiation
-
1.
The marginal tentorial artery arises from the cavernous portion of the ICA at the identical location as a PPTA [58].
-
2.
The meningohypophyseal trunk rises normally from the posterior aspect of the ICA, about half an inch distal to the origin of the PPTA [89]. Ohshiro et al. [79] found that at the origin of the PPTA, the meningohypophyseal trunk and the artery of the inferior cavernous sinus branched off in an 83-year-old man.
-
3.
The middle meningeal artery may also arise as a branch of the intracavernous portion of the ICA, as reviewed in an anatomical paper [63].
-
4.
The persistent primitive maxillary artery can originate from the medial surface of the C5 portion of the carotid siphon and can supply the posterior pituitary anastomoses with the contralateral one [92].
-
5.
Congenital transverse carotid anastomosis originating from the lower portion of one carotid siphon coursed transversely in front of the pituitary stalk and terminated in the normally developed lower portion of the contralateral siphon [41]. In theory, the collateral vessel is thought to arise from a union between two PTAs that have lost their communication with the BA [35].
-
6.
“PTAV” is a direct communication between the ICA and some of the cerebellar arteries without any interposition of the BA. This artery variant can originate from the posterior portion of the intracavernous ICA [96, 112, 116, 136] or from precavernous ICA [70, 118, 120]. Anomalous cerebellar arteries are paired in two groups. While the anterior group vessels were blushing the AICA territory completely [24, 47, 112, 120], the posterior group vessels supplied the most of the PICA territory [18, 77, 95, 120]. Teal et al. [119] were the first to report a case where the ICA supplied the ipsilateral SCA circulation through a PTAV.
Course
The course of the PPTA is usually tortuous or multisegmental compared with other persistent primitive arteries [87].
Initially, the PPTA courses through cavernous sinus [2, 49, 104] and emanates from the posterior wall of the cavernous sinus [125], following either a para- or an intrasellar course [1, 51, 91, 92]. The PPTA reaches the posterior cranial fossa in two ways: (1) In about half of the cases, the PPTA penetrates the sella turcica, runs in its own groove, and perforates the dura mater near the clivus; (2) in the other half of the cases, it runs extradurally after leaving the cavernous sinus, between the sensory trigeminal root and the lateral side of the sella in a groove of the posterior clinoid process [88, 102]. The roof of this groove is formed by the petroclinoid ligament [25]. The PPTA is fixed where it penetrates the dural foramen, as well as is relatively fixed where it joins the BA [69].
Kida et al. [53], as quoted by Arakawa et al. [9], classified the PPTA, based on the position of the abducens nerve, into lateral and medial (sub)types. These (sub)types could relate to the PPTA [16] or the so-called trigemino-cerebellar variants [9]. Among all 48 PPTA cases in the study of O'uchi and O'uchi [87], there were 44 lateral and four medial (sub)types. When the PTA originates from the posterolateral aspect of the posterior bend of the cavernous ICA, it crosses underneath and distorts the abducens nerve, continuing between the abducens and trigeminal nerves [2, 16, 20]. When taking a medial course, the PPTA arises from the posteromedial aspect of the posterior bend of the cavernous ICA and pierces the clival dura at the dorsum sellae [20, 27, 38, 126]. The PPTA also proceeds in close medial juxtaposition to the trigeminal ganglion and penetrates through the sella turcica [16, 92, 102] or courses in close relationship with the sensory root of the trigeminal nerve [107] or with the trunk of trigeminal nerve [66, 88, 108].
Length
The lengths of the PPTA at the intracavernous and subarachnoid segments were 0.9 and 1.1 cm, respectively, in a 68-year-old male and 3 cm in a 73-year-old female cadaver [125]. The PPTA could be tortuous [59, 92, 114] or of a trident shape [20].
Branches
Some authors claim that no case has been reported in which adult PPTA branched [89], contrary to the fetal one [51] or trigemino-cerebellar variants [9].
Khodadad [51] suggested that the PTAs might become of functional and clinical significance if they persisted, or it is quite possible that some or all of PPTA branches gradually become occluded after birth. Ohshiro et al. [79] noted that a PPTA in the posterior fossa branched into two branches on the way to the BA; one branch sent a feeding artery to the left trigeminal root and a perforating artery to the pons, while the other branch perforated directly into the pons. Suttner et al. [110] noted that the PPTA gave off two branches: the inferior hypophyseal and dorsal meningeal arteries. Takase et al. [115] showed a branch of the PPTA to the brainstem, while Yamada et al. [139] discovered the AICA from the left PPTA. Tubbs et al. [125] described the superior hypophyseal artery originating from the ICA–PPTA junction.
Termination
Generally, a communication of the PPTA and BA can be between the origin of the SCA and AICA [16, 88, 91, 110, 139]. It was either at basilar midpoint [1, 15, 30, 37, 117, 121] or near the junction of the BA middle and upper thirds [25, 141], or at a point between its proximal three fifths and distal two fifths [51] or at the upper third of the BA below the level of SCA [3, 10, 16, 27, 64, 129, 131] and in prepontine cistern [20, 91, 108]. The distance from the origin of SCA to the union of PTA with the BA was about 1 cm inferior to the origins of the SCA [54]; it ranges from a minimum of 0 mm to a maximum of 12 mm [87].
A “trigemino-cerebellar variant” [55] or a “trigeminal artery variant” [16] represents a junction of the PPTA with one of the cerebellar arteries originating from BA. This variant can connect the ICA and SCA [20, 83] or AICA [4, 9, 61]. A fenestration of the AICA could be a remnant of the junction of a PPTA with the ipsilateral vessel [130] (Fig. 3).
Fenestration of the left anterior inferior cerebellar artery (AICA) in fetal case [130]. BA basilar artery, VA vertebral artery. Arrow, remnant of the persistent primitive hypoglossal artery
A “stapedo-trigemino-cerebellar variant” was a large anastomosis between the SCA and an accessory meningeal artery originating from the ICA. Two modifications in the development of this stapedo-trigemino-cerebellar variant were presented. One modification was that the artery takes over the territory of the intracranial branch of the primitive stapedial artery (the “stapedo-trigeminal'” variant) and the other was that the artery takes over the territory of the SCA [55].
Caliber
The PPTA arising from the BA communicated with cavernous ICA [4, 97, 102, 136]. The PPTA caliber could be relatively small [28, 40, 137], narrow [114], large [52, 135], or almost equal to the ICA [102, 105, 125]. The diameter of the PTA could be equal to the BA above their anastomosis [110]. The diameter of the PPTA can differ before, at, and after the dural foramen. Before the foramen, it might be 4 mm, at the foramen 1 mm, and after the foramen could be 2 mm [79].
The PPTA caliber ranged from 1.7 to 4 mm in eight cases reported by Piotin et al. [92]. Its caliber “in vivo” was 5 mm in a 30-year-old woman [50] and in a 68-year-old male [125]; its caliber was 9 mm in a 73-year-old female cadaver [125].
The condition of the carotid system
Internal carotid artery
Aplasia of the cervical and petrosal portions of the left ICA [48], or a segmental dilatation of the left ICA [104], or a tortuous and medial course of both ICAs [108] or a kinking of the ICA at two levels [90] was associated with PPTA.
Anterior cerebral artery
Uchino et al. [126] and Varghese [129] revealed an azygos anterior cerebral artery (ACA) and left PPTA. Wismer [138] revealed a hypoplastic pre-communicating part (A1) of both ACAs, while Takase et al. [115] discovered a hypoplasia of the left A1 in the presence of the right PPTA. The absence of the right A1 part [103] and two cases of fenestration of the post-communicating part (A2) of ACA were also noted [87].
Middle cerebral artery
Two cases of double middle cerebral artery (MCAs) are revealed in association with PPTA [87].
Posterior communicating artery
As a rule, if the PPTA joins the BA between the SCA and AICA, the PCoA may be absent; if it joins only SCA in that case, the PCoA is large and supplies the PCA [42]. There were descriptions of the PCoA aplasia [20, 39, 121, 129], ipsilateral [4, 45, 51, 62, 92, 125], contralateral [20, 40, 92], or bilateral [75, 92]. In some cases, the PCoA had significant [10, 32, 37, 52, 68, 101, 110, 135, 137] or normal caliber [33, 37, 40, 131]. It was also hypoplastic, ipsilateral [3, 14, 65, 138], or opposite [39, 76] with PPTA. A hypoplasia of both PCoAs was evidenced by some authors [8, 52, 60, 102, 141]. Varghese [129] showed termination of the right PCoA at the junction of the BA with the right PCA. Previous findings are presented in Fig. 4.
Drawings (a–j) of the posterior communicating artery (PCoA) status in the presence of the persistent primitive trigeminal artery (PPTA). Normal PCoAs (a); bilateral PCoA aplasia (b); unilateral PCoA aplasia, ipsilaterally (c) and contralaterally (d); bilateral PCoA hypoplasia (e); unilateral PCoA hypoplasia (f); aplasia and hypoplasia of the PCoA (g); significant caliber of the PCoA, bilaterally (h) and unilaterally (i); termination of the PCoA at the origin of ipsilateral posterior cerebral artery (j). P1 pre-communicating part, P2 post-communicating part of the posterior cerebral artery (PCA), SCA superior cerebellar artery, BA basilar artery, VA vertebral artery, AICA anterior inferior cerebellar artery
The condition of the vertebrobasilar system
Vertebral artery
There were findings of ipsilateral hypoplasia [10, 48, 50, 88, 121, 129, 131] or contralateral [101] or aplasia [76] of the VA with PPTA. Hypoplasia of both VAs is also documented [30, 44–46, 58, 60, 62, 65, 73, 102, 110, 135, 137].
In cases of unilateral [8, 25, 39, 89, 105, 137] or bilateral PPTA [75], there was a termination of one VA into ipsilateral PICA. One case of bilateral PPTA was associated with a hypoplasia of the left VA and an aplasia of the right VA [13], whereas another case was associated with aplasia of the left VA [6]. A hypoplastic right VA of subclavian origin and a large caliber of the left VA of aortic origin in the presence of the left PPTA [131], or termination of hypoplastic right VA at the atlas level [50], were also documented. There were also notice of bilaterally normal [4, 40, 51, 68, 94] or tortuous VAs [90] or unilaterally dilatated VA [31, 84] in the presence of the PPTA.
Basilar artery
The part of the BA caudal to the point of junction with PPTA can be normal [4, 40, 79, 87, 131], aplastic [75], hypoplastic [8, 15, 20, 27, 34, 44, 46, 49, 58, 61, 65, 87, 102, 110, 113, 128, 129], or very hypoplastic [45, 87]. The PPTA usually supplies blood to both PCAs and SCAs via the distal BA, and when it persists, there is no flow-related stimulus for the BA proximal to the anastomosis to develop along with the embryo. This fact could explain the frequent association of BA hypoplasia with PPTA [83]. The degree of BA hypoplasia might be proportional to the functional importance of the PTA in the blood supply of the posterior circulation. The existence of BA hypoplasia allows easier detection of a PPTA and can be considered as an ancillary sign of PPTA (Fig. 5). In addition, above the anastomosis, the BA might be doubled [40]. There were data about BA elongation and dilatation [31, 84] or its segmental agenesis [48, 136]. The termination of the BA in two SCAs [138] or fenestration [39, 87] or tortuousity [90] of the BA could also be associated with PPTA.
Double BA caliber distally from the PPTA junction. Combination of findings based on three postmortem specimens: a [102]; b [137]; c Case is obtained during the routine autopsies at the Department of Forensic medicine in Niš, in accordance with the rules of the internal Ethical Committee (No. 01-206-1). VA vertebral artery, BA basilar artery, ICA internal carotid artery, PPTA persistent primitive trigeminal artery, P1 posterior cerebral artery—pre-communicating part, P2 posterior cerebral artery—post-communicating part, PCAc posterior cerebral artery of carotid origin, PCAb posterior cerebral artery of basilar origin, ICoA “intermediate communicating artery,” PCoA posterior communicating artery
Cerebellar arteries
Some authors revealed duplication of the left or right SCA [110, 129] or common trunks of AICAs and PICAs [79] simultaneously with the presence of the PPTA.
Posterior cerebral artery
A case reported by Wismer [138] presented both PCAs as final branches of the PPTA, while Lin et al. [59] discovered the left PCA in continuation of the ipsilateral PPTA. Khodadad [51] described bilateral hypoplasia of the precommunicating part (P1) of the PCA in an 8-month-old fetus, while Khodadad [52] and Wismer [138] discovered bilateral hypoplasia of the P1 in adults. Hinck and Gordy [40] described unilateral aplasia of P1 part opposite to the PPTA side, while Pascual-Castroviejo and Lopez-Gutierrez [90] evidenced its unilateral hypoplasia. A rich leptomeningeal anastomosis between the left PCA and the left MCA was also revealed in the presence of ipsilateral PPTA [32].
Saltzman’s angiographic types of PPTA
Saltzman [99], as quoted in the literature [24, 58], classified a PPTA into two types according to the configuration of ipsilateral PCA, while Kida et al. [53], as quoted by Arakawa et al. [9] as well Uchino et al. [126], classified PPTA types into lateral and medial subtypes (Fig. 6).
Drawings of two Saltzman-type persistent primitive trigeminal artery (PPTA) modified according to Uchino et al. [126] and Lie [58]. Lateral (a) and medial (b) subtypes of Saltzman type I. Lateral (c) and medial (d) subtypes of Saltzman type II. Broken line marks a course of the PPTA. ICA internal carotid artery, BA basilar artery, PCoA posterior communicating artery, P1 pre-communicating part of the posterior cerebral arteries (PCAs), SCAs superior cerebellar arteries
In type I, the BA, PCAs, and SCAs are supplied by the PPTA. the PPTA inserts into the BA distally to the AICA, but proximal to SCA. In this most common type, so-called fetal type (posterior circulation depends on PPTA), the ipsilateral PCoA is absent and the distal basilar system is supplied by the PPTA [8, 14, 16, 27, 40, 41, 61, 65, 105, 129, 137]. A good filling of both PCAs and SCAs from the VA is described as an adult type (posterior circulation is independent of PPTA) [3].
In type II, there is filling of the BA, the SCAs, and PCA on the contralateral side; the PPTA joins the BA above the origin of the SCAs. The homolateral PCA is supplied by the PCoA [5, 8, 12, 24, 36, 40, 41, 61, 64, 70, 80, 85, 89, 95].
Occasionally, the PPTA represents a combination of these two types [36], as a type III, with the anastomosis supplying the SCA bilaterally as well as the contralateral PCA. In this case, the ipsilateral PCA is supplied by the PCoA [40, 70, 95], or both PCAs are supplied by PCoAs [80].
Association of carotid–vertebrobasilar anastomoses
Öertel [78], according to Djindjian et al. [25], revealed the simultaneous presence of the persistent primitive hypoglossal and trigeminal arteries. The PPTA was associated with the persistent primitive otic artery (PPOA) in a 54-year-old woman [141] as well as with the PPOA variant in a 17-year-old male [122]. Bilateral proatlantal type 1 artery and PPTA on the left side was revealed by magnetic resonance angiography [90].
Differentiation
The characteristics of other persistent carotid–vertebrobasilar anastomoses are reviewed with following data:
-
The PPOA should rise from the ICA, in the lateral portion of the petrous carotid canal, close to the medial turn; it should run through the internal acoustic meatus with the facial and vestibulocochlear nerves and then connect with the BA at a caudal point [132].
-
PPHA arises from the ICA as a large extracranial branch at the level from C1 to C3 vertebra; it enters the skull through the hypoglossal canal. The basilar artery is filled only beyond the point where this artery joins it, and the PCoAs are absent or not visible on the angiogram [133].
-
The PPIA originates either from the CCA bifurcation or from the ECA or ICA; the level of its origin ranged from C2 to C4 levels. It joins the VA in the suboccipital region and traverses the foramen magnum [134].
Association with unusual congenital anomalies
Khodadad [51] found a rudimentary right arm and absent right thumb in an 8-month-old fetus, while Okanischi et al. [84] revealed aplasia of the lower half of the cerebellar vermis in a 6-month-old boy. Klippel–Feil syndrome (congenital failure in segmentation of two or more cervical vertebrae) was associated with the left PPTA [88].
Status of the skull base
Schmid [102] draws an attention to the atrophy of the dorsum sellae, while Anderson and Sondheimer [7] and Lin et al. [59] described that PPTA indented it. Piotin et al. [92] identified the canal of the PPTA in the dorsum sellae on axial CT image. Tubbs et al. [125] pointed out that the petrous apex of the temporal bone was slightly eroded by the PPTA.
Physiology
As a rule, blood in the PPTA flows from the ICA to the BA [41]. Some authors have stated, as quoted by Ali et al. [5], that the proximal portion of ICA may become occluded in the fetus and that to maintain adequate blood supply to the forebrain, the PTA must persist and transport blood from the BA to the ICA in a retrograde fashion. In addition, angiographic studies of the VA have also disclosed a reversed type of flow [81, 85, 105, 114], similar to the circulation in the PCoA [60]. A filling of the PPTA from the cavernous ICA to the midpoint of the BA was also visible after recanalization, indicating a reversal flow in the PPTA [30]. In an early phase of the carotid angiogram, the posterior circulation could be visualized via a well-working ipsilateral PCoA and PPTA [15, 35, 37].
The PPTA may itself play a role in additional blood supply to the oculomotor nerve [57]. The PPTA is a possible cause of significant differences in retinal artery pressures [17].
Radiologically, PPTA induces an abnormal flow void coming off the posterior aspect of the cavernous ICA and running a somewhat horizontal course posteriorly to the BA; the combination of the vertical and horizontal segments of the ICA and the proximal portion of the PPTA creates the outline of the Greek letter “τ” or tau (τ) sign [19, 57].
Pathologic physiology and pathologic anatomy
Generally, carotid–vertebrobasilar anastomoses may cause a diversion of blood from the cerebral hemisphere to the infratentorial arteries in patients with a seizure disorder, leading to ipsilateral cerebral ischemia [42]. An obstruction of the PPTA perforating branch has also been implicated in the development of ischemic damage to the brainstem [3, 115].
In patients with a persistent carotid–basilar arterial anastomosis, there might be two possible mechanisms of brainstem ischemia: low-perfusion pressure in the vertebrobasilar system and embolization from the stenotic carotid lesions and/or the heart [82]. As angiograms of patients with a PPTA show flow direction of the anastomosis from the ICA to the BA, this explains why patients with carotid stenoses proximal to the PPTA may develop embolic infarcts in the territory of the posterior circulation [30]. Although the PPTA has been implicated as a conduit of the thrombus from the ICA to PCA [34, 94, 135], localized occlusive process may also occur [113].
Because the PPTA is fixed where it penetrates the dural foramen and where it joins the BA, it may be subject to strong shear forces and distortion, with the subsequent occurrence of traumatic angiospasm [69].
Koch et al. [54] added PPTA to the list of intracranial arteries that may help to compensate for subclavian steal. Dicuonzo et al. [23] assumed that the PPTA was the cause of the flow reduction in the left hemisphere, perhaps also to replace the reduced blood flow in the thread-like opposite VA.
Takase et al. [115] speculate that the specific hemodynamics of the PPTA, which directly branches and receives significant blood flow from the ICA, may induce the formation of aneurysms. The pathogenesis of the aneurysm is possibly the result of congenital defects of the medial layer of this primitive vessel added to a hemodynamic stress due to the anatomic location of the PPTA [4, 58].
In some instances [100], spontaneous subarachnoid hemorrhage has occurred, without any other source of bleeding found. Uzava et al. [127] speculated that the BA dysplasia due to the PPTA, and poorly controlled hypertension, resulted in an overload of the SCA vessels through the PPTA, finally leading to a hemorrhage in the right vermis.
Because of proximity to the III, V, and VI cranial nerves, the PPTA can be an unusual cause of trigeminal neuralgia and oculomotor or abducens nerve palsy. The dilated trunk of the PPTA could only be a causal factor responsible for abducens nerve palsy [104] or more severe compression of the trigeminal nerve [22]. The instance under report is considered as a lateral variant where the PPTA compressed and distorted the root entry zone of the trigeminal nerve, precipitating the facial pain syndrome [20].
An association of the PPTA and arteriovenous malformation (AVM) is also described [37, 74, 80, 86, 122]. Tomsick et al. [122] stressed that an AVM serves to remind us that subarachnoid hemorrhage in a patient with PPTA may be due to a vascular malformation. Harman et al. [38] assumed that the reason for the hormonal disorder was related to the compression of the pituitary gland by the PPTA.
Clinical significance
There is no sex predilection for persistent carotid–basilar anastomoses, and they may be discovered in patients of any age, on either side, and may be multiple. The PPTA among these anastomoses is the most common case and accounts for approximately 80–85% of persistent anastomoses [16].
A headache was mostly the initial symptom for the PPTA evidence [2, 3, 6, 10, 13, 15, 17, 27, 45, 46, 52, 61, 62, 65, 68, 71, 72, 74–76, 80, 86, 100, 108, 121]. Other presentations, such as acute subarachnoid hemorrhage [50, 115, 120], consciousness disturbance [23, 33, 59, 69, 73, 117], sixth cranial nerve palsy [11, 49, 64, 77, 104, 137], different visual field defects [30, 94, 141], diplopia [4, 12, 19, 49, 56, 81], hemiparesis [4, 40, 82, 89, 103], and symptoms suggestive of vertebrobasilar insufficiency [26, 43, 88, 129], were some of the reasons for the PPTA evidence, too. Although no characteristic clinical syndrome is associated with this anomaly, some cases suggest that there is a higher incidence of this condition in patients with retarded mental development dating from infancy [17].
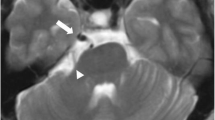
The interest for PPTA was promoted by the use of new radiological techniques in some institutes or clinics (Fig. 7). Small trigeminal arteries cannot be identified in spin-echo T1 and T2 sequences; it is only possible with MR angiography [126].
Some of the online presentation of a PPTA (arrow): three-dimensional TOF MRA—http://www.med.uc.edu/neurorad/webpage/coa.html; MRI—http://www.biomedsearch.com/nih/Twenty-classic-signs-in-neuroradiology/19881070.html; Axial three-dimensional maximum intensity—http://jkns.or.kr/fulltext/htm/0042005020f3.htm; CTI—http://neuroradiologyonthenet.blogspot.com/2009/11/pta.html
Morrison et al. [71] felt that we should attempt to preserve the continuity of the PPTA since we do not know how significant this vessel was to the patient's cerebral circulation. Knowing the presence of a PPTA is also important when planning a neuroradiologic intervention or performing procedures such as Wada's test or a test for carotid artery occlusion [16]. Cranial nerve displacement or distortion is less likely, but the identification of a PPTA with a trans-sellar course is crucial if trans-sphenoidal surgery is planned [49]. Failure to recognize an intrasellar PPTA could result in massive hemorrhage during trans-sphenoidal surgery for pituitary adenoma [92]. Recognition of the PPTA can be important in surgical procedures in the cavernous sinus or the posterior fossa and may prevent injury or disruption of the PPTA [98].
Davis and associates, in 1956 [21], first described an aneurysm of the PPTA. Aneurysms of a PPTA are very rare, and only 29 cases have been reported until 2005 [3]. The citations from the literature indicate that there may be aneurysms in nearly 14% of all cases [4], as well only 2% from PPTA itself [4, 8, 12, 71]. The aneurysms of PPTA were at its origin [4, 19, 28, 29, 68, 72, 75, 77, 85, 124] and distal to it [71], or at its mid-portion [3, 26, 115] or at its junction with BA bifurcation [65]. Takase et al. [115] discovered the simultaneous presence of three aneurysms on the PPTA, ICA-AChA, and ACoA, respectively. Some authors discovered aneurysms of other arteries—left MCA [50, 79], left PCA [101], left PCoA [10], left ICA [137], and at the A1–A2 junction [122] simultaneous with PPTA. Alleyne et al. [6] revealed ACoA and left pericallosal artery aneurysms simultaneously with bilateral PPTAs. Zhang et al. [141] reported a case of a giant unruptured aneurysm of the cavernous ICA associated with the PPTA and the PPOA in the English literature for the first time.
If an aneurysm located at the junction of the PPTA–ICA ruptures [28], or if spontaneous or traumatic rupture of the PPTA occurred nears its anomalous origin, carotid–cavernous fistula resulted [43, 81, 106]. Carotid–cavernous fistula was the result of a spontaneous rupture such as the PPTA in many cases [11, 12, 64, 106, 140].
Eight reported cases of Moyamoya or Moyamoya-like disease associated with PPTA have been presented in the literature until 1991. All patients were Japanese and there was no sex predominance [117]. The HLA genotype of the patient may influence the course of the disease, including PPTA, as discussed by Suzuki et al. [112], reporting the first case of identical twins suffering not only from Moyamoya disease but also having a PPTA variant on the left side.
The PPTA occurs with a much higher frequency among children with posterior fossa malformations–hemangiomas–arterial anomalies–cardiac defects–eye abnormalities (PHACE) syndrome (12–16%) than reports from other large cerebral angiographic series (0.1–0.2%). The relative frequency with which this otherwise rare anomaly is seen in PHACE syndrome (association between infantile hemangiomas and brain anomalies) may point to the gestational timing of an error in vasculogenesis [67].
Concluding remarks
The author is aware of the morphofunctional and clinical significance of the carotid–vertebrobasilar anastomoses, as in the case of the otic [132], hypoglossal [133], proatlantal [134], as well as in the trigeminal artery, which is presented in this manuscript.
The PPTA usually originates from the cavernous portion of the ICA. The PPTA might reach the posterior cranial fossa in two ways: (1) in about half of the cases, it penetrates the sella turcica, runs in its own groove, and perforates the dura mater near the clivus; (2) in the other half of the cases, it runs extradurally after leaving the cavernous sinus, between the sensory trigeminal root and lateral side of the sella. It communicates with BA between the origin of the SCA and AICA.
The PPTA is by far the most common of the three types of persistent carotid–basilar anastomosis. The interest for PPTA was promoted by the use of new radiological techniques in some institutes or clinics. The citations from the literature indicate that there may be aneurysms in nearly 14% of all cases, as well only 2% from PPTA itself. The PPTA occurs with a much higher frequency among children with PHACE syndrome (12–16%). Although no characteristic clinical syndrome is associated with this anomaly, the anatomy of PPTA is significant for performing various treatment procedures.
References
Abe K, Suzuki T (1964) Persistence of embryonic carotid–vertebrobasilar anastomoses. Folia Psychiatr Neurol Japonica 18:257–276
Abe T, Fujita S, Ozawa H, Kawamura N, Shimazu M, Ikeda H, Izumiyama H, Matsumoto K (2000) Haemorrhagic nonsecreting pituitary adenoma associated with persistent primitive trigeminal artery. Acta Neurochir (Wien) 142:1423–1424
Agrawal D, Mahapatra AK, Mishra NK (2005) Fusiform aneurysm of a persistent trigeminal artery. Case report. J Clin Neurosci 12:500–503
Ahmad I, Tominaga T, Suzuki M, Ogawa A, Yoshimoto T (1994) Primitive trigeminal artery associated with cavernous aneurysm: case report. Surg Neurol 41:75–79
Ali S, Radaideh MM, Shaibani A, Russell EJ, Walker MT (2008) Persistent trigeminal artery terminating in the posterior inferior cerebellar artery: case report. Neurosurgery 62:E746–E748
Alleyne CH Jr, Krisht A, Yoo FK, Silverstein A, Colohan AR (1997) Bilateral persistent trigeminal arteries associated with cerebral aneurysms and aortic arch vessel anomaly. South Med J 90:434–438
Anderson RA, Sondheimer FK (1976) Rare carotid–vertebrobasilar anastomoses with notes on the differentiation between proatlantal and hypoglossal arteries. Neuroradiology 11:113–118
Apostolides PJ, Lawton MT, David CA, Spetzler RF (1997) Clinical images: persistent primitive trigeminal artery with and without aneurysm. Barrow Quarterly 13(4). http://www.thebarrow.org/Education_And_Resources/Barrow_Quarterly/204843
Arakawa T, Koizumi M, Terashima T, Honma S, Kawai K, Kodama K, Miki A (2007) Two anatomical autopsy cases of direct communication between a persistent primitive trigeminal artery and an anterior inferior cerebellar artery. Ann Anat 189:489–498
Baskaya MK, Roberts R, Rivera E, Nanda A (2001) Persistent primitive trigeminal artery associated with posterior communicating artery aneurysm and hypoplastic vertebral artery. Surg Radiol Anat 23:169–171
Berger MS, Hosobuchi Y (1984) Cavernous sinus fistula caused by intracavernous rupture of a persistent trigeminal artery. Case report. J Neurosurg 61:391–395
Bernstein K, Teitelbaum GP, Herman B, Giannotta SL (1998) Coil embolization of a trigeminal–cavernous fistula. AJNR Am J Neuroradiol 19:1953–1954
Binet EF, Young RF (1977) Bilateral persistent trigeminal arteries. Case report. J Neurosurg 47:619–622
Bosco D, Consoli D, Lanza PL, Plastino M, Nicoletti F, Ceccotti C (2010) Complete oculomotor palsy caused by persistent trigeminal artery. Neurol Sci 31:657–659
Boyko OB, Curnes JT, Blatter DD, Parker DL (1996) MRI of basilar artery hypoplasia associated with persistent primitive trigeminal artery. Neuroradiology 38:11–14
Caldemeyer KS, Carrico JB, Mathews VP (1998) The radiology and embryology of anomalous arteries of the head and neck. AJR 170:197–203
Campbell RL, Dyken ML (1961) Four cases of carotid–basilar anastomosis associated with central nervous system dysfunction. J Neurol Neurosurg Psychiatr 24:250–253
Chambers AA, Lukin R (1975) Trigeminal artery connection to the posterior inferior cerebellar arteries. Neuroradiology 9:121–123
Chan DTM, Boet R, Yu S, Poon WS (2004) Trispan-assisted coiling of a wide-necked persistent trigeminal artery aneurysm. Acta Neurochir (Wien) 146:87–88
Chidambaranathan N, Sayeed ZA, Sunder K, Meera K (2006) Persistent trigeminal artery: a rare cause of trigeminal neuralgia—MR imaging. Neurol India 54:226–227
Davis RA, Wetzel N, Davis L (1956) An analysis of the results of intracranial vascular lesions by carotid artery ligation. Ann Surg 143:641–649
De Bondt B-J, Stokroos R, Casselman J (2007) Persistent trigeminal artery associated with trigeminal neuralgia: hypothesis of neurovascular compression. Neuroradiology 49:23–26
Dicuonzo F, Palma M, Fiume M, Scarpello R, Lefons V, Maghenzani M, Carella A (2008) Cerebrovascular disorders in the prenatal period. J Child Neurol 23:1260–1266
Dimmick SJ, Faulder KC (2009) Normal variants of the cerebral circulation at multidetector CT angiography. RadioGraphic 29:1027–1043
Djindjian R, Hurth M, Bories J, Brunet P (1965) L'artére trigéminale primitive (Aspects artériographiques et signification à propos de 12 cas). La Presse Méd 73:2905–2910 (in French)
Eggers FM, Tomsick TA, Chambers AA, Lukin RR (1982) Aneurysms of persistent trigeminal arteries. Report of two cases. Neuroradiology 24:65–66
Ekinci G, Baltacioğlu F, Kiliç T, Çimşit Ç, Akpmar I, Pamir N, Erzen C (2001) A rare cause of hyperprolactinemia: persistent trigeminal artery with stalk-section effect. Eur Radiol 11:648–650
Enomoto T, Sato A, Maki Y (1977) Carotid–cavernous sinus fistula caused by rupture of a primitive trigeminal artery aneurysm. Case report. J Neurosurg 46:373–376
Flandroy P, Lacour P, Marsault C, Stevenaert A, Collignon J (1987) The intravascular treatment of a cavernous fistula caused by rupture of a traumatic carotid trigeminal aneurysm. Neuroradiology 29:308–311
Foerch C, Berkefeld J, Halbsguth A, Ziemann U, Neumann-Haefelin T (2006) Brain stem infarction caused by proximal internal carotid stenosis in a patient with a persisting primitive trigeminal artery. Cerebrovasc Dis 22:200–202
Fukuda M, Kameyama S, Takahashi H, Tanaka R (1998) Trigeminal neuralgia caused by the vertebral artery associated with primitive trigeminal artery and agenesis of the internal carotid artery: case report. Neurol Med Chir (Tokyo) 38:367–370
Fuse T, Niwa Y, Harada S (2000) Local intraarterial fibrinolytic therapy for embolic stroke associated with vascular anomalies. Two case reports. Neurol Med Chir (Tokyo) 40:641–644
Gannon WE, Kaplan HA (1961) Persistent trigeminal artery. A method for its demonstration. Radiology 77:839–841
Gasecki AP, Fox AJ, Lebrun LH, Daneault N (1994) Bilateral occipital infarctions associated with carotid stenosis in a patient with persistent trigeminal artery. Stroke 25:1520–1523
Given CA II, Huang-Hellinger F, Baker MD, Chepuri NB, Morris PP (2001) Congenital absence of the internal carotid artery: case reports and review of the collateral circulation. AJNR Am J Neuroradiol 22:1953–1959
Goyal M (2001) The tau sign. Radiology 220:618–619
Güner M, Erbayraktar SE (1996) Arteriovenous malformation and persistent trigeminal artery association: case report. Turk Neurosurg 6:33–36
Harman M, Kýymaz N, Ayakta H, Kayan M (2004) Compressive effect of large persistent trigeminal artery upon pituitary gland: importance of MRI and MRA. Eur J Radiol Extra 51:65–67
Hattori T, Inoue S, Sakai N (1997) Fenestration of the basilar artery associated with persistent primitive trigeminal artery. Case report. Neurol Med Chir (Tokyo) 37:841–843
Hinck VC, Gordy PD (1964) Persistent primitive trigeminal artery. One type of persistent carotid–basilar anastomosis. Radiology 83:41–45
Huber G (1980) Intracranial carotid anastomosis and partial aplasia of an internal carotid artery. Neuroradiology 20:207–212
Huber P (1982) Radiologic anatomy and topography of cerebral vessels. In: Huber P (ed) Krayebühl/Yaşargil cerebral angiography, 2nd edn. Thieme, New York, pp 57–60
Hurst RW, Howard RS, Zager E (1998) Carotid cavernous fistula associated with persistent trigeminal artery: endovascular treatment using coil embolization. Skull Base Surg 8:225–228
Iancu D, Anxionnat R, Bracard S (2010) Brainstem infarction in a patient with internal carotid dissection and persistent trigeminal artery: a case report. BMC Med Imaging 10:14. http://www.biomedcentral.com/1471-2342/10/14)
Ide M, Jimbo M, Yamamoto M, Hagiwara S (1996) Emergence of persistent primitive trigeminal artery into posterior cranial fossa via an unusually caudal site. Acta Neurochir (Wien) 138:1132–1133
Ikushima I, Arikawa S, Korogi Y, Uehara H, Komohara Y, Takahashi M (2002) Basilar artery aneurysm treated with coil embolization via persistent primitive trigeminal artery. Cardiovasc Intervent Radiol 25:70–71
Ito J, Takeda N, Suzuki Y, Takeuchi S, Osugi S, Yoshida Y (1980) Anomalous origin of the anterior inferior cerebellar arteries from the internal carotid artery. Neuroradiology 19:105–109
Jaeger HJ, Mehring U-M, Gissler HM, Mathias KD (2000) Congenital absence of the internal carotid artery and the basilar artery with persistent trigeminal artery associated with corctation of the aorta. Case report. Eur Radiol 11:1805–1809
Kalidindi RS, Balen F, Hassan A, Al-Din A (2005) Persistent trigeminal artery presenting as intermittent isolated sixth nerve palsy. Clin Radiol 60:515–519
Kandelj EI, Saribekian AS (1978) Sočetanie persistiruiuščej primitivnoj trigeminaljnoj arterii s anevrizmoj srednej mozgovoj arterii. Vopr Neirohir 2:50–52 (in Russian)
Khodadad G (1976) Persistent trigeminal artery in the fetus. Radiology 121:653–656
Khodadad G (1977) Trigeminal artery and occlusive cerebrovascular disease. Stroke 8:177–181
Kida MY, Ishida H, Dodo Y, Ikeda K, Kobayashi D, Nonaka M (1990) A case of the persistent trigeminal artery. Sapporo Med J 59:629–633 (in Japanese)
Koch S, Romano JG, Forteza A (2002) Subclavian steal and a persistent trigeminal artery. J Neuroimaging 12:190–192
Komiyama M, Kitano S, Sakamoto H, Shiomi M (1998) An additional variant of the persistent primitive trigeminal artery: accessory meningeal artery—antero-superior cerebellar artery anastomosis associated with Moyamoya disease. Acta Neurochir (Wien) 140:1037–1042
Kwon K-H, Kim KH, Jeon P, Byun HS, Kim JS, Hong S-C (2007) Endovascular treatment for a persistent trigeminal artery aneurysm presenting as isolated sixth nerve palsy. Neurointervention 2:113–116
Lee M-R, Chuang Y-M, Chen W-J, Lin C-P (2009) Meticulous blood pressure control is mandatory for symptomatic primitive trigeminal artery. Am J Emerg Med 27:634.e5–634.e7
Lie TA (1972) Congenital malformations of the carotid and vertebral arterial systems, including the persistent anastomoses. In: Vinken PJ, Bruyn GW (eds) Handbook of clinical neurology, vol 12. North Holland, Amsterdam, pp 289–339
Lin W-C, Kung L-L, Hsieh W-Y, Liu G-C (2001) Variant of persistent primitive trigeminal artery associated with giant internal carotid artery pseudoaneurysm. Chin J Radiol 26:173–178
Loevner L, Quint DJ (1992) Persistent trigeminal artery in a patient with Sturge–Weber syndrome. AJR 158:872–874
Luh GY, Dean BL, Tomsick TA, Wallace RC (1999) The persistent fetal carotid–vertebrobasilar anastomoses. AJR 172:1427–1432
Maeshima S, Terada T, Masuo O, Nakai K, Itakura T, Komai N (1999) Multiple cerebral aneurysms with persistent primitive trigeminal artery. J Clin Neurosci 6:52–54
Manjunath KY (2001) Anomalous origin of the middle meningeal artery—a review. J Anat Soc India 50(2). http://www.indmedica.com/journals.php?journalid=8&issueid=32&articleid=394&action=article
McKenzie JD, Dean BL, Flom RA (1996) Trigeminal–cavernous fistula: Saltzman anatomy revisited. AJNR Am J Neuroradiol 17:280–282
Menkü A, Akdemir H, Tucer B, Kurtsoy A (2004) Ruptured aneurysm associated with persistent primitive trigeminal artery: report of a case with three dimensional CT angiographic evaluation. Turk Neurosurg 14:21–24
Merry GS, Jamieson KG (1977) Operative approach to persistent trigeminal artery producing facial pain and diplopia. Case report. J Neurosurg 47:613–618
Metry D, Heyer G, Hess C, Garzon M, Haggstrom A, Frommelt P, Adams D, Siegel D, Hall K, Powell J, Frieden I, Drolet B (2009) Consensus statement on diagnostic criteria for PHACE syndrome. Pediatrics 124:1447–1456
Mohammed MI, Johnny S, Sandhu JS, Wakhloo AK (2002) Stent-assisted coil placement in a wide-necked persistent trigeminal artery aneurysm with jailing of the trigeminal artery: a case report. AJNR Am J Neuroradiol 23:437–441
Morioka M, Yoshida A, Yoshikawa M, Ushio Y (1996) Transient alpha coma following minor head trauma in a patient with primitive trigeminal artery—case report. Neurol Med Chir (Tokyo) 36:224–228
Morita A, Fukushima T, Miyazaki S, Shimizu T, Atsuchi M (1989) Tic douloureux caused by primitive trigeminal artery or its variant. J Neurosurg 70:415–419
Morrison G, Hegarty WM, Brausch CC, Castele TJ, White RJ (1974) Direct surgical obliteration of a persistent trigeminal artery aneurysm. Case report. J Neurosurg 39:249–251
Murai Y, Kobayashi S, Tateyama K, Teramoto A (2006) Persistent primitive trigeminal artery aneurysm associated with cerebellar hemangioblastoma—case report. Neurol Med Chir (Tokyo) 46:143–146
Myint PK, Anderson KN, Antoun NM, Warburton EA (2008) Eyelid apraxia associated with bilateral paramedian thalamic infarct. Age Ageing 37:343–344
Nakai Y, Yasuda S, Hyodo A, Yanaka K, Nose T (2000) Infratentorial arteriovenous malformation associated with persistent prinitive trigeminal artery—case report. Neurol Med Chir (Tokyo) 40:572–574
Naruse S, Odake G (1979) Primitive trigeminal artery associated with an ipsilateral intracavernous giant aneurysm—a case report. Neuroradiology 17:259–264
Nielsen BP, Jonson M (1967) Persistent primitive trigeminal artery demonstrated by vertebral arteriography. Radiology 101:47–51
Nishio A, Nishijima Y, Komiyama M, Hara M (2001) Primitive trigeminal variant aneurysm treated with Guglielmi detachable coils. Neurol Med Chir (Tokyo) 41:446–449
Öertel O (1922) Über die persistenz embryonaler verindungen zwischen der A. carotis interna und der vertebralis cerebralis. Verh Anat Ges 55:281–295
Ohshiro S, Inoue T, Hamada Y, Matsuno H (1993) Branches of the persistent primitive trigeminal artery—an autopsy case. Neurosurgery 32:144–148
Ohtakara K, Kuga Y, Murao K, Kojima T, Taki W, Waga S (2000) Posterior fosa arteriovenous malformation associated with persistent primitive trigeminal artery—case report. Neurol Med Chir (Tokyo) 40:169–172
Oka Y, Sadamoto K, Tagawa M, Kumon Y, Sakaki S, Fujita M (2000) Transvenous embolization of carotid–cavernous fistula associated with a primitive trigeminal artery—case report. Neurol Med Chir (Tokyo) 40:61–64
Okada Y, Shima T, Nishida M, Yamada T, Yamane K, Okita S, Kagawa R (1992) Bilateral persistent trigeminal arteries presenting with brain-stem infarction. Neuroradiology 34:283–286
Okahara M, Kiyosue H, Mori H, Tanoue S, Sainou M, Nagatomi H (2002) Anatomic variations of the cerebral arteries and their embryology: a pictorial review. Eur Radiol 12:2548–2561
Okanishi T, Saito Y, Miki S et al (2007) Lower brainstem dysfunction in an infant with persistent primitive trigeminal artery. Brain Dev 29:189–192
Onizuka M, Kazekawa K, Tsutsumi M et al (2006) Hyperform remodeling balloon for the balloon occlusion test of persistent primitive trigeminal artery aneurysm—case report. Neurol Med Chir (Tokyo) 46:541–543
Oran I, Parildar M, Memis A, Yunten N (2000) Catheter and MR angiography of persistent trigeminal artery associated with occipital arteriovenous malformation. Comput Med Imaging Graph 24:33–35
O'uchi E, O'uchi T (2010) Persistent primitive trigeminal arteries (PTA) and its variant (PTAV): analysis of 103 cases detected in 16,415 cases of MRA over 3 years. Neuroradiology 52:1111–1119
Paksoy Y, Seker M, Kalkan E (2004) Klippel–Feil syndrome associated with persistent trigeminal artery. Spine 29:E193–E196
Parkinson D, Shields CB (1974) Persistent trigeminal artery: its relationship to the normal branches of the cavernous carotid artery. J Neurosurg 40:244–248
Pascual-Castroviejo I, Lopez-Gutierrez JC (2007) Cutaneous hemangioma associated with persistence of the trigeminal and both proatlantal arteries. J Child Neurol 22:337–340
Pereira LP, Nepomuceno LAM, Coimbra PP, Neto SRO, Natal MRC (2009) Persistent trigeminal artery. Angio-tomography and angio-magnetic resonance finding. Arq Neuropsiquiatr 67:882–885
Piotin M, Miralbés S, Cattin F, Marchal H, Amor-Sahli M, Moulin T, Bonneville JF (1996) MRI and MR angiography of persistent trigeminal artery. Neuroradiology 38:730–733
Quain R (1844) The anatomy of the arteries of the human body and its applications to pathology and operative surgery, with a series of lithographic drawings. Taylor and Walton, London
Quencer RM, Simon J (1979) Transient bilateral occipital lobe ischemia: embolization through a trigeminal artery. Neuroradiology 18:273–275
Raphaeli G, Bandeira A, Mine B, Brisbois D, Lubicz B (2009) A rare variant of persistent trigeminal artery: cavernous carotid–cerebellar artery anastomosis—a case report and a systematic review. Cerebellum 8:445–447
Rhee SJ, Kim MS, Lee CH, Lee GJ (2007) Persistent trigeminal artery variant detected by conventional angiography and magnetic resonance angiography. Incidence and clinical significance. J Korean Neurosurg Soc 42:446–449
Romero JM, Lev MH, Chan S-T, Connelly MM, Curiel RC, Jackson AE, Gonzales RG, Ackerman RH (2002) US of neurovascular occlusive disease: interpretive pearls and pitfalls. RadioGraphics 22:1165–1176
Salas E, Ziyal IM, Sekhar LN, Wright DC (1998) Persistent trigeminal artery: an anatomic study. Neurosurgery 43:557–561 (Abstract)
Saltzman GF (1959) Patent primitive trigeminal artery studied by cerebral angiography. Acta Radiol (Stockh) 51:329–336
Samra K, Scoville WB, Yaghmai M (1969) Anastomosis of carotid and basilar arteries. Persistent primitive trigeminal and hypoglossal artery: report of two cases. J Neurosurg 30:622–625
Schlamann M, Doerfler A, Schoch B, Forsting M, Wanke I (2006) Balloon-assisted coil embolization of a posterior cerebral artery aneurysm via a persistent primitive trigeminal artery: technical note. Neuroradiology 48:931–934
Schmid AH (1974) Persistent trigeminal artery. An autopsy report. Neuroradiology 7:173–176
Schwartz NE, Albers GW (2007) Acute strokes in the setting of a persistent primitive trigeminal artery. J Neurol Neurosurg Psychiatry 78:745
Seemann JH (2005) Coil embolization for sixth cranial nerve palsy caused by persistent primitive trigeminal artery. The Internet J Neurosurgery 2(2). http://www.ispub.com/ostia/index.php?xmlFilePath=journals/ijns/vol2n2/ppta.xml
Shigemori M, Shirahama M, Yamamoto F, Hara K, Tokutomi T, Nakashima O (1980) Primitive trigeminal artery and intracranial carotid occlusion—case report. Kurume Med J 27:263–268
Shin YS, Kim SY, Kim BM, Park SI (2005) Ruptured aneurysm of the anomalous cerebellar artery originating from internal carotid artery presenting with carotid cavernous fistula: a case report. AJNR Am J Neuroradiol 26:1849–1851
Silver JM, Wilkins RH (1991) Persistent embryonic intracranial and extracranial vessels. In: Wilkins RH, Rengachary SS (eds) Neurosurgery update II: Vascular, spinal, pediatric, and functional neurosurgery. McGraw-Hill, Health Professions Divisions, New York, pp 50–58
Singh RK, Varshney S, Bist SS, Burathoki S, Gupta N (2008) Anomalous internal carotid artery and persistent trigeminal artery. J Anat Soc India 57:140–143
Streeter GL (1918) The developmental alterations in the vascular system of the brain of the human embryo. Contr Embryol Carneg Instn 8:5–38
Suttner N, Mura J, Tedeschi H, Ferreira ATM, Wen HT, de Oliveira E, Rhoton AL Jr (2000) Persistent trigeminal artery: a unique anatomic specimen—analysis and therapeutic implications. Neurosurgery 47:428–434
Sutton D (1950) Anomalous carotid–basilar anastomosis. Brit J Radiol 23:617–619
Suzuki S, Morioka T, Matsushima T, Ikezaki K, Hasuo K, Fukui M (1996) Moyamoya disease associated with persistent primitive trigeminal artery variant in identical twins. Surg Neurol 45:236–240
Suzuki S, Chang GY (2010) A case of primitive trigeminal artery infarction. Neurology 75:S66
Szdzuy D, Lehmann R (1971) Persistent trigeminal artery in vertebral angiography. Neuroradiology 2:100–101
Takase T, Tanabe H, Kondo A, Nonoguchi N, Tane K (2004) Surgically treated aneurysm of the trunk of the persistent primitive trigeminal artery—case report. Neurol Med Chir (Tokyo) 44:420–423
Tamura Y, Shimano H, Kuroiwa T, Miki Y (2003) Trigeminal neuralgia associated with a primitive trigeminal artery variant: case report. Neurosurgery 52:1217–1220
Tann E-C, Takagi T, Nagai H (1991) Intracranial carotid artery occlusion with telangiectasia (Moyamoya disease) associated with persistent primitive trigeminal artery—case report. Neurol Med Chir (Tokyo) 31:800–803
Tanohata K, Maehara T, Noda M, Katoh H (1987) Internal carotid–cerebellar artery anastomosis: so-called persistent trigeminal artery variant (Images). http://ir.library.osaka-u.ac.jp/metadb/up/LIBLSK001/JJRS-47-9-1119-1126.pdf
Teal JS, Rumbaugh CL, Bergeron RT, Scanlan RL, Segall HD (1972) Persistent carotid–superior cerebellar artery anastomosis: a variant of persistent trigeminal artery. Radiology 103:335–341
Temizöz O, Genchellac H, Ünlü E, Çağlı B, Özdemir H, Demir MK (2010) Digital subtraction angiography of a persistent trigeminal artery variant. Diagn Interv Radiol (Turkish) 16:245–247
Terlecki M, Szopinski P, Huba M (2007) Persistent trigeminal artery—CTA findings. http://www.eurorad.org/case.php?id=6254
Tomsick TA, Lukin RR, Chambers AA (1979) Persistent trigeminal artery: unusual associated abnormalities. Neuroradiology 17:253–257
Tortori-Donati P, Fondelli MP, Rossi A, Bava GL (1999) Intracranial contrast-enhancing masses in infants with capillary haemangioma of the head and neck: intracranial capillary haemangioma? Neuroradiology 41:369–375
Tsuboi K, Shibuya F, Yamada T, Nose T (1992) Giant aneurysm at the junction of the left internal carotid and persistent primitive trigeminal arteries—case report. Neurol Med Chir (Tokyo) 32:778–781
Tubbs RS, Shoja MM, Salter EG, Oakes WJ (2007) Cadaveric findings of persistent trigeminal arteries. Clin Anat 20:367–370
Uchino A, Sawada A, Takase Y, Kudo S (2003) MR angiography of anomalous branches of the internal carotid artery. AJR 181:1409–1414
Uzawa A, Aotsuka A, Terano T (2009) Cerebellar haemorrhage associated with persistent primitive trigeminal artery. J Clin Neurosci 16:152–154
Valença MM, Martins C, Andrade-Valença LPA (2008) Trigeminal neuralgia associated with persistent primitive trigeminal artery. Migrâneas cefaléias (Brasil) 11:30–32
Varghese SPJ (2007) Persistent trigeminal artery and associated vascular variations. Austral Radiol 51:B31–B33
Vasović L. Morfološke karakteristike arterijskog prstena mozga kod različitog porekla kičmenih arterija. Doctoral thesis, Medical Faculty, Niš (in Serbian)
Vasović L, Đorđević Z, Stefanović N, Pavlović S, Bakić V, Antić S (1990) Rare anomalies of the brain arteries. Persistence of the a. trigemini. Acta Med Medianae 1:91–96 (in Serbian)
Vasović L, Arsić S, Vlajković S, Jovanović I, Jovanović P, Ugrenović S, Anđelković Z (2010) Otic artery: a review of normal and pathological features. Med Sci Monit 16:RA101–RA109
Vasović L, Milenković Z, Jovanović I, Čukuranović R, Jovanović P, Stefanović I (2008) Hypoglossal artery: a review of normal and pathological features. Neurosurg Rev 31:385–396
Vasović L, Mojsilović M, Anđelković Z, Jovanović I, Arsić S, Vlajković S, Milenković Z (2009) Proatlantal intersegmental artery: a review of normal and pathological features. Childs Nerv Syst 25:411–421
Waller FT, Simons RL, Kerber C, Kiesel IO, Tanabe CT (1977) Trigeminal artery and microemboli to the brain stem: report of two cases. J Neurosurg 46:104–106
Willinsky R, Lasjaunis P, Berenstein A (1987) Intracavernous branches of the internal carotid artery (ICA). Comprehensive review of their variations. Surg Radiol Anat 9:201–215
Wise BL, Palubinskas AJ (1964) Persistent trigeminal artery (carotid–basilar anastomosis). J Neurosurg 21:199–206
Wismer GL (1989) Circle of Willis variant analogous to fetal type primitive trigeminal artery. Neuroradiology 31:366–368
Yamada Y, Kondo A, Tanabe H (2006) Trigeminal neuralgia associated with an anomalous artery originating from the persistent primitive trigeminal artery—case report. Neurol Med Chir (Tokyo) 46:194–197
Yang X, Mu S, Srivastava T, Wu Z (2007) Treatment of traumatic trigeminal–cavernous fistula by coil embolization and compression of carotid artery. Neurol India 55:396–398
Zhang C, Xie X, Yang Z, Wang C, You C, Mao B, He M, Sun H (2009) Giant cavernous aneurysm associated with a persistent trigeminal artery and persistent otic artery. Korean J Radiol 10:519–522
Acknowledgment
This work was supported by Ministry of Science and Technological Development of Republic of Serbia (contract grant number: 175092).
Conflict of interest statement
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vasović, L., Jovanović, I., Ugrenović, S. et al. Trigeminal artery: a review of normal and pathological features. Childs Nerv Syst 28, 33–46 (2012). https://doi.org/10.1007/s00381-011-1622-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-011-1622-7