Abstract
Introduction
Intraventricular/germinal matrix hemorrhage affects 7–30% of premature neonates, 25–80% of whom (depending on the grade of the hemorrhage) will develop hydrocephalus requiring shunting. Predisposing factors are low birth weight and gestational age.
Material
There is increasing evidence for the role of TGF-β1 in the pathogenesis of hydrocephalus, but attempts to develop treatment modalities to clear the cerebrospinal fluid (CSF) from blood degradation products have not succeeded so far. Ultrasound is a valuable screening tool for high-risk infants and magnetic resonance imaging is increasingly utilized to differentiate progressive hydrocephalus from ex vacuo ventriculomegaly, evaluate periventricular parenchymal damage, decide on the surgical treatment of hydrocephalus, and follow up these patients in the long term. Treatment of increasing ventriculomegaly and intracranial hypertension in the presence of hemorrhagic CSF can involve a variety of strategies, all with relative drawbacks, aiming to drain the CSF while gaining time for it to clear and the neonate to reach term and become a suitable candidate for shunting. Eventually, patients with progressive ventriculomegaly causing intracranial hypertension, who have reached term and their CSF has cleared from blood products, will need shunting.
Conclusion
Cognitive long-term outcome is influenced more by the effect of the initial hemorrhage and other perinatal events and less by hydrocephalus, provided that this has been addressed timely in the early postnatal period. Shunting can have many long-term side effects due to mechanical complications and overdrainage. In particular, patients with posthemorrhagic hydrocephalus are more susceptible to multiloculated hydrocephalus and encysted fourth ventricle, both of which are challenging to treat.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The most common cause of acquired infantile hydrocephalus is germinal matrix hemorrhage (GMH) [24]. The term indicates a localized hemorrhage in a temporary brain structure that affects almost exclusively premature infants. Two alternative terms are subependymal hemorrhage and periventricular–intraventricular hemorrhage, providing the topographic relationship with the ventricular system, beneath and within it, respectively. Intraventricular extension of a GMH occurs in 80% of cases [120] and can be observed primarily or secondarily as extension of the subependymal–parenchymal hemorrhage. The presence of blood and degradation products into the ventricles and the subarachnoid space can lead to ventricular dilatation through a multifactorial mechanism. The term ventriculomegaly refers to any abnormal increase of the ventricular size found on imaging studies. Similar is the definition of posthemorrhagic ventricular dilatation (PHVD), which describes a clinically silent dilatation after an intraventricular extension of a GMH. Posthemorrhagic hydrocephalus (PHH) is a term used to describe a progressive ventriculomegaly followed by symptoms and signs of raised intracranial pressure (ICP) [23].
The significant progress that has been achieved in the neonatal intensive care field during the last two decades raised the survival rates of premature (below 37 weeks of gestational age [GA]) and low birth weight (BW; below 2,500 g regardless of GA) infants [111]. PHH poses a serious threat to the neurodevelopmental outcome of the newborn and the efforts of the multidisciplinary team approach should focus on that.
Epidemiology
The incidence of GMH has decreased in the past 40 years [2, 26, 33, 80, 88, 93, 104]. This is becoming clear as an observation between the same groups of infants-at-risk that manage to survive. Two major factors influencing survival are GA and BW. Improved treatment and survival rates, especially in the lower GA group, are “offering” more candidates for GMH than earlier [71, 126, 136]. Thus, the prevalence of GMH remains high [23]. A possible underestimation bias can be explained by the fact that many cases of GMH are asymptomatic and their detection depends on screening and imaging criteria.
In the low GA group, the rate of GMH has decreased from 40% to 44% in the 1970s to 20% in the 1980s [68], although an overall incidence of 7–30% is more accurate today [58, 66]. According to BW, in the very low birth weight (VLBW) subgroup (<1,500 g), the incidence has declined from 35% to 50% in the 1980s to 15–30% in the 1990s [58, 81, 119]. Series from institutions around the world show a wider range of germinal matrix–intraventricular hemorrhage (GM-IVH) from 20% to 64% in the subgroups of VLBW and extremely low birth weight (ELBW <700 g) [48, 51, 65, 99].
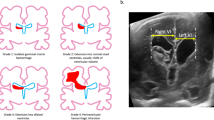
A widely used classification scheme is based on imaging findings and stratifies the degree of hemorrhage and subsequent ventricular dilatation [88]:
-
Grade I: GMH only (subependymal)
-
Grade II: GM-IVH (intraventricular extension without dilatation)
-
Grade III: GM-IVH (intraventricular extension with dilatation)
-
Grade IV: GM-IVH (intraventricular extension with dilatation plus parenchymal hemorrhage)
This system is mainly ultrasound based and can be adapted for computed tomography (CT) and magnetic resonance imaging (MRI) use as well. Ventricular dilatation and parenchymal hemorrhage are described as imaging findings with no pathophysiological correlation, although the higher grades pose higher morbidity and mortality rates. Infants with PHH have a significantly higher mortality rate than low BW infants without PHH (16–35% vs 6.5–13%). Grade I hemorrhage has 5% mortality rate and 5% chances for progressive ventriculomegaly, grade II has 10% and 20%, grade III has 20% and 55%, and grade IV has 50% and 80%, respectively [68].
The severity of GM-IVH correlates with the GA [122]:
-
GA (in weeks): 24 to 26 → 32% grade III and 19% grade IV
-
GA (in weeks): 31 to 32 → 11% grade III and 5% grade IV
The average incidence of ventriculomegaly in the entire GM-IVH population (all grades) is 40–50% [23, 81]. In cases of IVH (grades II–IV), the average incidence of ventriculomegaly is 55–80% and clinically significant PHH will develop in 26% to 80% of these cases [81, 89].
Pathophysiology
Germinal matrix hemorrhage–periventricular posthemorrhagic injury
During the last 12 weeks of gestation, a temporary zone at the external angles of both the lateral ventricles, just beneath the ependymal lining, is the source of the future neurons and glial cells of the forebrain. This germinal matrix (GM) is gelatinous, highly cellular, and vascularized. Because of the temporary nature of this structure, the capillary bed has minimal collagen basement membrane, no tight junctions, and no glial endfeet, although it receives a disproportionately large amount of cerebral blood flow (CBF) to uphold the high metabolic demands. Those fragile vessels with the impaired autoregulation are the site of the GMH. The GM reaches its maximal size by 23 weeks of gestation, decreases to half its size by 32 weeks, and almost involutes by 36 weeks [23, 68, 81]. The blood supply of the GM is from Heubner’s artery (branch of the anterior cerebral artery), lateral striate arteries (branch of the middle cerebral artery), and anterior choroidal artery (branch of the internal carotid or middle cerebral artery) [37]. The capillary bed drains into the terminal vein along with the medullary, choroidal, and thalamostriate veins, the confluence of whom drains into the internal cerebral vein, take a sharp bend, and the direction of blood flow is reversed abruptly [23, 68]. This creates an intramural stress condition and contributes to the vessel rupture and to further venous congestion [108]. The venous endothelial cell-lined channels are the most common site of rupture compared to the proximal capillary–venule junctions [79]. Macroscopically, the location of the hemorrhage before the 28th week is posterior to the body of the caudate nucleus and after that is more anterior, over the head of the caudate nucleus [23, 37].
Infants that are born prematurely with an active GM are in danger of hemorrhage because the fragile and immature vessels are vulnerable to respiratory and circulatory fluctuations. The major factors associated with GMH are increased cerebral perfusion pressure and hypoxia. Low oxygen delivery at the endothelial cells lining the capillaries makes them vulnerable to infarction and then disruption. Hypercapnia additionally dilates the thin wall vessels. Other factors are arterial hypertension especially when following systemic hypotension [121], increased cerebral venous pressure [82], dehydration followed by resuscitation with rapid infusion of hyperosmolar solutions, and coagulopathies [23].
Many risk factors, environmental, medical, obstetric, and neonatal, can result to the aforementioned conditions that cause GMH [4, 8, 27, 36, 54, 56, 61, 67, 70, 73, 86, 107, 126]:
-
rapid volume expansion, seizures, pneumothorax, pulmonary hemorrhage, low GA, low BW, absence of antenatal steroid exposure, antenatal maternal hemorrhage, maternal chorioamnionitis/infection/inflammation, maternal fertility treatment, neonatal transport, anemia, decreased blood glucose, cyanotic heart disease, respiratory distress syndrome/mechanical ventilation, arterial catheterization, early sepsis, hypotension treatment with pressor agents, severity of illness score, acidosis, maternal cocaine abuse, and prolonged labor.
Direct damage from the formation of hematoma into the brain tissue is expected after the initial bleeding. GM damage theoretically is better tolerated due to the plasticity of the developing brain in the particular area [23]. Further damage outside the GM region, superolateral to the head and body of the caudate nucleus, is a more serious insult and results from expansion of the initial bleeding or a periventricular hemorrhagic infarct (PHI). Those are separate entities but can also overlap. In a grade IV GM-IVH, the bleeding extension into the parenchyma can be immediate or secondary after several days. At the same period, hemorrhagic necrosis of the periventricular white matter is another contributing factor [30]. It is believed that the clot acts as space-occupying lesion which impairs venous drainage of that area due to the sharp bending of the terminal vein, which is further compounded when the ventricle expands and the ICP is raised. This occurs in 15% of all infants with GM-IVH [108]. Periventricular leukomalacia (PVL) is a remaining parenchymal lesion after an arterial ischemic injury. It affects the white matter and presents as multiple cystic foci with coagulation necrosis and loss of cellular architecture [70]; further progression of these foci result in porencephaly. The location corresponds to the PHI areas and a clear pathogenetic distinction between PVL and PHI is difficult. The white matter injury that accompanies GM-IVH is believed to represent the cause of the neurodevelopmental impairment suffered by these neonates [6, 70].
Common molecular pathways are involved in the initiation of the hemorrhage and the secondary white matter damage. The cyclooxygenase 2 (COX-2) system is induced by hypoxia, growth factors (transforming growth factor β1 [TGF-β1]), and inflammatory modulators (ΙL-6, IL-1β, and TNF-α). The resultant prostanoids mediate the production and release of the vascular endothelial growth factor (VEGF). Cytokines, nitric oxide, and VEGF mediate a cascade leading to the disruption of tight junctions, increased blood–brain barrier permeability, and microglial activation in the developing periventricular white matter [70]. Microglial activation releases reactive oxygen species which result in direct endothelial damage, alter hemostasis, and increase anaerobic metabolism [3, 14, 90, 96, 106].
The incidence and brain damage severity of GM-IVH may have a genetic predisposition. As with adult intracerebral hemorrhage and apolipoprotein E4 or E2 allele high incidence, proposed candidate genes include IL-6 (174 CC and 572 C alleles), TNF-α, factor V (Leiden), prothrombin (G20210A), and collagen IVA1 [28, 29, 31, 39, 60, 70]
Posthemorrhagic hydrocephalus
The presence of blood into the ventricular system can result in alterations of cerebrospinal fluid (CSF) circulation and ventricular dilatation—transient or progressive. Neonates with grades III and IV hemorrhage are in greater danger for developing PHH and that correlates with the blood amount in the ventricles. Large clots can directly obstruct the intraventricular CSF pathways (foramen of Monro, aqueduct, foramina of Magendie and Luschka) or cause reactive gliosis. The very likely presence of small transependymal channels provides another site for CSF reabsorption through small blood vessels within the parenchyma. Multiple clots can block those channels as well [124]. Furthermore, the rheological properties of CSF are altered as well and that impedes the circulation in the subarachnoid space and the reabsorption through the arachnoid villi [15]. At this point, some ventricular dilatation may occur and can be reversible if blood clots resolve quickly.
Concentrations of TGF-β1 in the CSF of infants who have suffered GM-IVH have been found to be high, especially in the PHH group [129]; in CSF of normal infants, it is undetectable. TGF-β1 is involved in the upregulation of genes for extracellular matrix protein synthesis such as fibronectin and laminin. It is linked also to wound healing, scar formation, and fibrotic diseases (e.g., cirrhosis) [136]. The origin of TGF-β1 in the CSF could be from the platelets or from the choroid plexus. In addition, high levels of C-propeptide of precollagen I have been found in CSF samples of preterm infants (VLBW) with PHH [41]. The above data suggest that IVH induces the production of extracellular matrix proteins and local collagen synthesis that promote fibrosing obliterative arachnoiditis, meningeal fibrosis, and subependymal gliosis [81]. This occurs 2 to 6 weeks after the initial bleeding and there is a tendency for more resistant ventricular dilatation. Extracellular matrix protein deposits are more prominent in the posterior fossa and can block the fourth ventricular outflow by the resultant adhesive arachnoiditis. The small diameter channels of transependymal reabsorption and the arachnoid villi are also vulnerable to blockage by fibrosis.
Besides the parenchymal injury from the initial bleeding and the following periventricular posthemorrhagic ischemia, further brain damage is anticipated by the ventriculomegaly. A typical mechanism describes fiber stretch and vessel compression, venous and arterial, with subsequent hypoxia due to increased ICP [68]. Further oxidative stress is caused by free radicals and inflammatory impact through a number of cytokines (TNF-α, IL-1β, IL-6, IL-8, and IFN-8) [7, 63, 101]. The neuropathological consequences affect neurons (swelling, impaired synaptogenesis), fibers (stretching, disruption, defective myelination), and neurotransmitters (lower levels of catecholamines, deficient synthesis) [21, 88, 98].
Clinical presentation
Premature infants are usually treated in the neonatal intensive care unit. The younger GA they are born, the more coexisting heath problems they bear. Cardiopulmonary insufficiency, metabolic disorders, and immune deficiency are the major risk factors and the neonates are often intubated under sedation. Under those circumstances, a smaller hemorrhage can go easily undetected and a high suspicion index is needed. Possible signs are hypotonia, seizures (often subclinical), ophthalmoplegia, irritability, and vomiting. Larger hemorrhages can present with further muscle tone changes (abnormal posturing), respiratory and cardiac irregularities (apnea, bradycardia, hypotension), metabolic acidosis, pupillary dilatation, and an unexplained drop of hematocrit (>10%). If the hemorrhage progresses to hydrocephalus, additional signs are bulging fontanelle, separation of sutures, distended scalp veins, upward gaze palsy (“sunset” eyes sign), and a rapidly increasing head circumference that crosses the centile curves with a rate larger than 1.5–2 cm/week or 2 mm/day. Head growth of the premature infant is increased during the “catch-up phase” when the infant is able to be fed adequately, and this has to be differentiated from abnormal head circumference increase. The time course from the initial hemorrhage to clinically evident ventriculomegaly and even further to progressive hydrocephalus varies from days to weeks [42, 53, 55]. Close evaluation and follow up of those infants cannot be supported only by clinical data and diagnostic tools are mandatory.
Diagnosis
Cranial ultrasonography (US) today has a very important role on the initial assessment and follow up of premature infants. Performed through the open anterior fontanelle (and occasionally posterior fontanelle) [109], it provides direct and accurate data about ventricular size, exact location and extension of the hematoma, cortical mantle thickness, and periventricular white matter condition. It is a noninvasive, low-cost, and easily available examination in most neonatal intensive care units. Disadvantages are diminished imaging of posterior fossa and operator dependency. With today’s advanced hardware, cranial ultrasonography has a sensitivity and specificity >91% each [5, 100]. Combined with clinical evidence, it is used to detect initial hemorrhage, which more often occurs at the first antenatal week, and further ventricular dilatation, which results in progressive hydrocephalus usually after the third week. This time interval is important because ventricular dilatation can occur without clinical evidence and contributes to an otherwise preventable brain damage. In addition, a primarily minor hemorrhage (grade I–II) can extend to a major one (grade IV) with subclinical evidence in an intubated and sedated infant. For these reasons, many recommendations have been formulated focusing on the screening usage of ultrasound [56, 60, 91, 92]. Infants at greater risk (lower GA, lower BW) should be scanned more often. An initial US is done before day 5 of life, a second one during the second week (days 10–14), and a third on day 28. Generally, at least one US per week should detect all cases of GM-IVH and delineate the ventricular size status. If ventricular dilatation is treated with surgical interventions (ventricular taps, reservoirs, drains, lumbar punctures [LP]), more frequent US should be considered.
Ventricular parameters used in clinical practice are the ventricular index (VI), anterior horn width (AHW), frontal horn ratio (FHR), ventricular axis (VA), ventricular height (VH), and thalamo-occipital distance (TOD) [11]. These measurements are applied in the coronal and sagittal plane. The most widely used is the VI, the distance between the falx and the lateral wall of the anterior horn in the coronal plane at the level of the third ventricle (foramen of Monro). Many studies provide reference ranges of such parameters on US in an attempt to distinguish normal from abnormal and to help clinicians to improve diagnostic and therapeutic evaluation [18, 59, 64, 105]. Ratios (e.g., FHR) are considered to be less useful for monitoring ventricular size compared to VI [92]. Progressive ventricular enlargement as measured by the VI that exceeds 4 mm above the 97th centile for GA is a strong indicator for therapeutic intervention by means of CSF drainage [59, 115, 116]. VI is also superior to other measurements as a reflection of ICP in neonates with PHVD. Interpretation of ventricular measurements should combine clinical evidence because, when these apply in very premature infants, considerable variation exists, asymmetry between the lateral ventricles is a common phenomenon, and operator dependency always concurs [11].
MRI has been employed in recent years in infants with GM-IVH. Although a critically ill preterm infant is difficult to transfer, maintain temperature, and endure the whole examination, valuable information can be extracted. The exact condition of periventricular white matter can be clarified (Fig. 1). On MRI, PVL is demonstrated early by restricted diffusion in affected areas Diffusion Weighted Imaging (DWI) and later by periventricular volume loss, ventriculomegaly, and gliosis [85]. Neurodevelopmental outcome is grossly affected by such findings and early consultation of parents can be more accurate. Furthermore, MRI can contribute to distinguishing ventricular enlargement due to atrophy from ventricular dilatation due to CSF accumulation. Round “ballooned” horns (Fig. 1), periventricular high signal on T2, and effaced subarachnoid spaces are typical findings of active hydrocephalus. More evidence can be elicited by more sophisticated techniques such as CBF measurement and apparent diffusion coefficient (ADC) values registration [57]. Distinction between progressive and compensated hydrocephalus is crucial in premature infants and it has been found that normal CBF and low ADC values (measured by MRI) are associated with a compensated–arrested state and could support a conservative treatment approach. The Doppler ultrasound measurement of cerebral arterial flow used mostly in the past was based on a similar theory. Arterial flow normalization after CSF drainage favored ventricular distention against “ex vacuo” ventriculomegaly [50].
T1-weighted (T1w) axial MR scans of a girl born at 24 weeks gestation who developed grade III IVH and eventually required shunting. A small clot can be seen at the medial (ventricular) surface of the thalamus (a), presumably the site of the original hemorrhage. A clot is also seen in the occipital horn of the right lateral ventricle (a, b) and the occipital horn of the left lateral ventricle (c). A low signal indicating possible early cystic change is seen in the periventricular white matter surrounding the left occipital horn (a, b)
Although CT scanning assisted the diagnostic workup of infants with GM-IVH when first applied, it is nowadays replaced by US and MRI. All the information that CT can provide is more easily and accurately obtained by the latest US devices, especially when combined with MRI. The major drawback of CT is ionizing radiation which is more hazardous when performed on the newborn. The role of CT is limited in cases of relevant emergency and lack of US assistance.
Prevention
Many trials and prevention strategies were based on the general theory that GM-IVH incidence would decrease when optimal resuscitation of the infant could minimize CBF fluctuations, hypoxia, hypercapnia, and increased venous pressure. The perinatal period is the most significant in the prevention of GMH and has been divided into prenatal and postnatal [23]. Prenatal strategies are focusing on the elimination of any stress condition by optimal obstetric management and smooth delivery, judicious usage of cesarean section, and administration of steroids and vitamin K. Postnatal approaches are almost exclusively pharmacological prevention strategies. Agents designed to modulate CBF to the preterm brain may alter other developing organs or impair neurogenesis [70] and cautious administration of these agents is mandatory.
-
Phenobarbital was believed to have neuroprotective effect by decreasing the metabolic rate of cerebral tissue, inhibiting seizures, and stabilizing blood pressure and production of free radicals. Antenatal administration failed to reduce GM-IVH, while postnatal administration could not be recommended for the prevention of GMH. Additionally, it was found to increase the risk for requiring mechanical ventilation [103, 127, 133].
-
Steroids are used routinely before delivery of premature infants. The main reason is to support respiratory function of the newborn, but it was found that the incidence and severity of GM-IVH were also decreased. Further studies demonstrated that this was not only due to the reduction of perinatal respiratory distress but directly due to potential stabilization of GM microvasculature [28, 62, 72].
-
Indomethacin is an anti-inflammatory agent used in preterm infants to close patent ductus arteriosus and prevent GM-IVH. Trials have shown that it decreases the rate and severity of hemorrhage in the newborn by inhibition of COX-1 and COX-2 isoforms and further decrease in prostaglandin synthesis, which possibly result in basement membrane maturation, improvement of autoregulation, and stabilization of the blood–brain barrier. It is associated with increased risk of intestinal perforation, and controversy exists about the long-term effects on neurodevelopmental outcome. Indomethacin protection against GM-IVH has been found more profound in male infants and that finding suggested that gender is an important issue in infantile GMH [40, 74–76, 114].
-
Recombinant activated factor VII (rFVII) is a hemostatic agent used in hemophiliac patients and other bleeding disorders such as major trauma, oral anticoagulation, and liver dysfunction. When administered, it binds to activated platelets and promotes a generous thrombin formation and further hemostasis. Small case series involving the administration of rFVII in neonates showed relevant safety and effectiveness and this deserves further investigation [9, 32, 78].
-
Other agents that were investigated are ethamsylate, ibuprofen, vitamin E, and pancuronium with results inadequate to support convincing evidence for the prevention of IVH in neonates and have limited use in current practice.
Management
Therapeutic approaches on a premature infant suffering from GM-IVH should focus on two objectives: (a) to protect the brain tissue from damage secondary to raised ICP and (b) to avoid the placement of a permanent shunt with the long-term complications and undesirable shunt dependency that this leads to [136]. Not all the cases of GM-IVH will evolve to progressive hydrocephalus. Eventually, 20% to 50% of them will develop ventricular dilatation, either transient or progressive, with the higher grades of hemorrhage having a greater risk and 50% of them will spontaneously arrest or resolve [120]. Ventricular enlargement is accommodated by cerebral tissue compliance [137], but if dilatation progresses, irrecoverable brain damage may occur. The clinician has to find the golden median between the two objectives during the first weeks of the infant’s life. The criteria for the first-tier interventions when ventricular dilatation is noticed are straightforward. VI measurements that gradually cross the 97th centile + 4 mm for the corrected GA are widely accepted as an indication for intervention. Other clinical data are taken into consideration such as tension of the anterior fontanelle, head circumference increase, squamosal suture diastasis >5 mm, and neurological status (irritability, tendon reflexes, ocular movement).
Interventions
When progressive ventricular dilatation is verified, first-tier interventions aim at CSF removal, which results in lowering intraventricular pressure. Blood and degradation products are also withdrawn, together with inflammatory cytokines and TGF-β1. Several options are available:
-
Serial LP are, in many neonatal ICUs, the first treatment option for rapid and direct CSF removal. As a prerequisite, the hydrocephalus has to be communicating without congenital anomalies affecting the area of the lumbar spine. Many infants cannot tolerate the longstanding lateral decubitus position required for the procedure. A minimum of 10 to 20 ml of CSF must be removed at each tap to be effective, daily for up to 3 consecutive weeks and initiated as soon as possible [23]. This makes serial LP rather strenuous both for the premature baby and the treating doctor. In clinical practice, after the first or second week of repeat LPs, these become less efficient because CSF is more difficult to emerge. Strong evidence has shown that this intervention fails to decrease numbers of shunt operations, disabled infants, and deaths. A trend towards increased CSF infection has been noticed in addition to the discomfort of the procedure. Routine use cannot be recommended but in selected cases could be useful in temporizing progressive hydrocephalus [128].
-
Ventricular taps offer direct access to the ventricles and are easily performed free handed or with ultrasound guidance. Serial taps are avoided in most centers because of the repeated brain trauma and the risk of porencephaly [44]. In addition, overall impact on the prevention of shunt operations and favorable outcome is not significant [125]. Their use is limited as a short-term option in infants with rapid ventriculomegaly and contraindicated LP.
-
External ventricular drainage (EVD) is an accepted option to rapidly relieve the raised intraventricular pressure and eliminate blood-filled CSF. It resembles the constant physiological drainage of CSF unlike the intermittent drainage offered by the serial taps. In most centers, placement of EVD requires transfer to the operating theaters and that is not always feasible. Shunt dependency remains high, up to 65% [17], and serious disadvantages are catheter dislodgment and high infection rates, which can reach 50% at 3 weeks of continuous usage. Such infections additionally increase shunt dependency and progression to multiloculated hydrocephalus [34].
-
Subcutaneous reservoirs (or ventricular access devices—VAD) connected to a ventricular catheter is a reliable option. New, low-profile devices allow placement even in VLBW infants and early commencement of CSF removal. It is preferable to drain smaller quantities of CSF (e.g., 10 ml/kg) daily rather than drain larger quantities less often. The frequency can be guided by clinical data (tension of anterior fontanelle, head circumference) and ultrasound measurements (VI). Generous drainage of CSF can lead to electrolyte abnormalities [69]. Repeated penetration of brain parenchyma or lumbar dura is avoided, compared to ventricular taps and LP. Infusion of intraventricular medication can be provided if needed. On the other hand, transfer to the operating room, skin erosion over the device (Fig. 2), and intermittent control of ICP are disadvantages. Infection rates are 8–12% and remain an issue [20, 34, 45, 81].
-
Ventricular–subgaleal shunt is an alternative option to VAD and centers with experience in this specific treatment option report minimal complications but temporary benefit of about a month. At the time of surgery, a generous subgaleal pocket is created to allow free drainage of CSF. Large fluid collection can lead to scalp expansion which later resolves. Infection rate may be smaller than VAD (6%) [49, 112].
-
Endoscopic third ventriculostomy (ETV) is thought to be ineffective in the treatment of neonatal PHH due to immaturity or blockage of CSF reabsorption sites by blood breakdown products [13, 22, 83].
-
Endoscopic coagulation of the choroid plexus has been tried in infants with slowly progressing, communicating hydrocephalus [94]. Two thirds of infants have been reported to have stabilized. In a recent small cohort, a combination of ETV and coagulation of choroid plexus in infants with PHH, previously shunted or not, showed promising evidence but only in cases where the prepontine cistern was unobstructed [123].
Complication of VAD. A subcutaneous reservoir connected to a ventricular catheter was surgically placed in the right frontal region of a premature neonate with PHH who required repeat tapping to control ventricular enlargement. The skin overlying the reservoir suffered pressure necrosis which necessitated the removal of the reservoir. Eventually, the child required permanent shunting
A different group of interventions combines medical and surgical treatment options and is based on the theory that lowering the intraventricular CSF accumulation is not the definite management to prevent PHH. Blood and blood degradation products have to be removed by means of fibrinolysis and draining. Animal experimental studies have shown that infusion of urokinase or tissue plasminogen activator (t-PA) in blood-containing ventricles prevented hydrocephalus [10, 87]. Clinical studies in adults have shown also effectiveness and safety in IVH caused by basal ganglia hypertensive extension. In premature infants, the purpose is to remove early and effectively the blood clot and decrease the rate of PHH, shunt requirement, and potential risk of venous infarction [23]. Several studies in infants have shown different results [35, 38, 46]. Upon randomization, two studies using streptokinase found similar results in both groups regarding shunt requirement and significant risk of infection. The treatment in these studies initiated after hydrocephalus was evident, several days after the GMH. In addition, t-PA administration has been shown to increase TGF-β1 concentration in CSF, an important factor in PHH pathogenesis [136]. On the basis of these results, intraventricular fibrinolytic therapy with streptokinase in preterm neonates, when PHH is established, cannot be recommended and this practice has been abandoned [131, 134].
An interesting intervention, which has been developed in Bristol, aims at removing intraventricular blood, removing TGF-β1, lowering intraventricular pressure, and removing nonprotein-bound iron and inflammatory cytokines [130, 135]. Drainage, irrigation, and fibrinolytic treatment (DRIFT) uses standard inclusion criteria (GM-IVH plus VI 4 mm above the 97th centile and age <28 days) and is performed using two ventricular catheters: one right frontal for the infusion of t-PA, artificial CSF, and antimicrobial agents and one left occipital for draining at a rate that keeps ICP below 7 mmHg. The procedure lasts usually 3 days, until clear CSF is drained. Short-term evidence, at hospital discharge and at 6 months, have shown equal results in the DRIFT group and control group regarding shunt requirement, but a significant reduction in severe cognitive disability was noticed in the DRIFT group at 2 years follow up [132]. Doubts about overall safety of the intervention and small numbers of patients restrict this treatment option into specialized centers only and has not found widespread use yet.
Pharmaceutical agents used in the past in the management of PHH are diuretics, mostly acetazolamide and furosemide and less often isosorbide and glycerol. Acetazolamide and furosemide manage to reduce CSF production by almost a half [77] in some cases, but side effects (electrolyte disorders) and long-term administration (up to 6 months) make their use problematic. Further investigation by large trials has shown that the combination of the two agents significantly increased the risk of impairment or disability at 1 year with no decrease in death or ventriculoperitoneal (VP) shunt requirement [47, 132]. They cannot be recommended as an option for the management of PHH.
The first-tier interventions aim to stabilize the progressive ventricular dilatation and, by that, the ongoing brain damage. They offer also time for the infant to gain weight and the clinician to deal with concomitant health problems and help the immature immune system to gain strength. If the mentioned interventions fail to control the progressive ventricular dilatation, then the next and well-established treatment option is VP shunting [43]. During the last decades, different VP systems have been developed in an attempt to simulate as much as possible the normal CSF absorption rate. Although considerable progress has been achieved, placing a VP shunt in an infant is a form of conviction in long-term medical follow up and possible surgical operations. Shunt dependency, infection (localized, meningitis, ventriculitis), skin erosion, shunt malfunction (migration, disconnection, obstruction), ventricular isolation, overdrainage syndromes, and intra-abdominal complications are important issues that can affect shunted patients [23]. In this group of premature infants with PHH, the complication rate is even higher and shunt revisions are increased (20–50%) [34]. Furthermore, the infection rate is higher as the age decreases (younger than 35 days) [110] and complications relevant to poor wound healing, CSF leakage, and necrotizing enterocolitis are more frequent as the infant’s weight at the time of operation is lower [34, 52, 113]. Most authors provide as minimum acceptable weight for VP shunt insertion >2,000 g. Other criteria for VP placement are CSF protein levels ideally <100 mg/dl and no evidence of infection and abdominal impairment [12]. Regarding the VP shunt type and specific valve mechanism that is more suitable to infants with PHH, no convincing evidence exists [19, 97]. As a general rule, a low profile valve with an antisiphoning mechanism, low pressure setting, and minimal interconnections (two-piece shunt system) is sufficient.
In our institution, a typical approach for a preterm infant suffering from GM-IVH (e.g., grade III) begins with serial LP performed by the neonatologists. Usually, they are performed two to three times weekly and the CSF amount removed is about 10 ml/kg. These are effective for a week or two and are accompanied by close clinical and ultrasound investigations (US every 2 or 3 days). The next step is insertion of a low-profile subcutaneous reservoir. Reservoir tapping is performed by a neurosurgeon with the aseptic technique. We prefer more frequent taps (once a day or day after day) and draining of smaller amounts of CSF (maximum of 10 ml/kg). Again, clinical (fontanelle tension, head circumference) and ultrasound monitoring (usually twice a week) are paramount in order to decide if ventriculomegaly persists and further intervention is necessary. This period can last up to 3 to 4 weeks, until the infant reaches term. At this point, an MRI scan is performed so that a more detailed imaging of the parenchyma, white matter, and posterior fossa can be assessed. Persistent or progressive hydrocephalus after this period is treated with VP shunting. Commonly, VP shunt insertion is performed after the infant has grown to term, the infant’s weight is over 2,000 g, there is no other concurrent morbidity, and CSF cell count is normal. CSF protein levels were previously regarded as essential to be normal to avoid shunt obstruction, but this view has not been substantiated.
Outcome
Long-term neurodevelopmental outcome of infants suffering from GM-IVH has been and still is an important topic of research in pediatric literature. Major issues under investigation are the extent of hemorrhage (grade), coexistent parenchymal injury (Fig. 3), and progression of compensated ventriculomegaly. The severity of GM-IVH, as determined by its grade, is an important factor for the mortality rate. Neurological disabilities are mainly influenced by the presence and extent of the parenchymal injury [117, 118]. Spasticity and intellectual deficits result from posthemorrhagic infarction, while PVL is mainly responsible for spastic diplegia, typically more severe in the lower than in the upper limps [68]. Mental retardation, seizures, and cerebral palsy are the most frequent disabilities. The timing of GM-IVH seems to affect neurodevelopmental outcome as infants who develop hemorrhage in <6 h of life have more chances to suffer from cerebral palsy and lower IQ scores [118]. Follow up should extend to childhood or even adolescence in order to unveil minor cognitive and neuropsychological deficits.
Assessment of brain damage following neonatal IVH with MR scan late after the event. a T1w axial MR scan of a 5-year-old boy who suffered grade IV IVH at birth. A large area of porencephaly is seen in the left frontal lobe, as the parenchyma surrounding the left frontal horn was destroyed following the initial hemorrhage, which had as its epicenter probably the head of the caudate nucleus, and the area was filled with ventricular CSF. b T1w axial MR scan of a 2-year-old girl who suffered a grade IV IVH at birth. The area surrounding the left occipital horn has suffered necrosis and cystic change, secondary to the initial insult. The child required shunting
The impact of progressive ventriculomegaly on neurodevelopmental outcome depends on the need for shunt insertion and the possible complications of this treatment option. Infants developing ventriculomegaly without signs of raised ICP have more favorable outcome than those with signs of progressive ventricular dilatation and shunt insertion [25]. A recent study suggests that ELBW children (<1,000 g) with severe IVH that require shunt insertion are at greater risk for adverse neurodevelopmental and growth outcomes compared with children without shunt, with or without severe IVH [1].
Shunts placed in premature infants have higher rates of infection and obstruction. An acceptable infection rate in full-term infants and older children is 5–7%, while in the preterm–low BW infants group, the infection rate is as high as 20–30% [95]. The immaturity of the infant’s immune system is the main reason for this disparity. Shunt infection and ventriculitis have serious impact on the child’s neurodevelopmental outcome and can reduce IQ scores by 10 points [102]. Furthermore, they contribute to subependymal inflammatory gliosis and formation of intraventricular deposits upon which exudates and debris collect. These are the basis for intraventricular septa formation which can obstruct CSF outflow routes and cause multiloculated hydrocephalus [84] (Fig. 4). This entity is very challenging and difficult to treat. Surgical options are cystoperitoneal shunting, stereotactic cyst aspiration, craniotomy for open fenestration of septations, and neuroendoscopic cyst fenestration. The goal is to simplify cystoventricular anatomy, usually by endoscopic cyst fenestration and then shunt a single compartment with one catheter. A specific form of ventricular compartmentalization is the isolated or encysted fourth ventricle. It can be seen in infants after IVH, but ventricular infection significantly increases the rate of this complication. The blockage of the aqueduct and the foramina of Magendie and Luschka by arachnoid scarring, subependymal gliosis, and septal formation in combination with continuous CSF production by the fourth ventricular choroid plexus results in the isolation and expansion of fourth ventricle (Fig. 5). Compression of adjacent brain stem and vermis can be suspected clinically by signs such as ataxia, cranial nerve palsies, hemiplegia, vomiting, and breathing abnormalities. Treatment options are surgical and aim in restoring communication between the fourth and the third ventricles or in permanent relief of the fourth ventricle’s expansion by shunting. The latter can be done by a ventricular catheter inserted with neuronavigation or with an open technique and shunted to the peritoneum. The catheter should float freely into the ventricle but there is a danger of entering the brain stem after the ventricle’s shrinkage. Neuroendoscopic techniques can reopen the occluded aqueduct and restore communication between the third and fourth ventricles. Cerebral aqueductoplasty can be performed by a frontal or a suboccipital approach and placement of a stent into the aqueduct has been found to be more effective in preventing repeated occlusion, compared to aqueductoplasty alone [16].
Multiloculated hydrocephalus secondary to protracted EVD drainage of PHH, with several episodes of infection of the drain. CT scan of a 4-year-old girl who at birth developed grade IV IVH. She was treated with placement of EVD. Eventually, the drain became infected and required several replacements and antibiotic treatment. The child gradually developed multiloculated hydrocephalus and required endoscopic fenestration of the cystic areas in order to manage the hydrocephalus with one shunt only
Encysted fourth ventricle. Sagittal T1w MR scan of the child of Fig. 1. The fourth ventricle is excessively dilated, as its inlet and outlets have been occluded by arachnoid scarring and free flow of CSF is inhibited
Overall, with modern neonatal treatment and proactive management of active hydrocephalus, the outcome of premature infants with PHH has improved in the last two decades. Nevertheless, prematurity is associated also with other health problems, including cardiac, respiratory, and neurodevelopmental defects, which can themselves affect the long-term development of the child.
References
Adams-Chapman I, Hansen NI, Stoll BJ, Higgins R (2008) Neurodevelopmental outcome of extremely low birth weight infants with posthemorrhagic hydrocephalus requiring shunt insertion. Pediatrics 121(5):e1167–e1177
Ahmann PA, Lazzara A, Dykes FD, Brann AW Jr, Schwartz JF (1980) Intraventricular hemorrhage in the high-risk preterm infant: incidence and outcome. Ann Neurol 7(2):118–124
Akundi RS, Candelario-Jalil E, Hess S, Hull M, Lieb K, Gebicke-Haerter PJ, Fiebich BL (2005) Signal transduction pathways regulating cyclooxygenase-2 in lipopolysaccharide-activated primary rat microglia. Glia 51(3):199–208
Ancel PY, Marret S, Larroque B, Arnaud C, Zupan-Simunek V, Voyer M, Roze JC, Matis J, Burguet A, Ledesert B, Andre M, Pierrat V, Kaminski M (2005) Are maternal hypertension and small-for-gestational age risk factors for severe intraventricular hemorrhage and cystic periventricular leukomalacia? Results of the EPIPAGE cohort study. Am J Obstet Gynecol 193(1):178–184
Babcock DS, Han BK (1981) The accuracy of high resolution, real-time ultrasonography of the head in infancy. Radiology 139(3):665–676
Bax M, Tydeman C, Flodmark O (2006) Clinical and MRI correlates of cerebral palsy: the European Cerebral Palsy Study. JAMA 296(13):1602–1608
Bejar R, Saugstad OD, James H, Gluck L (1983) Increased hypoxanthine concentrations in cerebrospinal fluid of infants with hydrocephalus. J Pediatr 103(1):44–48
Beverley DW, Chance GW, Coates CF (1984) Intraventricular haemorrhage—timing of occurrence and relationship to perinatal events. Br J Obstet Gynaecol 91(10):1007–1013
Brady KM, Easley RB, Tobias JD (2006) Recombinant activated factor VII (rFVIIa) treatment in infants with hemorrhage. Paediatr Anaesth 16(10):1042–1046
Brinker T, Seifert V, Dietz H (1992) Subacute hydrocephalus after experimental subarachnoid hemorrhage: its prevention by intrathecal fibrinolysis with recombinant tissue plasminogen activator. Neurosurgery 31(2):306–311, discussion 311–302
Brouwer MJ, de Vries LS, Pistorius L, Rademaker KJ, Groenendaal F, Benders MJ (2010) Ultrasound measurements of the lateral ventricles in neonates: why, how and when? A systematic review. Acta Paediatr 99(9):1298–1306
Brydon HL, Bayston R, Hayward R, Harkness W (1996) The effect of protein and blood cells on the flow-pressure characteristics of shunts. Neurosurgery 38(3):498–504, discussion 505
Buxton N, Macarthur D, Mallucci C, Punt J, Vloeberghs M (1998) Neuroendoscopy in the premature population. Childs Nerv Syst 14(11):649–652
Chao CC, Hu S, Molitor TW, Shaskan EG, Peterson PK (1992) Activated microglia mediate neuronal cell injury via a nitric oxide mechanism. J Immunol 149(8):2736–2741
Cherian S, Whitelaw A, Thoresen M, Love S (2004) The pathogenesis of neonatal post-hemorrhagic hydrocephalus. Brain Pathol 14(3):305–311
Cinalli G, Spennato P, Savarese L, Ruggiero C, Aliberti F, Cuomo L, Cianciulli E, Maggi G (2006) Endoscopic aqueductoplasty and placement of a stent in the cerebral aqueduct in the management of isolated fourth ventricle in children. J Neurosurg 104(1 Suppl):21–27
Cornips E, Van Calenbergh F, Plets C, Devlieger H, Casaer P (1997) Use of external drainage for posthemorrhagic hydrocephalus in very low birth weight premature infants. Childs Nerv Syst 13(7):369–374
Davies MW, Swaminathan M, Chuang SL, Betheras FR (2000) Reference ranges for the linear dimensions of the intracranial ventricles in preterm neonates. Arch Dis Child Fetal Neonatal Ed 82(3):F218–F223
Davis SE, Levy ML, McComb JG, Sposto R (2000) The delta valve: how does its clinical performance compare with two other pressure differential valves without antisiphon control? Pediatr Neurosurg 33(2):58–63
de Vries LS, Liem KD, van Dijk K, Smit BJ, Sie L, Rademaker KJ, Gavilanes AW (2002) Early versus late treatment of posthaemorrhagic ventricular dilatation: results of a retrospective study from five neonatal intensive care units in The Netherlands. Acta Paediatr 91(2):212–217
Del Bigio MR (1993) Neuropathological changes caused by hydrocephalus. Acta Neuropathol 85(6):573–585
Drake JM (1993) Ventriculostomy for treatment of hydrocephalus. Neurosurg Clin N Am 4(4):657–666
Duncan C, Chiang V (2008) Intraventricular hemorrhage and posthemorrhagic hydrocephalus. In: Albright A, Pollack I, Adelson P (eds) Principles and practice of pediatric neurosurgery, vol 2, 2. Thieme Medical Publishers, Inc., New York, pp 145–161
Fernell E, Hagberg G, Hagberg B (1994) Infantile hydrocephalus epidemiology: an indicator of enhanced survival. Arch Dis Child Fetal Neonatal Ed 70(2):F123–F128
Fletcher JM, Landry SH, Bohan TP, Davidson KC, Brookshire BL, Lachar D, Kramer LA, Francis DJ (1997) Effects of intraventricular hemorrhage and hydrocephalus on the long-term neurobehavioral development of preterm very-low-birthweight infants. Dev Med Child Neurol 39(9):596–606
Fok TF, Davies DP, Ng HK (1990) A study of periventricular haemorrhage, post-haemorrhagic ventricular dilatation and periventricular leucomalacia in Chinese preterm infants. J Paediatr Child Health 26(5):271–275
Garland JS, Buck R, Leviton A (1995) Effect of maternal glucocorticoid exposure on risk of severe intraventricular hemorrhage in surfactant-treated preterm infants. J Pediatr 126(2):272–279
Gopel W, Gortner L, Kohlmann T, Schultz C, Moller J (2001) Low prevalence of large intraventricular haemorrhage in very low birthweight infants carrying the factor V Leiden or prothrombin G20210A mutation. Acta Paediatr 90(9):1021–1024
Gopel W, Hartel C, Ahrens P, Konig I, Kattner E, Kuhls E, Kuster H, Moller J, Muller D, Roth B, Segerer H, Wieg C, Herting E (2006) Interleukin-6-174-genotype, sepsis and cerebral injury in very low birth weight infants. Genes Immun 7(1):65–68
Gould SJ, Howard S, Hope PL, Reynolds EO (1987) Periventricular intraparenchymal cerebral haemorrhage in preterm infants: the role of venous infarction. J Pathol 151(3):197–202
Gould DB, Phalan FC, Breedveld GJ, van Mil SE, Smith RS, Schimenti JC, Aguglia U, van der Knaap MS, Heutink P, John SW (2005) Mutations in Col4a1 cause perinatal cerebral hemorrhage and porencephaly. Science 308(5725):1167–1171
Greisen G, Andreasen RB (2003) Recombinant factor VIIa in preterm neonates with prolonged prothrombin time. Blood Coagul Fibrinolysis 14(1):117–120
Grogaard JB, Lindstrom DP, Parker RA, Culley B, Stahlman MT (1990) Increased survival rate in very low birth weight infants (1500 grams or less): no association with increased incidence of handicaps. J Pediatr 117(1 Pt 1):139–146
Gurtner P, Bass T, Gudeman SK, Penix JO, Philput CB, Schinco FP (1992) Surgical management of posthemorrhagic hydrocephalus in 22 low-birth-weight infants. Childs Nerv Syst 8(4):198–202
Haines SJ, Lapointe M (1999) Fibrinolytic agents in the management of posthemorrhagic hydrocephalus in preterm infants: the evidence. Childs Nerv Syst 15(5):226–234
Hall RW, Kronsberg SS, Barton BA, Kaiser JR, Anand KJ (2005) Morphine, hypotension, and adverse outcomes among preterm neonates: who's to blame? Secondary results from the NEOPAIN trial. Pediatrics 115(5):1351–1359
Hambleton G, Wigglesworth JS (1976) Origin of intraventricular haemorrhage in the preterm infant. Arch Dis Child 51(9):651–659
Hansen AR, Volpe JJ, Goumnerova LC, Madsen JR (1997) Intraventricular urokinase for the treatment of posthemorrhagic hydrocephalus. Pediatr Neurol 17(3):213–217
Harding D, Brull D, Humphries SE, Whitelaw A, Montgomery H, Marlow N (2005) Variation in the interleukin-6 gene is associated with impaired cognitive development in children born prematurely: a preliminary study. Pediatr Res 58(1):117–120
Harding DR, Humphries SE, Whitelaw A, Marlow N, Montgomery HE (2007) Cognitive outcome and cyclo-oxygenase-2 gene (−765 G/C) variation in the preterm infant. Arch Dis Child Fetal Neonatal Ed 92(2):F108–F112
Heep A, Stoffel-Wagner B, Soditt V, Aring C, Groneck P, Bartmann P (2002) Procollagen I C-propeptide in the cerebrospinal fluid of neonates with posthaemorrhagic hydrocephalus. Arch Dis Child Fetal Neonatal Ed 87(1):F34–F36
Holt PJ (1989) Posthemorrhagic hydrocephalus. J Child Neurol 4:S23–S31
Horinek D, Cihar M, Tichy M (2003) Current methods in the treatment of posthemorrhagic hydrocephalus in infants. Bratisl Lek Listy 104(11):347–351
Hudgins RJ (2001) Posthemorrhagic hydrocephalus of infancy. Neurosurg Clin N Am 12(4):743–751, ix
Hudgins RJ, Boydston WR, Gilreath CL (1998) Treatment of posthemorrhagic hydrocephalus in the preterm infant with a ventricular access device. Pediatr Neurosurg 29(6):309–313
Hudgins RJ, Boydston WR, Hudgins PA, Morris R, Adler SM, Gilreath CL (1997) Intrathecal urokinase as a treatment for intraventricular hemorrhage in the preterm infant. Pediatr Neurosurg 26(6):281–287
International PHVD Drug Trial Group (1998) International randomised controlled trial of acetazolamide and furosemide in posthaemorrhagic ventricular dilatation in infancy. Lancet 352(9126):433–440
Kadri H, Mawla AA, Kazah J (2006) The incidence, timing, and predisposing factors of germinal matrix and intraventricular hemorrhage (GMH/IVH) in preterm neonates. Childs Nerv Syst 22(9):1086–1090
Karas CS, Baig MN, Elton SW (2007) Ventriculosubgaleal shunts at Columbus Children's Hospital: neurosurgical implant placement in the neonatal intensive care unit. J Neurosurg 107(3 Suppl):220–223
Kempley ST, Gamsu HR (1993) Changes in cerebral artery blood flow velocity after intermittent cerebrospinal fluid drainage. Arch Dis Child 69(1 Spec No):74–76
Kenet G, Kuperman AA, Strauss T, Brenner B (2011) Neonatal IVH—mechanisms and management. Thromb Res 127(Suppl 3):S120–S122
Kestle J, Drake J, Milner R, Sainte-Rose C, Cinalli G, Boop F, Piatt J, Haines S, Schiff S, Cochrane D, Steinbok P, MacNeil N (2000) Long-term follow-up data from the Shunt Design Trial. Pediatr Neurosurg 33(5):230–236
Kirkpatrick M, Engleman H, Minns RA (1989) Symptoms and signs of progressive hydrocephalus. Arch Dis Child 64(1):124–128
Kluckow M (2005) Low systemic blood flow and pathophysiology of the preterm transitional circulation. Early Hum Dev 81(5):429–437
Korobkin R (1975) The relationship between head circumference and the development of communicating hydrocephalus in infants following intraventricular hemorrhage. Pediatrics 56(1):74–77
Kuban KC, Leviton A, Pagano M, Fenton T, Strassfeld R, Wolff M (1992) Maternal toxemia is associated with reduced incidence of germinal matrix hemorrhage in premature babies. J Child Neurol 7(1):70–76
Leliefeld PH, Gooskens RH, Tulleken CA, Regli L, Uiterwaal CS, Han KS, Kappelle LJ (2010) Noninvasive detection of the distinction between progressive and compensated hydrocephalus in infants: is it possible? J Neurosurg Pediatr 5(6):562–568
Lemons JA, Bauer CR, Oh W, Korones SB, Papile LA, Stoll BJ, Verter J, Temprosa M, Wright LL, Ehrenkranz RA, Fanaroff AA, Stark A, Carlo W, Tyson JE, Donovan EF, Shankaran S, Stevenson DK (2001) Very low birth weight outcomes of the National Institute of Child Health and Human Development Neonatal Research Network, January 1995 through December 1996. NICHD Neonatal Research Network. Pediatrics 107(1):E1
Levene MI (1981) Measurement of the growth of the lateral ventricles in preterm infants with real-time ultrasound. Arch Dis Child 56(12):900–904
Leviton A, Dammann O (2004) Coagulation, inflammation, and the risk of neonatal white matter damage. Pediatr Res 55(4):541–545
Leviton A, Fenton T, Kuban KC, Pagano M (1991) Labor and delivery characteristics and the risk of germinal matrix hemorrhage in low birth weight infants. J Child Neurol 6(1):35–40
Leviton A, Kuban KC, Pagano M, Allred EN, Van Marter L (1993) Antenatal corticosteroids appear to reduce the risk of postnatal germinal matrix hemorrhage in intubated low birth weight newborns. Pediatrics 91(6):1083–1088
Leviton A, Paneth N, Reuss ML, Susser M, Allred EN, Dammann O, Kuban K, Van Marter LJ, Pagano M, Hegyi T, Hiatt M, Sanocka U, Shahrivar F, Abiri M, Disalvo D, Doubilet P, Kairam R, Kazam E, Kirpekar M, Rosenfeld D, Schonfeld S, Share J, Collins M, Genest D, Shen-Schwarz S et al (1999) Maternal infection, fetal inflammatory response, and brain damage in very low birth weight infants. Developmental Epidemiology Network Investigators. Pediatr Res 46(5):566–575
Liao MF, Chaou WT, Tsao LY, Nishida H, Sakanoue M (1986) Ultrasound measurement of the ventricular size in newborn infants. Brain Dev 8(3):262–268
Lim LW, Volkodav OV (2009) Germinal matrix hemorrhage of prematurity: treatment approaches and outcomes in a single institutional review in the Ukraine. Pediatr Neurosurg 45(2):132–140
Limbrick DD Jr, Mathur A, Johnston JM, Munro R, Sagar J, Inder T, Park TS, Leonard JL, Smyth MD (2010) Neurosurgical treatment of progressive posthemorrhagic ventricular dilation in preterm infants: a 10-year single-institution study. J Neurosurg Pediatr 6(3):224–230
Linder N, Haskin O, Levit O, Klinger G, Prince T, Naor N, Turner P, Karmazyn B, Sirota L (2003) Risk factors for intraventricular hemorrhage in very low birth weight premature infants: a retrospective case-control study. Pediatrics 111(5 Pt 1):e590–e595
Luciano M, Pattisapu JV, Wickremesekera A (2004) Infantile posthemorhhagic hydrocephalus. In: Winn HR (ed) Youmans neurological surgery. Saunders, Philadelphia, pp 3405–3417
MacMahon P, Cooke RW (1983) Hyponatraemia caused by repeated cerebrospinal fluid drainage in post haemorrhagic hydrocephalus. Arch Dis Child 58(5):385–386
McCrea HJ, Ment LR (2008) The diagnosis, management, and postnatal prevention of intraventricular hemorrhage in the preterm neonate. Clin Perinatol 35(4):777–792, vii
McIntire DD, Bloom SL, Casey BM, Leveno KJ (1999) Birth weight in relation to morbidity and mortality among newborn infants. N Engl J Med 340(16):1234–1238
Ment LR, Oh W, Ehrenkranz RA, Philip AG, Duncan CC, Makuch RW (1995) Antenatal steroids, delivery mode, and intraventricular hemorrhage in preterm infants. Am J Obstet Gynecol 172(3):795–800
Ment LR, Oh W, Philip AG, Ehrenkranz RA, Duncan CC, Allan W, Taylor KJ, Schneider K, Katz KH, Makuch RW (1992) Risk factors for early intraventricular hemorrhage in low birth weight infants. J Pediatr 121(5 Pt 1):776–783
Ment LR, Stewart WB, Ardito TA, Huang E, Madri JA (1992) Indomethacin promotes germinal matrix microvessel maturation in the newborn beagle pup. Stroke 23(8):1132–1137
Ment LR, Stewart WB, Scott DT, Duncan CC (1983) Beagle puppy model of intraventricular hemorrhage: randomized indomethacin prevention trial. Neurology 33(2):179–184
Ment LR, Vohr BR, Makuch RW, Westerveld M, Katz KH, Schneider KC, Duncan CC, Ehrenkranz R, Oh W, Philip AG, Scott DT, Allan WC (2004) Prevention of intraventricular hemorrhage by indomethacin in male preterm infants. J Pediatr 145(6):832–834
Miner ME (1986) Acetazolamide treatment of progressive hydrocephalus secondary to intraventricular hemorrhage in a preterm infant. Childs Nerv Syst 2(2):105–106
Mitsiakos G, Papaioannou G, Giougi E, Karagianni P, Garipidou V, Nikolaidis N (2007) Is the use of rFVIIa safe and effective in bleeding neonates? A retrospective series of 8 cases. J Pediatr Hematol Oncol 29(3):145–150
Moody DM, Brown WR, Challa VR, Block SM (1994) Alkaline phosphatase histochemical staining in the study of germinal matrix hemorrhage and brain vascular morphology in a very-low-birth-weight neonate. Pediatr Res 35(4 Pt 1):424–430
Murphy BP, Inder TE, Rooks V, Taylor GA, Anderson NJ, Mogridge N, Horwood LJ, Volpe JJ (2002) Posthaemorrhagic ventricular dilatation in the premature infant: natural history and predictors of outcome. Arch Dis Child Fetal Neonatal Ed 87(1):F37–F41
Muszinski C (2010) Posthemorrhagic hydrocephalus. In: Malluci C, Sgouros S (eds) Cerebrospinal fluid disorders. Informa Healthcare USA, Inc., New York, pp 141–153
Nakamura Y, Okudera T, Fukuda S, Hashimoto T (1990) Germinal matrix hemorrhage of venous origin in preterm neonates. Hum Pathol 21(10):1059–1062
O'Brien DF, Seghedoni A, Collins DR, Hayhurst C, Mallucci CL (2006) Is there an indication for ETV in young infants in aetiologies other than isolated aqueduct stenosis? Childs Nerv Syst 22(12):1565–1572
Oi S, Abbott R (2004) Loculated ventricles and isolated compartments in hydrocephalus: their pathophysiology and the efficacy of neuroendoscopic surgery. Neurosurg Clin N Am 15(1):77–87
Osborn AG (ed) (2005) Brain. Diagnostic imaging, 3rd edn. Amirsys, Salt Lake City
Pagano M, Leviton A, Kuban K (1990) Early and late germinal matrix hemorrhage may have different antecedents. Eur J Obstet Gynecol Reprod Biol 37(1):47–54
Pang D, Sclabassi RJ, Horton JA (1986) Lysis of intraventricular blood clot with urokinase in a canine model: part 3. Effects of intraventricular urokinase on clot lysis and posthemorrhagic hydrocephalus. Neurosurgery 19(4):553–572
Papile LA, Burstein J, Burstein R, Koffler H (1978) Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr 92(4):529–534
Papile LA, Burstein J, Burstein R, Koffler H, Koops BL, Johnson JD (1980) Posthemorrhagic hydrocephalus in low-birth-weight infants: treatment by serial lumbar punctures. J Pediatr 97(2):273–277
Parfenova H, Levine V, Gunther WM, Pourcyrous M, Leffler CW (2002) COX-1 and COX-2 contributions to basal and IL-1 beta-stimulated prostanoid synthesis in human neonatal cerebral microvascular endothelial cells. Pediatr Res 52(3):342–348
Perlman JM, Rollins N (2000) Surveillance protocol for the detection of intracranial abnormalities in premature neonates. Arch Pediatr Adolesc Med 154(8):822–826
Perry RN, Bowman ED, Murton LJ, Roy RN, de Crespigny LC (1985) Ventricular size in newborn infants. J Ultrasound Med 4(9):475–477
Philip AG, Allan WC, Tito AM, Wheeler LR (1989) Intraventricular hemorrhage in preterm infants: declining incidence in the 1980s. Pediatrics 84(5):797–801
Pople IK, Ettles D (1995) The role of endoscopic choroid plexus coagulation in the management of hydrocephalus. Neurosurgery 36(4):698–701, discussion 701–692
Reinprecht A, Dietrich W, Berger A, Bavinzski G, Weninger M, Czech T (2001) Posthemorrhagic hydrocephalus in preterm infants: long-term follow-up and shunt-related complications. Childs Nerv Syst 17(11):663–669
Rezaie P, Dean A, Male D, Ul N (2005) Microglia in the cerebral wall of the human telencephalon at second trimester. Cereb Cortex 15(7):938–949
Robinson S, Kaufman BA, Park TS (2002) Outcome analysis of initial neonatal shunts: does the valve make a difference? Pediatr Neurosurg 37(6):287–294
Roland EH, Hill A (1997) Intraventricular hemorrhage and posthemorrhagic hydrocephalus. Current and potential future interventions. Clin Perinatol 24(3):589–605
Sajjadian N, Fakhrai H, Jahadi R (2010) Incidence of intraventricular hemorrhage and post hemorrhagic hydrocephalus in preterm infants. Acta Med Iran 48(4):260–262
Sauerbrei EE, Digney M, Harrison PB, Cooperberg PL (1981) Ultrasonic evaluation of neonatal intracranial hemorrhage and its complications. Radiology 139(3):677–685
Savman K, Blennow M, Hagberg H, Tarkowski E, Thoresen M, Whitelaw A (2002) Cytokine response in cerebrospinal fluid from preterm infants with posthaemorrhagic ventricular dilatation. Acta Paediatr 91(12):1357–1363
Sgouros S, Malluci C, Walsh AR, Hockley AD (1995) Long-term complications of hydrocephalus. Pediatr Neurosurg 23(3):127–132
Shankaran S, Papile LA, Wright LL, Ehrenkranz RA, Mele L, Lemons JA, Korones SB, Stevenson DK, Donovan EF, Stoll BJ, Fanaroff AA, Oh W (1997) The effect of antenatal phenobarbital therapy on neonatal intracranial hemorrhage in preterm infants. N Engl J Med 337(7):466–471
Sheth RD (1998) Trends in incidence and severity of intraventricular hemorrhage. J Child Neurol 13(6):261–264
Sondhi V, Gupta G, Gupta PK, Patnaik SK, Tshering K (2008) Establishment of nomograms and reference ranges for intra-cranial ventricular dimensions and ventriculo-hemispheric ratio in newborns by ultrasonography. Acta Paediatr 97(6):738–744
Stanimirovic D, Satoh K (2000) Inflammatory mediators of cerebral endothelium: a role in ischemic brain inflammation. Brain Pathol 10(1):113–126
Synnes AR, Chien LY, Peliowski A, Baboolal R, Lee SK (2001) Variations in intraventricular hemorrhage incidence rates among Canadian neonatal intensive care units. J Pediatr 138(4):525–531
Takashima S, Mito T, Ando Y (1986) Pathogenesis of periventricular white matter hemorrhages in preterm infants. Brain Dev 8(1):25–30
Taylor GA (2001) Sonographic assessment of posthemorrhagic ventricular dilatation. Radiol Clin North Am 39(3):541–551
Taylor AG, Peter JC (2001) Advantages of delayed VP shunting in post-haemorrhagic hydrocephalus seen in low-birth-weight infants. Childs Nerv Syst 17(6):328–333
The Victorian Infant Collaborative Study Group (1997) Improved outcome into the 1990s for infants weighing 500–999 g at birth. Arch Dis Child Fetal Neonatal Ed 77(2):F91–F94
Tubbs RS, Smyth MD, Wellons JC 3rd, Blount JP, Grabb PA, Oakes WJ (2003) Alternative uses for the subgaleal shunt in pediatric neurosurgery. Pediatr Neurosurg 39(1):22–24
Tuli S, Drake J, Lawless J, Wigg M, Lamberti-Pasculli M (2000) Risk factors for repeated cerebrospinal shunt failures in pediatric patients with hydrocephalus. J Neurosurg 92(1):31–38
van Bel F, Klautz RJ, Steendijk P, Schipper IB, Teitel DF, Baan J (1993) The influence of indomethacin on the autoregulatory ability of the cerebral vascular bed in the newborn lamb. Pediatr Res 34(2):178–181
Ventriculomegaly Trial Group (1990) Randomised trial of early tapping in neonatal posthaemorrhagic ventricular dilatation. Arch Dis Child 65(1 Spec No):3–10
Ventriculomegaly Trial Group (1994) Randomised trial of early tapping in neonatal posthaemorrhagic ventricular dilatation: results at 30 months. Arch Dis Child Fetal Neonatal Ed 70(2):F129–F136
Vohr B, Allan WC, Scott DT, Katz KH, Schneider KC, Makuch RW, Ment LR (1999) Early-onset intraventricular hemorrhage in preterm neonates: incidence of neurodevelopmental handicap. Semin Perinatol 23(3):212–217
Vohr BR, Allan WC, Westerveld M, Schneider KC, Katz KH, Makuch RW, Ment LR (2003) School-age outcomes of very low birth weight infants in the indomethacin intraventricular hemorrhage prevention trial. Pediatrics 111(4 Pt 1):e340–e346
Vohr BR, Wright LL, Dusick AM, Mele L, Verter J, Steichen JJ, Simon NP, Wilson DC, Broyles S, Bauer CR, Delaney-Black V, Yolton KA, Fleisher BE, Papile LA, Kaplan MD (2000) Neurodevelopmental and functional outcomes of extremely low birth weight infants in the National Institute of Child Health and Human Development Neonatal Research Network, 1993–1994. Pediatrics 105(6):1216–1226
Volpe JJ (1981) Neonatal intraventricular hemorrhage. N Engl J Med 304(15):886–891
Volpe JJ (1989) Intraventricular hemorrhage in the premature infant—current concepts. Part I. Ann Neurol 25(1):3–11
Volpe JJ (2008) Neurology of the newborn. W.B. Saunders, Philadelphia
Warf BC, Campbell JW, Riddle E (2011) Initial experience with combined endoscopic third ventriculostomy and choroid plexus cauterization for post-hemorrhagic hydrocephalus of prematurity: the importance of prepontine cistern status and the predictive value of FIESTA MRI imaging. Childs Nerv Syst 27:1063–1071
Whitelaw A (1997) We need a new understanding of the reabsorption of cerebrospinal fluid. Acta Paediatr 86(2):133–134
Whitelaw A (2000) Repeated lumbar or ventricular punctures for preventing disability or shunt dependence in newborn infants with intraventricular hemorrhage. Cochrane Database Syst Rev (2):CD000216
Whitelaw A (2001) Intraventricular haemorrhage and posthaemorrhagic hydrocephalus: pathogenesis, prevention and future interventions. Semin Neonatol 6(2):135–146
Whitelaw A (2001) Postnatal phenobarbitone for the prevention of intraventricular hemorrhage in preterm infants. Cochrane Database Syst Rev (1):CD001691
Whitelaw A (2001) Repeated lumbar or ventricular punctures in newborns with intraventricular hemorrhage. Cochrane Database Syst Rev (1):CD000216
Whitelaw A, Christie S, Pople I (1999) Transforming growth factor-beta1: a possible signal molecule for posthemorrhagic hydrocephalus? Pediatr Res 46(5):576–580
Whitelaw A, Evans D, Carter M, Thoresen M, Wroblewska J, Mandera M, Swietlinski J, Simpson J, Hajivassiliou C, Hunt LP, Pople I (2007) Randomized clinical trial of prevention of hydrocephalus after intraventricular hemorrhage in preterm infants: brain-washing versus tapping fluid. Pediatrics 119(5):e1071–e1078
Whitelaw A, Jary S, Kmita G, Wroblewska J, Musialik-Swietlinska E, Mandera M, Hunt L, Carter M, Pople I (2010) Randomized trial of drainage, irrigation and fibrinolytic therapy for premature infants with posthemorrhagic ventricular dilatation: developmental outcome at 2 years. Pediatrics 125(4):e852–e858
Whitelaw A, Kennedy CR, Brion LP (2001) Diuretic therapy for newborn infants with posthemorrhagic ventricular dilatation. Cochrane Database Syst Rev (2):CD002270
Whitelaw A, Odd D (2007) Postnatal phenobarbital for the prevention of intraventricular hemorrhage in preterm infants. Cochrane Database Syst Rev (4):CD001691
Whitelaw A, Odd DE (2007) Intraventricular streptokinase after intraventricular hemorrhage in newborn infants. Cochrane Database Syst Rev (4):CD000498
Whitelaw A, Pople I, Cherian S, Evans D, Thoresen M (2003) Phase 1 trial of prevention of hydrocephalus after intraventricular hemorrhage in newborn infants by drainage, irrigation, and fibrinolytic therapy. Pediatrics 111(4 Pt 1):759–765
Whitelaw A, Thoresen M, Pople I (2002) Posthaemorrhagic ventricular dilatation. Arch Dis Child Fetal Neonatal Ed 86(2):F72–F74
Wigglesworth JS, Pape KE (1980) Pathophysiology of intracranial haemorrhage in the newborn. J Perinat Med 8(3):119–133
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tsitouras, V., Sgouros, S. Infantile posthemorrhagic hydrocephalus. Childs Nerv Syst 27, 1595–1608 (2011). https://doi.org/10.1007/s00381-011-1521-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-011-1521-y