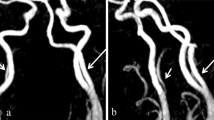
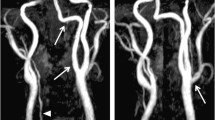
Abstract
The hypoglossal artery is rarely described member of carotid–basilar family anastomoses. Together with a caudal end of the primitive internal artery, trigeminal, otic, and proatlantal intersegmental arteries, it represents the remnant of vascular channels’ unsuccessful involution which function normally stops in human embryo with 12 to 14 mm crown–rump length. The persistence of hypoglossal artery alone is usually incidental and asymptomatic finding during the routine angiography, while during autopsies or surgical operations, its presence is frequently associated with other vascular or organic abnormalities and diseases. The aim of this review is to document the hypoglossal artery developmental morphology, as well as the normal anatomical and clinical aspects and better understanding of its persistence overall significance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The first literature data
Batujeff [8] made the first anatomical description of this vestigial vessel, while Öertel [61], according to Lie [49], suggested the term—hypoglossal artery for the vessel, which follows hypoglossal nerve on its pathway through its bony canal. Synonyms were the last occipital intersegmental or the last hypoglossal intersegmental artery [64].
In cases when any of these primitive vascular channels persist in adult life, the result is the presence of persistent carotid–basilar or carotid–vertebral anastomosis [68]. Therefore, from the anatomical and clinical points of view [84], there are two forms of the hypoglossal artery: (1) the embryonic or primitive form and (2) the persistent fetal or adult form.
Primitive hypoglossal artery
Development
Padget [63] has authoritatively demonstrated the origins of the hindbrain circulation. Two longitudinal neural arteries arise, ventral to the developing hindbrain on both sides of midline as successors to the primitive hindbrain channel; their fusion produce basilar artery (BA). When the embryo is 4 mm long, the primitive hypoglossal, trigeminal, otic, and proatlantal intersegmental arteries are presegmental branches of dorsal aorta that serve as anastomoses between primitive internal carotid and bilateral longitudinal neural arteries (Fig. 1).
Modified Lie' schema [49] of the primitive carotid–vertebrobasilar anastomoses according to Padget [63] in human embryo of 4 mm—PIA Proatlantal intersegmental artery, PHA primitive hypoglossal artery, POA primitive otic artery, PTA primitive trigeminal artery, ICA Primitive internal carotid artery, LNA longitudinal neural artery, CIA1 first cervical intersegmental artery
The primitive otic artery is, normally, the first one, which will regress during the further development of the embryo. Soon afterwards, posterior communicating artery (PCoA) develops and renders trigeminal redundant; primitive hypoglossal artery (PHA) involution is usually preceded by the involution of latter cited artery. Caudal parts of the primitive carotid artery incorporate in the PCoA and posterior cerebral artery (PCA), and they can be identified in the 8-week-old fetus. The next phase is development of the vertebral arteries (VAs), which arise from longitudinal anastomoses between transversely running cervical intersegmental arteries and which function is to supply blood to the caudal hindbrain. Vertebral artery on each side accomplishes this by incorporation of the proatlantal intersegmental artery; the latter artery, henceforth, represents the transversely directed portion of the VA or so-called suboccipitosegmental artery.
If the embryo has an inappropriate development of the VA or inappropriate fusion between the VA and BA, the blood circulation of the posterior brain is supplied mainly by the persistent anastomotic vessel. It is referred to as an accessory basivertebral anastomosis or the primitive lateral basivertebral anastomosis (LBVA), through which the VA and BA communicate with each other [5]. When the embryo is between 11 and 14 mm long, the plexus vasculosus similar to moyamoya vessels may be visible sometimes [45].
Malobabić and Milisavljević [51] cited the old embryologists who have reported that the first two or three segmental arteries supplying the brain stem and the spinal cord represent arteries of the hypoglossal nerve. One of the embryonic vessel among the rootlets of the hypoglossal nerve which goes from the primitive internal carotid artery (ICA) dorsally and caudally (as the future PCoA and then as the PCA) can join the BA and remains separated forming the first part of the VA on the corresponding side. Such longitudinal anastomosis connects the PHA with the suboccipitosegmental artery from which the horizontal segment of the VA usually arises. Lie [49] stressed that, according to Schmeidel [69], the PHA represents the complex of blood vessels consisting of 2–3 channels, while Padget [63] considered the PHA as a single vessel trunk.
There are three main topographic segments of the PHA [55]: (1) the proximal segment lies on the primitive ICA. (2) The middle segment that consists of lateral anastomotic channels (Paget’s primitive lateral–basivertebral anastomosis) connects the first and the third division of the PHA. Intracranially, the PHA enters the hypoglossal canal ventrally of the 12th cranial nerve rootlets, keeping its central position in this canal [6]. (3) The distal segment, as a transversal anastomotic vessel, connects the middle part of the PHA with the neural longitudinal arteries, i.e., with the precursors of the BA.
Involution
Padget [63] pointed out that the PHA usually disappears between 5- and 6-mm- or between 7- and 12-mm-long embryo, i.e., during the 29th day, after gestation. Lie [49] described that this artery disappears after the primitive otic artery when embryo is ±5 mm long. The hypoglossal artery can also involutes at 5.5-mm or 8-mm stage [53], when its life span is less than 1 week and rarely persists at birth [12].
Persistent primitive hypoglossal artery
The first clinical diagnosis
Matsumura et al. [54] considered Lindgren [50] to be the first who radiologically diagnosed persistent primitive hypoglossal artery (PPHA). On the other hand, Andoh et al. [5] stressed that Begg [9] reported the first case of the PPHA established by carotid arteriography in 1961.
Hypotheses of the PPHA topography
The first hypothesis by Morris and Moffat [55] presented that the PPHA has three segments: (1) the proximal segment which represents the PHA, (2) the middle segment which gives rise to posterior inferior cerebellar artery (PICA), and (3) the distal segment which originates from the primitive vertebrobasilar connection. The second hypothesis is in agreement with Lie’s criteria [49]. The author has mentioned the four essential criteria in establishing the diagnosis of PPHA: (1) hypoglossal artery arises from ICA as a large extracranial branch at the level from C1 to C3 vertebra, (2) it enters the skull through the hypoglossal canal, (3) basilar artery is filled only beyond the point where this artery joins it, and (4) the posterior communicating arteries are absent or not visible on the angiogram.
The third hypothesis includes modified conditions of Brismar [12]: (1) hypoglossal artery arises as an extracranial branch of ICA, (2) this artery passes through the hypoglossal canal, and (3) basilar artery originates from PPHA. The fourth hypothesis is established on the following facts: the persistent artery is composed of the proximal segment, derived from the PHA, and the distal segment, which consists of the lateral anastomotic channel portions, which give rise to the posterior inferior cerebellar artery [58]. The author also speculated that the variant presents the result of proximal segment persistence and communicates with the stem of PICA via distal segment, and next, about the disconnection of PICA origin with the VA, due to aplasia of ipsilateral VA, and the involution of distal segment, which is connected to the BA.
Incidence
This is a rare embryological anastomosis between the ICA and BA, and reported angiographic frequency ranges from 0.027% to 0.1% [5, 10, 12, 17, 21, 33, 37, 54, 59, 62, 75, 86, 89, 93]. Hundred and thirty-four cases of PPHA were described in the literature until 1990 [92]. On the other hand, Khodadad [42] identified only one case of the PPHA among 555 fetuses, but Vasović [81, 82] dissected even four samples of PPHA remnants in 206 fetal brains. According to Arnould et al. [6], the left PPHA was more frequent (65%) than the right one. Murayama et al. [56] described the first case of the PPHA on both sides. Significant predominance of one sex was not described. The age at which diagnosis was established ranged mainly between 20 and 40 years [72].
Patterns of the PPHA origin
The PPHA as a branch of the ICA
The origin of PPHA is different from the origin of ICA cervical portion. Hypoglossal artery may arise from ICA at the level of atlas [6, 25, 44]. It typically originates from the cervical portion of ICA between the C1 and C2 vertebrae [5, 31, 33, 55, 73], or from C2 level [2, 16, 32, 65, 70, 72, 76, 77, 80, 85, 91, 92]. This artery was revealed to branch off at the C3 vertebra level [42, 54, 59, 88] or between C3 and C5 vertebrae [32]. There have been also reports about the PPHA as a “terminal or dorsal” branch of ICA [70, 80]. Oelerich and Schuierer [60] reported the unique origin of PPHA from the petrosal segment of ICA.
Katayama et al. [40] identified PPHA by conventional angiography as an anomalous artery branching off from the C5 portion of the ICA. Dilenge et al. [19] presented angiographically an ectatic extracranial remnant of PPHA on the ICA trunk. Arnould et al. [6] supported the opinion of the previous authors that “an aneurysmal nodus” of the ICA, at the level of axis, has been the consequence of PPHA implantation and that the persistence of its carotid or proximal segment was followed by the disappearance of its distal segment. Teal et al. [78] reported a case with ICA origin of PICAs distal part, identifying it as the variant of PPHA. However, Murayama et al. [56] and Andoh et al. [5] found two patients with PICA originated from the extracranial segment of ICA and which further passed through the hypoglossal canal without an interposed segment of the BA. They nominated it as a PPHA variant.
The PPHA as a branch of the common carotid artery
Stern et al. [75] found the origin of the PPHA from the right common carotid artery (CCA).
The PPHA as a branch of the external carotid artery
The external carotid artery gives rise to PPHA at the level of axis’ body [4, 68] or between the bodies of C2–C3 vertebrae [57].
Course and termination
The hypoglossal artery arises from the ICA under a sharp angle, turns dorsally about 3 cm away from the anterior edges of the first two cervical vertebrae [70], then twists dorsally and inferiorly [16] or dorsally and medially from the ICA [65, 87] to enter the hypoglossal canal (Figs. 2 and 3). In case that the PPHA has the common trunk with the proatlantal intersegmental artery, the anomalous vessel should pass also through the hypoglossal canal [77]. Sometimes, the PPHA crosses over the anterior aspect of the ICA medially from the facial and lingual arteries, the posterior belly of the digastric muscle and the trunk of the hypoglossal nerve. After giving off the rare lateral branch, the otic artery, for example [36], the medial branch of the PPHA crosses the ICA, directed itself to the hypoglossal canal.
Modified Gray’ schema [28] of carotid arteries on the right lateral aspect of the head and neck (a). Black circles mark sites of carotid origin of the PPHA at the level of corresponding cervical vertebrae according to literature data. Modified picture (b) of three-dimensional computed tomography angiography demonstrates the right PPHA that entering the posterior fossa through ipsilateral hypoglossal canal and continuities in BA [34]. CCA Common carotid artery, ICA internal carotid artery, ECA external carotid artery, SA subclavian artery, VA vertebral artery, BA basilar artery, C1–C6 cervical vertebrae
Modified Morris and Moffat' schema [55] of relation of the PPHA and hypoglossal nerve (XII) in hypoglossal canal of occipital bone. left PICA posterior inferior cerebellar artery, left VA vertebral artery, BA basilar artery
The hypoglossal artery appeared to arise from the posterior surface of external carotid artery (ECA), immediately opposite to the lingual artery. It extends rostrally and posteriorly, behind the ICA in its cervical segment, as the occipital branch of ECA. After making a loop opposite the atlantooccipital region, it enters the hypoglossal canal [68].
Normally, the 12th cranial nerve (hypoglossal nerve), the meningeal branch of the ascending pharyngeal artery, and a plexus of emissary veins traverse the hypoglossal canal [43]. In this canal, PPHA is situated dorsolaterally in relation to the rootlets of hypoglossal nerve [49] or it pierces these rootlets when it is located in the subarachnoid space. The majority of rootlets pass medially from PPHA, while only two rootlets travel caudally from the artery, which give the impression that PPHA passes through the hypoglossal nerve rootlets [55]. Opposite to the latter rootlets, Arnould et al. [6] stressed that, like in the primitive type, PPHA goes partially through the rootlets of the hypoglossal nerve or swings up ventrally. The artery passed dorsolaterally from the hypoglossal rootlets in case that the lateral anastomotic channel has its part in their formation [49]. Only Batujeff [8], according to Morris and Moffat [55], reported the presence of the PPHA and two rootlets (an accessory and main) of the hypoglossal nerve in the same canal. Andoh et al. [5] stressed that PPHA can pass through the anterolateral aspect of the basiocciput above the occipital condyles.
An artery, which is referred to as the variant of PPHA, can also goes through this canal [57, 79]. This artery can make anastomosis with the BA at the lower clivus [70]. A large PPHA usually continues as the BA [2, 31, 40, 53, 59, 68, 80, 85, 86]. Therefore, the basilar system could be visualized through the PPHA [76]. However, there were the cases in which PPHA provided blood flow to the BA and all its major branches except the PICA [66, 91].
PPHA variants
The cases of PPHA origin from the ECA [4, 57, 68] or from the CCA [75] are the examples which not fulfill the first of the four Lie' conditions [49]. The cases described by Teal et al. [78] and Trandafilović [79] are examples which fulfill only the second of four Lie’ conditions [49]. The cases of PICA origin from ICA and its course via hypoglossal canal without an interposed segment of BA [5, 55, 77] are examples which fulfill the first and second of four Lie’ conditions [49].
Differentiation between primitive and/or persistent forms
Morris and Moffat [55] supported the opinion that PPHA differs from PHA. They observed during postmortem dissections that PPHA lied laterally and dorsally in relation to the hypoglossal nerve rootlets in the hypoglossal canal, while in embryo, PHA is situated medial and ventral to the rootlets.
Andoh et al. [5] speculated that PPHA variant results at first from the persistence of the PHA near the ICA origin, which communicates with the stem of the PICA via the LBVA, and next, disconnection of the PICA origin from the VA and disappearance of the LBVA connected to the BA. The PHA has not branched, in contrast to the PPHA. [6, 42].
Anatomically, there are no any differences between the fetal type and the adult one of the PPHA [42]. However, the PPHA in human fetuses may have the same function like the VA with large caliber, giving off all important branches to the brain stem and the cerebellum. Judging based on the diameter of the BA, one may have the impression that the BA is the continuation of the PPHA. This artery can be located ventrally to the hypoglossal nerve in the same canal [42]. The second, remnants of the second or the third embryonic segment may lead to the presence of segmental duplication of the VA in its intracranial (V4) segment [51, 82] (Figs. 4 and 5).
Modified picture (a) and original one (b) of a persistence of lateral basivertebral anastomosis (1), in the form of a perivertebral branch [82] in human fetus. Right anterior inferior cerebellar artery (2); right vertebral artery (3); left vertebral artery (4); basilar artery (5)
Modified picture (a) and original one (b) of a fetal anterior spinal artery origin (2) from PPHA remnant (1), in the form of an intervertebral branch [82]. Left vertebral artery (3), right vertebral artery (4); basilar artery (5)
Caliber
The size of the PPHA probably depends upon the size of VA on both sides and whether or not it terminates as the PICA or contributes the primary supply to the BA [78]. The diameter of the PPHA at the point of origin is between 4 and 7 mm [6, 18, 32, 55, 80]. There were extreme exceptions. Arnould et al. [6] identified the diameter of 7 mm, while Jukic-Basic et al. [36] found the diameter of 1.5 mm. The caliber of the PPHA is comparable with the caliber of the ICA either in the fetus [42] or in the postnatal life [68]. Sometimes, it may be slightly larger than the ICA [55], i.e., for 1 mm [70]. However, this rule is valid even in the case of the persisting variant of PPHA [57].
The diameter of the PPHA was equal to both of the ICA and the BA in a case reported by Constans et al. [16]. However, Oelerich and Schuierer [60], using digital subtraction magnetic resonance (MR) and computed tomography (CT) angiography, were able to confirm the existence of a large vessel originating from ICA and connecting with the caudal part of the BA, as a peculiar “hair pin” bend at the anastomosis.
Branches
The side branches of the PPHA were reported as follows:
-
The meningeal artery was a very rare branch of PPHA [8] according to Lie [49]
-
The posterior inferior cerebral artery as a branch of PPHA [16, 52, 60] arises from its extracranial segment [80] or from its intracranial segment [65]. This artery may arise at the point where the PPHA joins the opposite VA [6] or from the connection of the PPHA with the lateral anastomotic canal [69], according to Lie [49]. It has been considered that the origin of PICA from the second segment of PPHA appears because of the omission of the first division of the PPHA involution and the definitive involution of the PHA third segment [55]. The stem of the future PICA can originate from one of several VA branches and these branches may join primitive LBVA. Late retention of different LBVA remnants may be the reason for the variable origins of PICA [5]
-
The anterior spinal artery has the origin from the ipsilateral PPHA [55] or from its segmental remnant [82] (see Fig. 5). This rare vascular anomaly may have the common trunk with the anterior spinal artery in the corresponding hypoglossal canal [6]
-
The posterior spinal artery with one “root” on the PPHA is also described [55]
-
The persistent otic artery, as a branch of the PPHA, was very rare collateral that described in the literature [36]
Association of PPHA with other vascular variations
The anomalies of posterior circulation present the rule, rather than the exception stated in a case of the PPHA [60].
Condition of the VA
Khodadad [42], in his study of PPHA based on 50 dissected fetal brains, observed that in 90% of the specimens, both VAs were hypoplastic, or one of the VAs was hypoplastic while the opposite artery was absent. In the rest of 10% of cases, VA was absent on one side, which was associated with normal VA on the other side. Agnoli [1] reported in his review of PPHA that 79% of 80 observed cases had unilaterally or bilaterally hypoplastic VA. According to Lasjaunias and Berenstein [47], the ipsilateral VA must be hypoplastic in the presence of PPHA. Fujita et al. [23], pointed to the interrelation dependence of PPHA and VA calibers, stressing that in the case of PPHA large caliber, the proximal segments of both VAs remain hypoplastic or the ipsilateral VA is absent. Conforto et al. [15] stressed that the absence of VAs in cervical Doppler provides an important clue to diagnosis of the PPHA.
Combination of three modified schemas and a fetal case. Batujeff' case [8] is presented at schema 1; Öertel' case [61] is presented at schema 2; Morris and Moffat' case [55] is presented at schema 3. HC Hypoglossal canal, PPHA persistent primitive hypoglossal artery, VA vertebral artery, PICA posterior inferior cerebellar artery, PSA posterior spinal artery, BA basilar artery, CB-a unnamed carotid–basilar anastomosis. In the fetal case [79], some vascular components in the posterior cranial fossa (4) are presented. VA Left vertebral artery, PPHA left persistent primitive hypoglossal artery, PICA left posterior inferior cerebellar artery, Tj the tuberculum jugulare
However, the presence of PPHA, its variation [16, 22, 33, 57, 67] or its common trunk with the proatlantal intersegmental artery [77], was associated with hypoplastic ipsilateral VA. Beside, the VA may be aplastic on the PPHA side [33] and hypoplastic on the other side [31, 32, 59, 73, 85, 86, 88] or vice versa [54]. Recently, cervical Doppler, in a case with right PPHA, did not identify the presence of VA on both sides [15].
In a case with right PPHA [2], the right tapered VA was present at the level of the C2 body upper portion while intracranial branches were not visible. In another case [7], the right VA was hypoplastic, while left one proximally supplied one of two left PICA origins and distally was connected with the PPHA. In some cases, VA was hypoplastic [6, 39, 60, 65–67, 76, 80, 90] or aplastic bilaterally [38], or only the VA on opposite side was aplastic [6] or hypoplastic [59]. In rare cases, PPHA continued into BA, while both hypoplastic VAs provided flow for PICAs and were not connected with BA [66]. Huber and Rivoir [32] observed left PPHA associated with right hypoplastic VA that terminated in the PICA, as well as the absence of its connection with the BA. There was also a description of the PPHA ending at distal left VA [72, 79]. Öertel [61], according to Lie [49], described the case of VA with normal diameter on the side with PPHA (Fig. 6).
Condition of the ICA
Anatomically, the most significant findings were the equal diameter of ICA and PPHA [42], as well as the absence of left ICA and presence of the PPHA [90] or the association of PPHA on one and hypoplastic ICA on the other side [59, 66].
Clinically, Gilmartin [26] found the stenosis of the ICA first part; Arnould et al. [6] reported an elongation of both ICAs (dolicho carotid arteries); Sunada et al. [76] reported a severe stenosis extending from the ICA to the PPHA origin, while Oelerich and Schuierer [60] found the moderate stenosis and elongation of the ICA ipsilaterally with the PPHA. Hatayama et al. [31] and Katoh et al. [39] demonstrated severe stenosis of left ICA and PPHA just distal to the stenosis. Elhamady et al. [22] reported a case of proximal ICA stenosis simultaneous with PPHA presence and retrograde flow from the vertebrobasilar system to distal ICA. Nishida et al. [59] demonstrated the hypoplastic left ICA, which was severely stenosed at the entrance of the carotid siphon, as well as an origin of PPHA from the right ICA.
Condition of the anterior cerebral artery
Gerlach et al. [25] reported the simultaneous absence of right anterior cerebral artery (ACA) and presence of PPHA on the same side. The asymmetry of ACAs diameter was identified in fetal brains too [42]. Poorly developed anterior communicating artery was present together with left PPHA [39].
Condition of the posterior communicating artery
Seventy eight percent of 80 PPHA cases described in review were with bilaterally hypoplastic or unopacified PCoA [1]. The PPHA could be the blood source for BA and the left middle cerebral artery via the left PCoA [59]. Angiograms on which BA is opacified and supplied with blood by PPHA may be without [33, 38, 57, 65] or with bilaterally visualized PCoAs [85]. The PCoA can be hypoplastic [60, 88] and/or aplastic on the side of the PPHA [6, 12, 18, 32, 65, 92]. Khodadad [42], during the fetal brains dissection, observed both hypoplastic PCoAs, while others authors described the absence of both PCoAs in adults [7, 31, 33, 39, 67, 75, 86].
Condition of cerebellar arteries
Uniquely, anterior inferior cerebellar artery might be associated with left PPHA [6, 80] or, as Perret et al. [65] described, it might be in combination with the aplastic right posterior inferior cerebellar artery. According to Batujeff [8], who cited by Udvarhelyi and Lai [80], posterior inferior cerebellar artery could be extracranial branch of PPHA. Hatayama et al. [31] and Andoh et al. [5] cited the termination of PPHA in PICA without BA interposed segment presence. However, PICA can terminate into ipsilateral VA while PPHA is present on the other side [2]. Additionally, PICA as an extracranial or intracranial branch of the PPHA, may also represent the supplementary variation or appear as a part of Arnold–Chiari malformation [33, 54, 80].
Association of PPHA with other carotid–vertebrobasilar anastomoses
Öertel [60], who cited, reported a case of PPHA association with persistent trigeminal artery on the left side by Dechaume et al. [18]. The PPHA and the proatlantal intersegmental artery were present in a case reported by Suzuki et al. [77]. Jukic-Basic et al. [36] confirmed PPHA as primitive otic artery point of origin.
Association of PPHA with some other persistent vessels
The association of PPHA and patent ductus arteriosus (PDA) was described as a unique case in a 30-year-old woman who had operation due to an arteriovenous malformation [59]. In a case described by Huynh-Le et al. [33], angiography depicted a strong and large emissary vein on PPHA side.
Association of PPHA with osseous variations
Dechaume et al. [18] described the hypoplasia of the cervical vertebrae transversal foramina. The long dens of C2 vertebra was present with left PPHA [65, 88]. Wiedner and Schreyer [90] presented having had wedged cervical and thoracic vertebrae with a butterfly appearance associated with the PPHA. Dolichocephalic and slight hypoplastic cranial base was described in a 48 year-old lady together with the left PPHA [88].
Some authors only noted that the hypoglossal canal was larger on the PPHA side [7, 25, 31, 60]. Wardwell et al. [87] defined as abnormal the difference of more than 3 mm between the right and left canals. One of the most reliable findings for PPHA diagnosis establishment is the vessel’s course through the hypoglossal canal external foramen. Matsui et al. [53] reported the enlargement of this foramen, while according to Wardwell et al. [87], it was up to 18 mm. Shapiro [71] and Huynh-Le P et al. [33] stressed that when a little hypoglossal canal intracanalicular enlargement is identified, PPHA should be considered in the differential diagnosis. However, Perret et al. [65] described that hypoglossal canal was three times larger on PPHA side in relation to the canal on the opposite side. Wiedner and Schreyer [90] described hypoglossal canal's diameter of 10.5 mm, while Stern et al. [75] and Sunada et al. [76] were able to notify an 8-mm-large canal with persistent ipsilateral PPHA. The enlarged hypoglossal canal with a thin sclerotic narrow margin is also important in the differentiation of hypoglossal neurinoma and PPHA [87]. The thin-slice CT scans in case with left PPHA revealed a thin sclerotic margin of left hypoglossal canal and that the diameter of canal middle part was 5.9 mm wide, while on the right side it was 3.0 mm wide [5].
Contrast enhanced CT revealed left hypoglossal and condylar canals enlargement, as well as ipsilateral PPHA presence [33]. Perret et al. [65] first described basilar impression associated with the left PPHA in a 55 year-old man. The congenital anomalies of the hand's phalanges were associated with the left PPHA [65, 88].
Association of PPHA with organic variations or malformations
The association of kidney malformations (e.g., double calices, polycystic disease) with PPHA is a part of general malformations [6]. Matsui et al. [53] described the PPHA presence in a case with atrial septal defect.
Diagnostic procedures in the revelation of the PPHA
Dissection of fetal and adult brains revealed PPHA presence [36, 42, 81–83]. Different diagnostic procedures, like routine or selective angiography, digital subtraction angiography, three-dimensional (3D) reconstructed computed tomography, magnetic resonance imaging, and magnetic resonance angiography (MRA), are able to clearly demonstrate the vascular anatomy of PPHA and other anatomical structures, which are in close relation with this artery such as ICA, VA, BA, PICA, or hypoglossal canal. A modified reverse Stenvers projection is useful not only for the PPHA passage through the hypoglossal canal confirmation but also to assess any difference of size between the right and left canals [76]. The increase of incidentally found PPHA cases was correlated with the increased use of 3D-CTA and MRA together with its variations, such as time-of-flight MR angiography or angiographic projection images using a maximum-intensity-projection program, in the examination of cerebral vessels [2, 5, 30, 33, 34, 38, 53, 59, 60, 86].
Clinical relevance
Many authors claimed that PPHA is usually present an incidental and asymptomatic observation during the carotid arteriography and is without clinical significance [4, 25, 36, 60, 65, 70, 78, 85, 86, 90]. It is obvious that PPHA is present at birth and will remain in the postnatal life. The findings on positron–emission tomography revealed the greatest values of regional cerebral blood flow in the cerebellar hemispheres between 3 and 8 years of age [14]. Changes in regional cerebral blood flow during brain maturation in children indicates a prominent role of the brain regions supplied by the posterior circulation (brainstem, cerebellum, and visual cortex) during early brain development. It was stressed that the undiagnosed presence of vertebral arteries including the PPHA vascular abnormalities in early childhood might have disastrous outcome during neck and head surgery [27].
Actually, PPHA represents complementary and compensatory artery for the vertebrobasilar system, even though it may be the main source of the blood supply to the brain stem and the cerebellum, especially in case of the aplastic or hypoplastic VAs [11, 38]. Udvarhelyi and Lai [80] have demonstrated the contrast reflux from PPHA into ipsilateral VA. Warot et al. [88] cited literature data that steal phenomenon from ICA is obvious in a case with PPHA presence when it operates as a large VA. A case report by Elhamady et al. [22] illustrated the first description of the reversal flow phenomenon in PPHA. Low flow in the ICA proximal to the second bifurcation and low stump pressure of PPHA strongly indicated low perfusion in the posterior circulation, which could be a cause of vertigo, although thromboembolism due to migration of thrombus at the additional bifurcation or thrombus at the atheromatous plaque through PPHA could also be the cause [31]. Beside, during the cross clamping of carotid arteries the brainstem, cerebellum and ipsilateral cerebral hemisphere can be deprived of blood supply in case VA and PCoAs absence [48, 89].
Stern et al. (1978) [75] described atherosclerotic plaques presence both on PPHA and CCA. Well-known fact is that if proximal occlusion of ICA is applied, clipping of ICA caudally to the origin of PPHA should be avoided [25]. Moreover, PPHA can provide a pathway for cerebral embolism in patients with carotid stenoses [5]. Calcified plaques were present in ICA just proximal to the PPHA take off, but significant stenosis was not present [86]. Hähnel et al. [30] described the case of PPHA isolated stenosis by 3D-CT angiography application.
Katayama et al. [40] stressed that in case of moyamoya vessels occurrence hemodynamic stress present in the PPHA may disturb its spontaneous closure. The particular significance of the PPHA origin from the ECA is that this artery could, under such conditions, became the main source of blood supply to the brain stem and its surgical or spontaneous occlusion could cause infarction of that brain vital area [68].
Arnould et al. [6] reported a finding from the literature about the fibrous regression and partial separation of the elastic lamina in the wall of the left PPHA. Stern et al. [75] found the atheroma with ulceration foci on the CCA dorsal wall and in the proximal part of the PPHA. Calcified plaques were present in the ICA, just proximal to the PPHA take off, but significant stenosis was not present [86]. Some authors reported that the association of PPHA and Arnold–Chiari (type I) malformation is the result of basioccipital structures and hindbrain early stages developmental disorders [54].
The segmental fragility of the PPHA wall can cause subarachnoid hemorrhage [88]. This vascular wall fragility, as well as hemodynamic stress, may be involved in aneurysm formation [67]. According to our knowledge, only few cases of PPHA and arteriovenous malformation association have been reported [24, 29, 59, 72, 74, 92]. The PPHA might cause glossopharyngeal neuralgia [41]. Rotational movements of the neck can cause syncope repeated attacks due to PPHA presence [35]. Udvarhelyi and Lai [80] proved the association of occipital region headaches with the left PPHA presence. Nishida et al. [59] described similar finding when right PPHA was present. The PPHA persisted in a patient with glioblastoma multiforme [23] and in the case of temporal bone chondroblastoma [10].
Udvarhelyi and Lai [80] gave the first description of an aneurysm at the bifurcation of the left PPHA and the right VA, which size was 10 × 8 mm, while Bongartz et al. [13] reported the first successful direct operative treatment of a rare PPHA aneurysm. Yamamoto et al. [91] reported a case with aneurysm at a junction of PPHA and BA. Huber and Rivoir [32] presented the giant aneurysm (25 × 15 cm) at the left PPHA and BA arterial fork, while Kodama et al. [44] confirmed angiographically small (5 mm) aneurysm on the PPHA trunk, slightly dorsally from the hypoglossal canal. Kuramitsu et al. [46] reported two saccular aneurysms located on PPHA and the BA bifurcation; Al-Memar and Thrush [3] described an aneurysm, which arose from the stump of a persistent right PPHA, just outside the skull base, while Duffill et al. [20] and Baltsavias et al. [7] presented a large ruptured aneurysm of the left PPHA. Yamamoto et al. [91] and Huynh-Le et al. [33] confirmed an aneurysm at the junction of the PPHA and proximal PICA.
The angiograms made by Sakai et al. [67] and Komiyama et al. [45] showed a basilar bifurcation aneurysm associated with the PPHA, while Kanematsu et al. [38] presented ruptured aneurysm arising from the BA fenestration associated with the PPHA. Hatayama et al. [31] revealed a large ICA aneurysm and the PPHA arising from the cervical ICA on the left side during the left carotid angiography. Andoh et al. [5] showed a fusiform aneurysm of the right VA with simultaneous presence of ipsilateral PPHA during the right vertebral arteriography.
The incidence of cases with PPHA associated intracranial aneurysm (26.9%) is relatively high [92]. Their mean age is 43 years, somewhat younger than the mean age of cases with other locations aneurysms [91].
According to Kanematsu' report, 41 cases of the PPHA associated with ruptured aneurysms in different locations have been described in the literature [38]. However, only 16 patients with an aneurysm on a persistent PHA itself have been reported in the literature [7].
Authors are aware of morphofunctional and clinical significance of other carotid–vertebrobasilar anastomoses (caudal end of the primitive ICA, proatlantal intersegmental, trigeminal, and otic artery) [81–84], which will be described in their future reviews.
References
Agnoli AL (1982) Vascular anomalies and subarachnoid hemorrhage associated with persisting embryonic vessels. Acta Neurochir 60:183–199
Ahn JH, Choe WJ, Park HI, Lee CH (2005) Persistent hypoglossal artery. J Korean Neurosurg Soc 38:312–315
Al-Memar A, Thrush D (1998) Unilateral hypoglossal nerve palsy due to aneurysm of the stump of persistent hypoglossal artery (extract). J Neurol Neurosurg Psychiatry 64:405
Anderson AR, Sondheimer KF (1976) Rare carotid–vertebrobasilar anastomoses with notes on the differentiation between proatlantal and hypoglossal arteries. Neuroradiology 11:113–118
Andoh K, Tanohata K, Moriya N, Hagiwara H, Lee J, Sato M, Yoshida T, Nagashima T (2001) The posterior inferior cerebellar artery arising from the extracranial segment of the internal carotid artery via the hypoglossal canal without an interposed segment of the basilar artery. Clin Imaging 25:86–89
Arnould G, Tridon P, Laxenaire M, Picard L, Weber M, Gougaud G (1968) L’artère hypoglosse primitive. Ètude anatomique et radiologique. À propos de deux observations. Rev Neurol 118:372–379
Baltsavias GM, Chourmouzi D, Tasianas N, Drevelengas A, Damianovski D, Jovkovski S (2007) Ruptured aneurysm of a persistent primitive hypoglossal artery treated by endovascular approach—case report and literature review. Surg Neurol 68:338–343
Batujeff N (1889) Eine seltene Arterienanomalie: Ursprung der A. basilaris aus der A. carotis interna. Anat Anz 4:282–285
Begg AC (1961) Radiographic demonstration of the “hypoglossal artery”: a rare type of persistent anomalous carotid–basilar anastomosis. Clin Radiol 12:187–189
Ben Salem D, Allaoui M, Dumousset E, Ponnelle T, Justrabo E, Martin D, Sautreaux JL (2002) Chondroblastoma of the temporal bone associated with a persistent hypoglossal artery. Acta Neurochir 144:1315–1318
Bohmfalk GL, Story JL (1977) Aneurysms of the persistent hypoglossal artery. Neurosurgery 1:291–296
Brismar J (1976) Persistent hypoglossal artery, diagnostic criteria. Acta Radiol Diagn 17:160–166
Bongartz EB, Nau HE, Grote W (1977) Successful operative treatment of an aneurysm on a left persistent hypoglossal artery (abstract). Neurochirurgia 20:169–173
Chiron C, Raynaud C, Mazière B, Zilbovicius M, Laflamme L, Masure M-C, Dulac O, Bourguignon M, Syrota A (1992) Changes in regional cerebral blood flow during brain maturation in children and adolescents. J Nucl Med 33:696–703
Conforto AB, de Souza M, Puglia P, Yamamoto FI, da Costa Leite C, Scaff M (2007) Bilateral occipital infarcts associated with carotid atherosclerosis and a persistent hypoglossal artery. Clin Neurol Neurosurg 109:364–367
Constans JP, Dilenge D, Jolivet B (1964) Une cas de persistance d’une artère hypoglosse embryionnaire. Neurochirurgie 10:297–301
Debaene P, Farnarier P, Dufour M, Legre J (1972) Hypoglossal artery, a rare abnormal carotid–basilar anastomosis. Neuroradiology 4:233–238
Dechaume JP, Levy A, Kofman J, Michel D, Rambaud G, Verger D, Bigay JG, Buffard P (1966) À propos des anastomoses carotido–basilaire anormales. Considèrations cliniques et radiologiques. Neurochirurgie 12:777–788
Dilenge D, Constans JP, Dionis PJ (1966) La carotide interne extracranienne. Atlas Radiol Clin Presse Mèd 74(13):1–4
Duffill J, Lang DA, Dwyer ON (1996) Subarachnoid haemorrhage in a child from an aneurysm of a persistent primitive hypoglossal artery (abstract). Br J Neurosurg 10:607–610
Eadie MJ, Jamieson KG, Lennon EA (1964) Persisting carotid–basilar anastomosis. J Neurol Sci 1:501–511
Elhamady MHA, Basakaya MK, Sönmez OF, Morcos JJ (2007) Persistent primitive hypoglossal artery with retrograde flow from the vertebrobasilar system: a case report. Neurosurg Rev 30:345–349 DOI 10.1007/s10143-007-0092-6
Fujita N, Shimada N, Takimoto H, Satou T (1995) MR appearance of the persistent hypoglossal artery. Am J Neuroradiol 16:990–992
Garza-Mercado R, Cavazos E, Urrutia G (1990) Persistent hypoglossal artery in combination with multifocal arteriovenous malformations of the brain: case report. Neurosurgery 26:871–876
Gerlach J, Jensen HP, Spuler H, Viehweger G (1963) Traumatic carotico–cavernous fistula combined with persisting primitive hypoglossal artery. J Neurosurg 20:885–887
Gilmartin D (1963) Hypoglosal artery associated with internal carotid stenosis. Brit J Radiol 36:849–851
Giuffrè R, Sherkat S (2000) Maldevelopmental pathology of the vertebral artery in infancy and childhood. Child's Nerv Syst 16:627–632
Gray H (1918) Illustration, in: anatomy of the human body. Available from: http://content.answers.com/main/content/wp/en-commons/thumb/8/82/250px-Gray513.png
Gupta AK (2005) Cerebral arteriovenous malformation embolized through persistent primitive hypoglossal artery. Interv Neuroradiol 11:241–246
Hähnel S, Hartmann M, Jansen O, Sartor K (2001) Persistent hypoglossal artery: MRI, MRA and digital subtraction angiography. Neuroradiology 43:767–769
Hatayama T, Yamane K, Shima T, Okada Y, Nishida M (1999) Persistent primitive hypoglossal artery associated with cerebral aneurysm and cervical internal carotid stenosis. Neurol Med Chir 39:372–375
Huber P, Rivoir R (1974) Aneurysm on a persistent left hypoglossal artery. Neuroradiology 6:277–278
Huynh-Le P, Matsushima T, Muratani H, Hikita T, Hirokawa E (2004) Persistent primitive hypoglossal artery associated with proximal posterior inferior cerebellar artery aneurysm. Surg Neurol 62:546–51
Hypoglossal canal and artery, in: image. Available from: www.jkns.or.kr/fulltext/htm/0042005150f3.htm
Jackson FE (1964) Syncope associated with persistent hypoglossal artery. J Neurosurg 21:139–141
Jukic-Basic N, Jelic M, Basic V, Jukic T, Vinter I (2001) Persistent hypoglossal artery. J Anat 198:315–316
Kanai H, Nagai H, Wakabayashi S, Hashimoto N (1992) A large aneurysm of the persistent primitive hypoglossal artery. Neurosurgery 30:794–797
Kanematsu M, Satoh K, Nakajima N, Hamazaki F, Nagahiro S (2004) Ruptured aneurysm arising from a basilar artery fenestration and associated with a persistent primitive hypoglossal artery. Case report and review of the literature. J Neurosurg 101:532–535
Katoh M, Kamyama H, Kobayashi N, Makino K, Takano K, Tokumitsu N, Takamura H (1999) Severe stenosis of the internal carotid artery presenting as loss of consciousness due to the presence of a primitive hypoglossal artery: a case report. Surg Neurol 51:310312
Katayama W, Enomoto T, Yanaka K, Nose T (2001) Moyamoya disease associated with persistent primitive hypoglossal artery. Report of a case. Pediatr Neurosurg 35:262–265
Kempe LG, Smith DR (1969) Trigeminal neuralgia, facial spasm, intermedius and glossopharyngeal neuralgia with persistent carotid basilar anastomosis. J Neurosurg 31:445–451
Khodadad G (1977) Persistent hypoglossal artery in the fetus. Acta Anat 99:477–481
Kirdani MA (1967) The normal hypoglossal canal. Am J Roentgenol 99:700–704
Kodama N, Ohara H, Suzuki J (1976) Persistent hypoglossal artery associated with aneurysms. Report of two cases. J Neurosurg 45:449–451
Komiyama M, Nakajima H, Nishikawa M, Yasui T, Kitano S, Sakamoto H, Fu Y (1999) High incidence of persistent primitive arteries in moyamoya and quasi-moyamoya diseases. Neurol Med Chir 39:416–422
Kuramitsu T, Yamada F, Hoshino M (1982) Persistent hypoglossal artery with multiple aneurysms. A case report (abstract). Neuroradiology 24:126
Lasjaunias P, Berenstein A (1987) Surgical neuroangiography functional anatomy of craniofacial arteries, vol. 1. Springer, Berlin Heidelberg New York Tokyo, pp 123–126
Lasjaunias P, Santoyo-Vazueza A (1984) Segmental agenesis of the internal carotid artery: angiographic aspects with embryological discussion. Anat Clin 6:133–141
Lie TA (1972) Congenital malformations of the carotid and vertebral arterial systems, including the persistent anastomoses. In: Vinken PJ, Bruyn GW (eds) Handbook of clinical neurology. vol. 12. North Holland, Amsterdam, pp 289–339
Lindgren E (1950) Percutaneous angiography of the vertebral artery. Acta Radiol 33:389–404
Malobabić S, Milisavljević M (1984) A rare relationship between the vertebral artery (a. vertebralis) and the hypoglossal nerve (n. hypoglossus). Srp Arh Celok Lek 112:1079–1084
Marinković S, Milisavljević M, Antunović V (2001) Arterije mozga i kičmene moždine. Anatomske i kliničke karakteristike (In Serbian). Bit inženjering, Beograd, p 17
Matsui H, Udaka F, Kubori T, Oda M, Nishinaka K, Kameyama M (2005) Persistent primitive hypoglossal artery with atrial septal defect. Intern Med 44:507–508
Matsumura M, Nojiri K, Yumoto Y (1985) Persistent primitive hypoglossal artery associated with Arnold–Chiari type I malformation. Surg Neurol 24:241–244
Morris ED, Moffat DB (1956) Abnormal origin of the basilar artery from the cervical part of the internal carotid and its embryological significance. Anat Rec 125:701–708
Murayama Y, Fujimoto N, Matsumoto K (1985) Bilateral persistent primitive hypoglossal arteries associated with a large ruptured aneurysm on one side. Surg Neurol 24:498–502
Nakamura M, Kobayashi S, Yoshida T, Kamagata M, Sasaki T (2000) Persistent external carotid–vertebrobasilar anastomosis via the hypoglossal canal. Neuroradiology 42:821–823
Nakanishi N, Sugino T, Morikawa K, Ohkawa N, Fukusumi A (2004) The posterior inferior cerebellar artery arising from the internal carotid artery directly: a variant of the persistent primitive hypoglossal artery (abstract). No To Shinkei 56:253–257
Nishida C, Ashikaga R, Araki Y, Nakamatsu K, Ono Y, Fujii K, Nishimura Y (2000) Persistent hypoglossal artery associated with arteriovenous malformation: case report. Eur Radiol 33:59–62
Oelerich M, Schuierer G (1997) Primitive hypoglossal artery: demonstration with digital subtraction-, MR- and CT angiography. Case report. Eur Radiol 7:1492–1494
Öertel O (1922) Über die persistenz embryonaler verindungen zwischen der A. carotis interna und der vertebralis cerebralis. Verh Anat Ges 55:281–295
Ouriel K, Green RM, DeWeese JA (1988) Anomalous carotid–basilar anastomoses in cerebrovascular surgery. J Vasc Surg 7:774–777
Padget DH (1948) The development of the cranial arteries. Contrib Embryol 32:207–261
Padget DH (1954) Designation of the embryonic intersegmental arteries in reference of the vertebral and subclavian stem. Anat Rec 119:349–356
Perret J, Groslambert R, Crouzet G, Bonneton M, Fau R (1970) Ètude radiologique d'une nouvelle observation d'artère hypoglosse. Rev Neurol 123:49–53
Pinkerton JA, Kendrick CD, Hibbard BZ (1980) Primitive hypoglossal artery and carotid endarterectomy. Stroke 11:658–660
Sakai K, Tanaka Y, Tokushige K, Tanabe A, Kobayashi S (1998) Basilar bifurcation aneurysms associated with persistent primitive hypoglossal artery. Neurosurg Rev 21:290–294
Samra K, Scoville WB, Yaghmai M (1969) Anastomosis of carotid and basilar arteries. Persistent primitive trigeminal artery and hypoglossal artery: report of two cases. J Neurosurg 30:622–625
Schmeidel G (1933) Die entwicklung der arteria vertebralis des menschen. Gegenbaurs Morphol Jahrb 71:315–435
Scott HS (1963) Carotid basilar anastomosis—persistent hypoglossal artery. Brit J Radiol 36:847–849
Shapiro R (1979) Enlargement of the hypoglossal canal in the presence of a persistent hypoglossal artery. Radiology 133:395–396
Shibata Y, Hyodo A, Saito A, Yoshii Y, Nose T (1991) Large arteriovenous malformation associated with persistent primitive hypoglossal artery: case report. Neurol Med Chir 31:804–808
Springer TD, Fishbone G, Shapiro R (1974) Persistent hypoglossal artery associated with superior cerebellar artery aneurysm. Case report. Neurosurg 40:397–399
Stanley OS (1990) Unilateral sudden hearing loss as a result of anomalous carotid anatomy. J Vasc Surg 12:341–344
Stern J, Correll JW, Bryan N (1978) Persistent hypoglossal artery and persistent trigeminal artery presenting with posterior fossa transient ischaemia attacks. Report of 2 cases. J Neurosurg 49:614–619
Sunada I, Yamamoto S, Matsuoka Y, Nishimura S (1991) Endarterectomy for persistent primitive hypoglossal artery: case report. Neurol Med Chir 31:104–108
Suzuki S, Nobechi T, Itoh I, Yakura M, Iwashita K (1979) Persistent proatlantal artery and occipital artery originating from internal carotid artery. Neuroradiology 17:105–109
Teal JS, Rumbaugh CL, Segall HD, Bergeron RT (1973) Anomalous branches of the internal carotid artery. Radiology 106:567–573
Trandafilović M (2007) Peculiarities of the hypoglossal artery in a foetal period. Abstract book of international medical students' congress in Novi Sad. p 120
Udvarhelyi GB, Lai M (1963) Subarachnoid haemorrhage due to rupture of an aneurysm on a persistent left hypoglossal artery. Br J Radiol 36:843–847
Vasović L (1990) Morfološke karakteristike arterijskog prstena mozga kod različitog porekla kičmenih arterija (teza). Medicinski Fakultet, Niš, Serbia
Vasovic LP (2004) Reevaluation of the morphological parameters according to 11 different duplications of the fetal vertebral artery at prevertebral (V1) and intracranial (V4) parts. Cells Tissues Organs 176:195–204
Vasovic LP (2004) The tenth vascular component in a rare form of the cerebral arterial circle of fetuses. Cells Tissues Organs 178:231–238
Vasović L, Milenković Z (2005) Funkcionalna i patološka anatomija karotidobazilarnih i karotidovertebralnih anastomoza (in Serbian). SVEN, Niš, pp 64–79
Vlychou M, Georganas M, Spanomichos G, Kanavaros P, Artinopoulos C, Zavras GM (2003) Angiographic findings and clinical implications of persistent primitive hypoglossal artery. Case report. BMC Medical Imaging 3:http//www.biomedcentra.com/1471–2342/3/2
Wagner AL (2001) Isolated stenosis of a persistent hypoglossal artery visualized at 3D CT angiography. Am J Neuroradiol 22:1613–1614
Wardwell GA, Goree JA, Jimenez JP (1973) The hypoglossal artery and hypoglossal canal. Am J Roentgenol 118:528–533
Warot P, Lambert P, Gozet G (1970) À propos d’une observation d’artère hypoglosse primitive. Rev Neurol 123:53–56
Widmann MD, Sumpio BE (1992) Persistent hypoglossal artery: an anomaly leading to false-positive carotid duplex sonography. Ann Vasc Surg 6:176–178
Wiedner F, Schrueyer H (1976) Seltene anomalien zerebraler gefaße (einseitige aplasie der a. carotis interna, Persistenz der A. hypoglossica). Fortschr Röntgenstr 124:245–249
Yamamoto S, Sunada I, Matsuoka Y, Hakuba A, Nishimura S (1991) Persistent primitive hypoglossal aneurysms. Report of two cases. Neurol Med Chir 31:199–202
Yamanaka K, Noguchi K, Hayasaki K, Matsuoka Y (1990) Persistent primitive hypoglossal artery associated with arteriovenous malformation: case report. Neurol Med Chir 30:949–955
Yokota N, Yokoyama T, Ryu H (1999) Aneurysm of persistent primitive hypoglossal artery. Br J Neurosurg 13:608–610
Author information
Authors and Affiliations
Corresponding author
Additional information
Comments
Tamas Dóczi, Pécs, Hungary
This is an interesting review of normal and pathological aspects of the hypoglossal artery. Persistent carotid–vertebrobasilar anastomoses can be explained by an interruption of the vertebrobasilar system embryogenesis. Different types of persistent anastomoses (hypoglossal, type I and II proatlantal, trigeminal, and otic arteries) have been described in the literature. Each anastomosis was not well known but has typical potential clinical risks. This manuscript has reviewed developmental morphology of the hypoglossal artery on the basis of a literature search. Several figures help the readers to understand the anatomical details. It provides no significant original contribution to the topic. However, persistent carotid–vertebrobasilar anastomoses are rarely seen, and they have major clinical significance. Reading the review on this topic may turn in clinical situation to be extremely important to the practicing readers of Neurosurgical Review. I think that the review of not only one of the anomalous channels is interesting and useful but also all of the persistent carotid–vertebrobasilar anastomoses.
Kiyohiro Houkin, Sapporo, Japan
This paper has reviewed the normal anatomy and variation of the hypoglossal artery. It has been believed that the hypoglossal artery is rare anomaly. However, it has turned out that this anomalous artery is often revealed in clinical scene due to the recent development of the diagnostic modality. In this sense, this paper has timely highlighted the novel contemporary significance of the hypoglossal artery.
This paper seems to be the most in-depth review of this specific artery in terms of its anatomy, pathology, and embryology. The most educational point of this paper is its schematic drawings that introduce us to visually understand this complicated anomalous artery. The hypoglossal artery is clinically important as the collateral circulation of the cerebrovascular occlusive disease and complicated cerebral aneurysm. Therefore, radiological images including angiography and operative photography of the hypoglossal artery would have added more practical and attractive knowledge to this paper. In any case, I am confident that this paper is one of the representative reviews on the hypoglossal artery. I also expect the authors to review another anomalous artery.
Rights and permissions
About this article
Cite this article
Vasović, L., Milenković, Z., Jovanović, I. et al. Hypoglossal artery: a review of normal and pathological features. Neurosurg Rev 31, 385–396 (2008). https://doi.org/10.1007/s10143-008-0145-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-008-0145-5