Abstract
Background
Ventriculoperitoneal shunt infection remains a significant problem. The introduction of antibiotic-impregnated shunt (AIS) systems in the prevention of shunt infection may represent a potential advance; however, there are no randomized controlled trials to establish a robust evidence-based practice. Previously published single-institution cohort studies have provided varying results on the efficacy of AIS systems in the prevention of shunt infection. In this study, we evaluate combined outcomes from three paediatric neurosurgical units in the use of AIS systems for paediatric patients with hydrocephalus.
Methods
The three units established independent databases with data collected from varying time frames. All procedures, where a complete AIS system or part was implanted into patients from 0–16 years in age, were included. The primary outcome measure was shunt infection rate. Shunt procedures were classified as de novo (DNS) and clean revision (CRS). An infant (<1 year) de novo insertion subgroup was also analyzed. AIS shunts were compared to a historical control of non-AIS shunts and results were analysed by centre using an odds ratio with a 95% confidence interval and combined across centres by meta-analysis.
Results
A total of 581 AIS implantation procedures were performed in all three units. The comparative non-AIS historical cohort comprised of 1,963 procedures. The pooled effect estimate indicated a clinical advantage for AIS shunts compared to non-AIS shunts, odds ratio (OR), 0.60 (95% CI 0.38, 0.93). The de novo infant group comprised 153 AIS systems, and 465 de novo shunts in the historical non-AIS cohort. Again the pooled effect estimate indicated a clinical advantage for AIS shunts compared to non-AIS shunts, OR 0.38 (95% CI, 0.17; 0.85); however, there was a large overlap of confidence intervals in the results from the different sites indicating the uncertainty in the treatment effect estimates. Over 80% of organisms were gram positive in the infected AIS cohort with a median time to infection of 19 days. Two rifampicin-resistant organisms and three MRSA organisms were detected.
Conclusion
Data from this exclusively paediatric multi-centre historical control study suggest that AIS may significantly reduce infection rates in de novo and clean revision shunt implants. Although the possibility of bias cannot be excluded due to study design, this is the largest study on an exclusively paediatric cohort comparing standard shunts to AIS implants. Future double-blinded RCTs are needed to confirm AIS efficacy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction and background
Shunt infection remains a significant problem and there is significant associated morbidity. The reported infection rates range from 3% to 27% [2, 7, 9–11, 20, 24]. Despite extensive research into contributing factors such as surgical technique, length of operation, surgeon grade and no-touch technique, very low infection rates have not been consistently achieved [8–10, 13, 17, 32]. Most shunt infections occur within 2 months of surgery with Gram positive organisms accounting for over 90% of infections [2, 11, 25, 33].
Antibiotic-impregnated shunt (AIS) catheters have been tested extensively in vitro, showing promising sustained antibacterial activity. Codman® Bactiseal™ antibiotic shunt catheters are impregnated with clindamycin and rifampicin and aim to reduce infection rates with Gram positive organisms. Antibiotics have been shown to be effective in vitro up to 56 days [3–5, 18]. Although there have been reports of the impact of these catheters on the infection rate in vivo, the published data is varied in its conclusion and most studies do not report significant reduction in infection rates [14–16, 19, 22, 23, 26].
We report our multi-centre with historical controls, experience of permanent ventriculoperitoneal (VP) shunt catheters impregnated with clindamycin and rifampicin (Codman® Bactiseal®, Johnson & Johnson, Raynham, MA, USA) in a large population of, exclusively, children and neonates involving multiple surgeons of all grades and both elective and emergency procedures across three tertiary paediatric neurosurgery institutions. We have assessed the clinical impact of antibiotic-impregnated catheters on overall infection rates in clinical practice and include a subanalysis of the infant subgroup aged below 1 year.
Materials and methods
The three units established and maintained their own individual databases on patients aged 0–16 years of age undergoing permanent VP shunting for any hydrocephalus aetiology. The study period and historical cohorts are described below. The historical control group periods vary between the centres in accordance with the individual units’ database establishment dates.
Leeds
Established prospective databases for each cohort were maintained. The AIS database cohort period included all consecutive patients undergoing AIS implantation from December 2003 until April 2007. The historical cohort (non-AIS) included all consecutive patients undergoing shunt implantation from October 1997 to December 2003.
Liverpool
Established retrospective databases for each cohort were maintained. The AIS cohort period included all consecutive patients undergoing AIS implantation from October 2003 until December 2006. The historical cohort included consecutive patients undergoing shunt implantation (non-AIS) in January to December 2002.
London GOS
Established prospective databases for each cohort were maintained. The AIS database cohort period included all consecutive patients aged 0–16 years undergoing AIS implantation from June 2005 until June 2006 and 0–16 years from June 2006 until June 2007. The historical cohort (non-AIS) included consecutive patients undergoing shunt implantation (0–16 years) from January 1993–December 2003.
Study shunts, methods and definitions
A database of all shunts implanted, in patients aged 16 and under, subsequent to the introduction of antibiotic-impregnated catheters to our institutions was established. All shunt procedures were undertaken using Bactiseal™ catheters regardless of the aetiology of hydrocephalus. Patients who had implantation of a complete de novo antibiotic-impregnated internalized shunt system or replacement of non-infected non-AIS part to AIS part or following external drainage for non-infectious cause were included. The study groups were divided into those children receiving de novo (DNS) or following clean revision (CRS). A variety of valve types were implanted. All patients received antibiotic prophylaxis at induction of anaesthesia; Leeds and Liverpool used a single dose of cefuroxime, and GOSH used flucloxacillin and amikacin [teicoplanin and amikacin if a known methicillin-resistant Staphylococcus aureus (MRSA) carrier] at induction and for 24 h after. There was a variation between surgeons in all three units with regard to the degree of perioperative hair shaving ranging from no hair shaving to wide margins. Operations in all three units were performed either by neurosurgical consultants or registrars in elective or emergency settings.
Laboratory procedures used for cerebrospinal fluid (CSF) microscopy and cultures were not standardized at each individual unit between control and study periods. Extended primary culture was used during the study period but enrichment CSF cultures were not performed for all CSF samples. Full antibiotic sensitivity data was not available for all organisms.
Primary diagnosis, clinical history, prior CSF diversion surgery and all episodes of shunt malfunctions were recorded. In addition, a consultant microbiologist at each individual unit reviewed all microbiological data.
VP shunt infection was diagnosed if a patient had signs and symptoms of shunt infection with either (1) an organism cultured from CSF or shunt apparatus or (2) raised CSF white cell count and a clinical response to treatment for infection following shunt removal and appropriate antibiotic therapy.
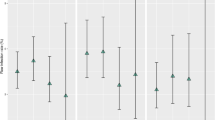
Data are presented in forest plots showing the number of infections and the number of procedures for both the AIS and non-AIS groups for each site. This is considered for de novo and clean revisions combined and subgroup analyses are presented for de novo shunts only, clean revision shunts only and children under 1 year de novo shunts. Odds ratios (OR) and 95% confidence intervals (CI) are presented for each site which are indicated on the plot with the square representing the OR and lines representing the 95% CI. The data are combined using meta-analysis and the pooled treatment effect estimate and confidence interval are shown on the plot as a diamond.
Results
Leeds
This unit performed a total of 200 AIS shunt procedures in 98 children with an age range of 1 day to 16 years between December 2003 and April 2007. There were 12 (6%) infected shunts. The median follow-up was 22 months ranging from 5 to 47 months. There were 55 de novo shunts implanted and, in this group, there were four infections (7.3%). There were 145 clean shunt revisions where partial or entire new AIS systems were implanted with eight infected cases (5.5%). In the Historical cohort, there were 109 de novo shunts implanted, and in this group, there were six infections (5.5%). There were 300 clean shunt revisions where partial or entire new standard systems were implanted with 20 infected cases (6.6%).
Liverpool
This unit performed a total of 160 AIS shunt procedures in 112 children with an age range of 1 day to 16 years between October 2003 and December 2006. There were ten (6.3%) infected shunts. The median follow-up was 19 months ranging from 8 to 42 months. There were 47 de novo shunts implanted, and in this group, there were four infections (8.5%). In the first half of the study period from 2003 to 2005, there were no infections seen in this group. There were 113 clean shunt revisions where partial or entire new AIS systems were implanted with six infected cases (5.3%). In the Historical cohort, there were 32 de novo shunts implanted, and in this group, there were six infections (18.8%). There were 45 clean shunt revisions where partial or entire new standard systems were implanted with two infected cases (4.4%).
London GOS
This unit performed a total of 221 AIS shunt procedures in 165 children with an age range of 1 day to 16 years between June 2005 and June 2007. There were eight (3.8%) infected shunts. The median follow-up was 14 months ranging from 5 to 27 months. There were 116 de novo shunts implanted, and in this group, there were four infections (3.4%). There were 105 clean shunt revisions where partial or entire new AIS systems were implanted or part was changed with four infected cases (3.8%). In the historical cohort there were 657 de novo shunts implanted, and in this group, there were 60 infections (9.1%). There were 820 clean shunt revisions for shunt malfunction where partial or entire new standard systems were implanted with 61 infected cases (7.4%).
Figure 1 summarizes and compares the individual units’ AIS and historical non-AIS cohort shunt implantation and infection rate. For this outcome, an OR less than 1 indicates a clinical advantage for AIS shunts. There was no difference in the surgical procedure/technique in the historical cohort other than the type of catheter used. The overall pooled treatment effect estimate is statistically significant and favours AIS shunts for de novo shunts and clean revisions combined [Fig. 1, OR 0.60 (95% CI 0.38, 0.93)], de novo shunts [Fig. 1, OR 0.50 (95% CI 0.25, 0.99)] and for the subgroup analysis of less than 1 year de novo shunts [Fig. 1, OR 0.38 (95% CI 0.17, 0.85)]. Although the pooled treatment effect estimate is not statistically significant for clean revisions only, the result still favours AIS shunt [Fig. 1, OR 0.70 (95% CI 0.39, 1.27)].
Table 1 shows the caseload for de novo shunts and clean revisions for each unit for both AIS and the non-AIS historical cohort.
Infecting organisms in AIS systems
Only London GOS and Liverpool collected complete data on causative organisms for AIS infections. Antibiotic sensitivity data was not complete. Over 80% of the causative pathogens were Gram positive organisms, which included three MRSA infections and two rifampicin-resistant organisms. The median time to infection in the entire cohort was 19 days (ranging from 1 to 75 days).
Discussion
Despite being the largest paediatric multi-centre study with historical controls, the worthwhile difficulties in proving a new intervention without proper randomized studies is clearly demonstrated. We have attempted to overcome the limitations of single-institution studies by presenting data for three sites.
When analysed separately, only the prospective London GOS group data demonstrated a statistically significant reduction in total DNS and CRS infections rates when comparing AIS versus non-AIS shunts—8.2% reduced to 3.6%. In contrast, both Leeds and Liverpool data did not reveal any statistically significant difference in DNS and CRS AIS infection rates compared to historical non-AIS controls, although the pooled treatment effect estimate was statistically significant indicating a clinical advantage for AIS shunts (Fig. 1). This is due to the weight given to the London site.
The results are presented individually by centre but also combined within a meta-analysis (Fig. 1). There was a large overlap between the confidence intervals for each centre and consequently low levels of heterogeneity; however, as this is not a meta-analysis of randomized controlled trials, results should be interpreted with caution [30].
Analysis of the infant subgroup in the three centres suggests that AIS implantation may confer a benefit; however, this only achieved statistical significance in the London site. The multi-unit data confirms that gram positive organisms remain the prevalent infective pathogen. It was of interest to note the three MRSA infections and also the important finding of two rifampicin-resistant organisms in the GOS group.
A limitation of this data is that the number of participants is not taken into account in the analysis and therefore one participant may have more than one shunt procedure and so the analysis has not allowed for multiple shunts per person.
This may artificially narrow the confidence intervals, decreasing the precision; however, the confidence intervals are very wide here indicating large uncertainty in the effect estimates. In addition, data was not available to allow analyses to account for varying lengths of follow-up for each individual meaning that time to event or analyses using person–years follow-up were not possible.
Differences in the historical cohort periods also need to be considered with the differing lengths of time (i.e. Liverpool, 12 months; Leeds, 63 months and London, 120 months) and also the differences in the way the data was collected, retrospectively and prospectively. This re-enforces the need for cautious interpretation of single-unit studies and historical cohorts as it is difficult to be able to generalise the results.
Ventriculoperitoneal shunt infection continues to be a major source of morbidity and mortality for patients with hydrocephalus [11, 33]. The paediatric population and, in particular, neonates and infants are the most vulnerable, and infection rates of up to 15% in children less than 6 months in age have been reported [7, 9–11, 20, 21].
Attempts to curtail this seemingly inevitable part of neurosurgical practice have included strict operative protocols, including length of operative time, number of personnel present in the theatre during the operation, operating list order (first on list), surgeon seniority, operating technique (non-touch) and prophylactic antibiotics [8–10, 13, 17, 32]. Within the United Kingdom neurosurgical practice, VP shunt insertion in the emergency setting does not always allow for all these strict protocols to be adhered to, in particular operating list order and surgeon seniority. In a previous study, it has been noted that the organisms responsible for shunt infection were rarely detected in the operative wound at the time of shunt insertion, leading the authors to conclude that the vulnerable period for bacterial colonization of shunts may not be restricted to the operative procedure as is commonly believed, but may extend throughout the postoperative period of wound healing [28, 29, 31].
The Bactiseal® antimicrobial-impregnated catheter system has been developed and introduced to prevent infection, mainly in the early postoperative period when most infections occur. It is impregnated with 0.15% clindamycin and 0.054% rifampicin. Initial in vivo studies have shown this combination to be effective against sensitive staphylococcal species colonization (such as S. epidermidis, S. aureus, S. haemolyticus and S. hominis) with persisting in vivo antibiotic activity up to 3 months postimplantation [18]. Nevertheless, its clinical efficacy in significantly reducing shunt infections in clinical practice is uncertain and there remains a reluctance to use AIS components because of their increased cost not necessarily translating to clear patient benefit [26, 27].
There have been several studies of varying conclusions which have been reported in the literature. Govender et al. [14] found a significant (p = 0.04) reduction with antibiotic-impregnated catheters, from 16.7% to 6%. In their randomized study which did not include neonates, they reported the absence of any staphylococci in any of their AIS infections which was in contrast to other published reports. In a retrospective review, Sciubba et al. reported a 2.4-fold decreased paediatric shunt infection rate (12–1.4%) when changing from conventional (n = 208) to impregnated (n = 145) catheters with a follow-up period of 6 months [26].
A statistically significant reduction in infection rates has also been reported by Pattavilakom et al. [19] where in adult and paediatric patients the infection rate fell from 6.5% in 551 procedures with standard catheters to 1.2% in 243 procedures with impregnated catheters. A recent single-centre study by Eymann et al. [12] compared 171 adults and 26 paediatric patients who had AIS catheters inserted between January 2002 and December 2006 against 98 adults and 22 paediatric patients who had standard catheters inserted between January 1998 and December 2001. The overall infection rate fell from 5.8% to 1% and was statistically significant (P = 0.0145). The study included a cost–benefit analysis and concluded that antibiotic-impregnated catheters are cost-effective if the shunt infection risk can be reduced by 50% where the initial infection risk is higher than 4% [12].
Kan and Kestle [16] reported a study in children where converting from conventional (n = 80) to AIS (n = 80) catheters reduced the shunt infection rate in children from 8.8% to 5.0%. In a similar smaller study, Aryan et al. [1] found a reduction in infection rate in children from 15.2% (Std, n = 46) to 3.1% (AIS, n = 32). Both these studies were not statistically significant.
Infection rates can vary drastically within an institute over time with peaks and troughs of postoperative complications. Previously published preliminary Liverpool single-unit data demonstrated a 0% infection rate in AIS group 1 patients over a consecutive 2-year period [15]; however this was significantly altered by a cluster of infections over a subsequent 2-week period that increased the AIS infection rate to 10%. Similar peaks and troughs can be demonstrated in the 10-year audit of the GOSH historical cohort [6]. This demonstrates the difficulty and the need for caution when interpreting data in the literature from single units over a relatively short period of time.
More recently, Richards et al. [23] published their retrospective review of the UK shunt registry database utilising a matched-pair study design comparing AIS against standard shunts. They allowed for a 10% leeway in matching for age and identified 994 matched pairs. They analysed procedures that took place between 2000 and 2006 with the median difference in date of surgery between the two groups being 0.3 years. The matched-pair cohort was slightly biased towards paediatric neurosurgery, and their data also indicated more frequent use of AIS catheters for de novo implantations. Their data demonstrated a 4.7% infection rate in standard shunts (n = 47) and an infection rate of 3.0% in AIS implantations (n = 30). This result was statistically significant and is the largest cohort of patients analysed thus far. The authors acknowledged that a matched-pair study design does not entirely eliminate bias and the data does not include causative pathogens or a standardised definition of an infection.
While our statistically significant multi-centre study with historical controls is the largest analysis of an exclusively paediatric cohort, the study design with mixed prospective and retrospective data is open to potential bias and confounding factors. The results and limitations of our and other published studies lead us to concur with Richards et al. that there is a need for a future prospective, randomised, multi-centre study. The only next step is an adequately powered multi-centre randomised controlled trial with an agreement of definitions of shunt infection, standardisation of techniques and antibiotic prophylaxis. In particular, it will allow for more robust data on vulnerable groups such as neonates that are potentially most likely to benefit from the perceived advantage of AIS implants.
Conclusion
This study provides further evidence of the positive effect of AIS in reducing infection rates for de novo and clean revision VP shunt implants in children. Despite being the largest study of its kind in an exclusively paediatric cohort, there are significant caveats related to study design. Nevertheless, we feel the data presented is an advance compared to single-institution studies and can serve to guide practice until a multi-unit prospective, randomized controlled trial provides more robust evidence.
References
Aryan HE, Meltzer HS, Park MS, Bennett RL, Jandial R, Levy ML (2005) Initial experience with antibiotic-impregnated silicone catheters for shunting of cerebrospinal fluid in children. Childs Nerv Syst 21:56–61
Bayston R (1989) Hydrocephalus shunt infections. Chapman and Hall, London
Bayston R, Grove N, Siegel J, Lawellin D, Barsham S (1989) Prevention of hydrocephalus shunt catheter colonisation in vitro by impregnation with antimicrobials. J Neurol Neurosurg Psychiatry 52:605–609
Bayston R, Lambert E (1997) Duration of protective activity of cerebrospinal fluid shunt catheters impregnated with antimicrobial agents to prevent bacterial catheter-related infection. J Neurosurg 87:247–251
Bayston R, Ashraf W, Bhundia C (2004) Mode of action of an antimicrobial biomaterial for use in hydrocephalus shunts. J Antimicrob Chemother 53:778–782
Belli A, Thompson DNP, Harkness WFJ, Pitt M, Hayward RD (2001) Shunt infection rates: surgical skill or statistical serendipity? Analysis of 1,106 cases. Br J Neurosurg 15:82, Abstract
Borgbjerg BM, Gjerris F, Albeck MJ, Borgesen SE (1995) Risk of infection after cerebrospinal fluid shunt: an analysis of 884 first time shunts. Acta Neurochir (Wien) 136:1–7
Choksey MS, Malik IA (2004) Zero tolerance to shunt infections: can it be achieved? J Neurol Neurosurg Psychiatry 75:87–91
Choux M, Genitori L, Lang D, Lena G (1992) Shunt implantation: reducing the incidence of shunt infection. J Neurosurg 77:875–880
Drake JM, Sainte-Rose C (1995) The shunt book. Blackwell Science, Cambridge, Mass
Enger PO, Svendsen F, Wester K (2003) CSF shunt infections in children: experiences from a population-based study. Acta Neurochir (Wien) 145:243–248
Eymann R, Chehab S, Strowitzki M, Steudel W-I, Keifer M (2008) Clinical and economic consequences of antibiotic-impregnated cerebrospinal fluid shunt catheters. J Neurosurg Pediatrics 1:444–450
Fan-Havard P, Nahata MC (1987) Treatment and prevention of infections of cerebrospinal fluid shunts. Clin Pharm 6:866–880
Govender ST, Nathoo N, van Dellen JR (2003) Evaluation of an antibiotic-impregnated shunt system for the treatment of hydrocephalus. J Neurosurg 99:831–839
Hayhurst C, Cooke R, Williams D, Kandasamy J, O'Brien DF, Mallucci CL (2008) The impact of antibiotic-impregnated catheters on shunt infection in children and neonates. Childs Nerv Syst 24(5):557–562, Epub 2007 Oct 26
Kan P, Kestle J (2007) Lack of efficacy of antibiotic-impregnated shunt systems in preventing shunt infections in children. Childs Nerv Syst 23:773–777
Kulkarni AV, Drake JM, Lamberti-Pasculli M (2001) Cerebrospinal fluid shunt infection: a prospective study of risk factors. J Neurosurg 94:195–201
Pattavilakom A, Kotasnas D, Korman TM, Xenos C, Danks A (2006) Duration of in vivo antimicrobial activity of antibiotic-impregnated cerebrospinal fluid catheters. Neurosurgery 58:930–935
Pattavilakom A, Xenos C, Bradfield O, Danks RA (2007) Reduction in shunt infection using antibiotic impregnated CSF shunt catheters: an Australian prospective study. J Clin Neurosci 14(6):526–531
Pople IK, Bayston R, Hayward RD (1992) Infection of cerebrospinal fluid shunts in infants: a study of etiological factors. J Neurosurg 77:29–36
Ratilal B, Costa J, Sampaio C (2008) Antibiotic prophylaxis for surgical introduction of intracranial ventricular shunts: a systematic review. J Neurosurg Pediatrics 1:48–56
Ritz R, Roser F, Morgalla M, Dietz K, Tatagiba M, Will BE (2007) Do antibiotic-impregnated shunts in hydrocephalus therapy reduce the risk of infection? An observational study in 258 patients. BMC Infect Dis 8(7):38
Richards HK, Seeley HM, Pickard JD (2009) Efficacy of antibiotic-impregnated shunt catheters in reducing shunt infection: data from the United Kingdom Shunt Registry. J Neurosurg Pediatr 4(4):389–393
Ronan A, Hogg GG, Klug GL (1995) Cerebrospinal fluid shunt infections in children. Pediatr Infect Dis J 14:782–786
Schiff SJ, Oakes WJ (1989) Delayed cerebrospinal-fluid shunt infection in children. Pediatr Neurosci 15:131–135
Sciubba DM, Stuart RM, McGirt MJ, Woodworth GF, Samdani A, Carson B, Jallo GI (2005) Effect of antibiotic-impregnated shunt catheters in decreasing the incidence of shunt infection in the treatment of hydrocephalus. J Neurosurg 103:131–136
Sciubba DM, Lin LM, Woodworth GF, McGirt MJ, Carson B, Jallo GI (2007) Factors contributing to the medical costs of cerebrospinal fluid shunt infection treatment in pediatric patients with standard shunt components compared with those in patients with antibiotic impregnated components. Neurosurg Focus 22(4):E9
Sciubba DM, McGirt MJ, Woodworth GF, Carson B, Jallo GI (2007) Prolonged exposure to antibiotic-impregnated shunt catheters does not increase incidence of late shunt infections. Childs Nerv Syst 23:867–871
Shapiro S, Boaz J, Kleiman M, Kalsbeck J, Mealey J (1988) Origin of organisms infecting ventricular shunts. Neurosurgery 22:868–872
Taggart DP, D'Amico R, Altman DG (2001) Effect of arterial revascularisation on survival: a systematic review of studies comparing bilateral and single internal mammary arteries. Lancet 358(9285):870–875
Thompson DN, Hartley JC, Hayward RD (2007) Shunt infection: is there a near-miss scenario? J Neurosurg 106(1 Suppl):15–19
Walters BC, Hoffman HJ, Hendrick EB, Humphreys RP (1984) Cerebrospinal fluid shunt infection. Influences on initial management and subsequent outcome. J Neurosurg 60:1014–1021
Wang KW, Chang WN, Shih TY, Huang CR, Tsai NW, Chang CS, Chuang YC, Liliang PC, Su TM, Rau CS, Tsai YD, Cheng BC, Hung PL, Chang CJ, Lu CH (2004) Infection of cerebrospinal fluid shunts: causative pathogens, clinical features, and outcomes. Jpn J Infect Dis 57:44–48
Conflict of interest
None.
Disclosure
The authors confirm that no financial support has been received for the inception or preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kandasamy, J., Dwan, K., Hartley, J.C. et al. Antibiotic-impregnated ventriculoperitoneal shunts—a multi-centre British paediatric neurosurgery group (BPNG) study using historical controls. Childs Nerv Syst 27, 575–581 (2011). https://doi.org/10.1007/s00381-010-1290-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-010-1290-z