Abstract
Purpose
Hydrocephalus is a common disorder of defective cerebrospinal fluid (CSF) turnover. The identification of the aquaporin water channels (AQPs) led to the study of their role in the composition of biological fluids including CSF. The purpose of this study is to review the potential role of aquaporins in the pathogenesis, compensation, and possibly treatment of hydrocephalus.
Methods
We performed a MEDLINE search using the terms “aquaporin AND hydrocephalus.” The search returned a total of 20 titles. Eleven studies fulfilled the criteria for this review.
Results
Most studies were performed in animal models. The expression of AQPs in hydrocephalus is significantly altered. Aquaporin-1 levels at the choroid plexus are decreased in most models of hydrocephalus while CSF production and intracranial pressure are reduced in AQP1 knockout mice. In contrast, the expression of AQP4 in hydrocephalus is increased at its sites of expression. Aquaporin-4 knockout mice show a decreased clearance of brain edema via blood–CSF and blood–brain barrier (BBB) pathways and decreased survival in hydrocephalus models.
Conclusions
Aquaporin-1 is highly expressed at the choroid plexus and is related to CSF production. Aquaporin-4 is expressed at the ependyma, glia limitans, and at the perivascular end feet processes of astrocytes of the BBB, facilitating the water movement across these tissue interfaces. The observations obtained from animal studies and few cases in humans indicate an adaptive and protective role of AQPs in hydrocephalus by decreasing CSF production and increasing edema clearance. Aquaporins are attractive targets for the pharmaceutical treatment of hydrocephalus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Water homeostasis is a crucial step in the physiology of the central nervous system and pathophysiology of hydrocephalus [1]. Cerebrospinal fluid (CSF) not only provides a protective fluid layer for the brain, but it delivers nutrients and growth factors important for pruning and maintenance of neural networks and removes waste products secreted by the brain parenchyma [2–4].
The identification of the elusive water channel or aquaporins (AQPs) in the early 1990s allowed the scientific community to study the biology of CSF and blood production. Aquaporins are members of a family of transmembrane proteins that allow for the movement of water and small solutes through the fatty membranes of cells and by doing so contribute to the bulk of water homeostasis [5–9]. To date, 13 mammalian aquaporins have been cloned and identified in every tissue in the body. In the central nervous system, there are five members of the AQP family, AQP1, AQP4, AQP7, AQP9, and AQP11 [9–12]. Of the five expressed AQPs, only AQP1 and AQP4 are expressed in abundance, with AQP4 showing the highest expression pattern of any other member [9]. The presence of AQPs in the central nervous system has stimulated much interest among the scientific community in order to study the role of these proteins in normal CSF turnover as well as pathological conditions linked to CSF production and absorption such as brain edema and hydrocephalus [7, 9, 13].
The aim of this study is to review the pertinent literature concerning the role of aquaporins in cerebrospinal fluid turnover and pathophysiology of hydrocephalus. The information obtained from this process will be used in order to clarify any potential association between AQPs and hydrocephalus, define further experimental approaches, and focus on key pathophysiological steps as candidates for pharmacological treatment.
Why study the role of aquaporins in the pathophysiology of hydrocephalus?
A fundamental understanding of the mechanisms leading to hydrocephalus is crucial for its more effective treatment and classification. Early reports from Dandy classified hydrocephalus as “communicating” and “noncommunicating” based on the state-of-the-art understanding of the pathophysiology. In Dandy’s vernacular, communicating hydrocephalus was that form of the disease in which injection of a dye into the ventricles could be recovered from the spinal subarachnoid space. In noncommunicating hydrocephalus, injection of a dye into the ventricles could not complete the pathway of flow, suggesting an obstruction [14].
With the advent of new imaging modalities and experimental tools, it is now possible to dissect the various points of obstruction and further classify hydrocephalus into distinct pathologies. Recently, Rekate et al. proposed a contemporary classification of hydrocephalus based on a flow circuit model. The Rekate model, much like Dandy’s, rests on two simple assumptions: CSF is either overproduced (e.g., choroid plexus papillomas) or underrecycled via flow obstruction at the level of the foramen of Monro, aqueduct of Sylvius, outlets of fourth ventricle, basal cisterns, arachnoid granulations, or other points of absorption [15, 16]. Simplifying hydrocephalus into a disease of overproduction or impaired CSF absorption, it becomes an intuitive extension to assume that water channels are key players in the pathophysiology of this disease. Aquaporins are highly expressed at known points of CSF production and absorption such as the choroid plexus and extrachoroidal sites and ependymal cells, glia limitans interna, and CSF circulation at the cortical subarachnoid space like glia limitans externa, subpial zone, and meninges. In addition, aquaporin-4 is highly expressed at the end feet of the astrocyte processes that are part of the blood–brain barrier (BBB) [3, 7–9, 17–22].
A less noted but closely related pathophysiological process to hydrocephalus is the observation of interstitial brain edema subsequent to impaired CSF turnover and increased intracranial pressure (ICP). This type of edema, observed as transependymal CSF flow in neuroimaging studies, has been categorized as “hydrocephalic edema” [8, 23]. The reduction of intracranial pressure and the resolution of “hydrocephalic” brain edema are the mainstays of therapy for hydrocephalus. Again, given the role of water in the pathophysiology of this disease, it is not surprising to note that aquaporins demonstrate a distinct role in the resolution of this and other types of brain edema [8, 9, 24, 25]. Aquaporin-4 deficiency in the AQP4 knockout mice, studying vasogenic edema models like tumors and abscess formation, is associated with higher ICP, more brain water content, and unfavorable outcomes compared with controls. The AQP4 water channel presence in wild-type mice at the blood–CSF (ependyma, glia limitans interna, subpial zone, or glia limitans externa) and blood–brain barrier (astrocyte feet processes at BBB) interfaces, which serve as edema clearance pathways, indicated an active role for AQP4 in this setting. The interpretation of these results by the authors suggested that AQP4 acts adaptively and protectively in the resolution of vasogenic edema via pathways from brain parenchyma to CSF and blood. In contrast, aquaporin-4 deficiency in AQP4 knockout mice is associated with favorable outcome in models of cytotoxic brain edema (water intoxication, meningitis, and ischemia). In this model, AQP4 knockout mice and wild-type controls were injected with water into the peritoneum. The water injection lowered the osmolarity of plasma and resulted in profuse astrocyte end feet swelling, increased brain water content, and worse outcomes concerning survival and neurological impairment in wild-type controls expressing AQP4. The authors indicate that the presence of AQP4 has a different significance in cytotoxic versus vasogenic edema resolution. It has been suggested that AQP4 mediates osmotically driven water movement in cytotoxic edema models and drives water in the brain (due to the presence of osmotic gradients), leading to cerebral edema [8, 9, 24, 25]. Aquaporin-9 expression is increased in astrocytes in the peri-infract area in mice models of ischemia, indicating a role in the regulation of postischemic edema [26]. The role of AQPs in relation to the resolution of the third type of brain edema, the “hydrocephalic edema,” could be significant and could shed more light to the pathophysiology and treatment of hydrocephalus.
Distribution of aquaporins in the brain
The knowledge of distribution and localization of aquaporins in tissues is the first step in understanding their location-specific function. Aquaporin-1 (AQP1) is highly expressed at the apical surface (surface facing the ventricles) of the choroid plexus epithelium in mice, rats, sheep, opossum, and humans [7, 19, 21, 27–29]. A lower gradient of AQP1 expression has been reported at the basolateral (blood side) of the choroid plexus epithelium in rodents and humans [19, 28]. The higher apical expression of AQP1 has been used to rationalize its contribution to the transcellular transport of water molecules for the production of CSF [7, 19, 21, 27–29]. Current models of CSF production underline the role of the apical presentation of sodium–potassium ATPase and AQP1 at the choroid plexus as the most significant molecular mechanisms concerning water transport to the ventricles and CSF production [2, 4]. The weak basolateral expression of AQP1 at the choroid plexus could explain how water molecules enter the choroid plexus cells and are subsequently filtered into the ventricle to produce CSF [2, 4, 21, 27, 29, 30]. The polarized expression of AQP1 at the choroid plexus has been confirmed in humans [19, 21, 27]. Others have described a putative role for AQP4 in CSF production at the choroid plexus, but AQP1 predominates in this region [19, 21, 29]. Aquaporin-1 expression has also been described at the craniofacial lymphatics that accompany perineural sheaths in rodents, implying a role in water movement in these tissues [31, 32]. The contribution of perineural craniofacial lymphatics in CSF turnover has been widely recognized, but currently there are no studies about the expression of AQP1 at the lymphatics in hydrocephalus models [33, 34].
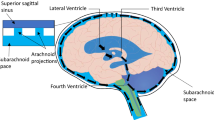
Aquaporin-4 is the most abundant water channel in the brain [7, 8, 20, 35]. Main areas of high distribution include the glia cells at the junction of fluid and brain parenchyma, at BBB and blood–CSF barriers like ependymal cells, glia limitans interna (astrocyte processes and ependyma bordering brain and subarachnoid CSF at the subpial zone), and glia limitans externa (astrocyte processes bordering brain and ventricular CSF) [7]. The location-specific distribution of AQP4 around tissue interfaces that are close to CSF and thus water (ventricles, subarachnoid space) indicates that these channels are significant for the regulation of water physiology in these areas. Specifically, aquaporin-4 is located at the perivascular end feet processes of astrocytes that form the blood–brain barrier (Fig. 1). Other areas of AQP-4 in brain are the cerebellum, the supraoptic and suprachiasmatic nucleus of the hypothalamus, hippocampal dentate gyrus and areas CA-1 and CA-3, neocortex, nucleus of stria terminalis, and medial habenular nucleus [7]. Mobasheri et al., using a tissue microarray approach, showed that AQP4 is expressed in meningeal tissues in humans [20]. This observation, in conjunction with the documented presence of AQP4 at the glia limitans externa, the glial area at the brain surface under the pia mater, in many species could possibly indicate an active role of AQP4 water channel at the cortical subarachnoid space or at the surface brain parenchyma in regulating the concentration of water and ions in the CSF.
Aquaporin-9 belongs to a specific subfamily of aquaporins named “neutral solute channels” [7]. Aquaporin-9 is permeable to water, glycerol, lactate, urea, purines, pyrimidines, and monocarboxylates. In the brain, AQP9 is present in astrocytes, hypothalamic tanycytes, cerebellar neurons, glia limitans, endothelial cells of pial vessels, and hippocampal area (CA2) [7, 10]. The existence of a water channel, like AQP9, in tissue areas that are close to CSF circulation like hypothalamic tanycytes, glia limitans, and pial endothelial cells suggests that they have a central role in water flux between cerebrospinal fluid and brain parenchyma [10].
Much less is known about functions of AQP7 and AQP11 in the brain, described in few reports. Although both proteins have been identified in the brain of rodents, AQP11 does not seem to possess the ability to transfer water [11, 12].
AQP1 and hydrocephalus
Since AQP1 distribution is dominant in choroid plexus and it demonstrates a specific localization at the apical (ventricular) side facing CSF, its role in the production of CSF is highly suspected. Oshio and colleagues compared cerebrospinal fluid production and intracranial pressure between normal mice (wild-type mice) and mice lacking AQP1 expression (AQP1 knockouts) [36, 37]. The osmotic water permeability of choroid epithelium cells was fivefold reduced in knockouts. The CSF production was reduced by 20–25% in AQP1 knockouts. Also, AQP1 knockouts demonstrated a 56% reduction in ICP in comparison with wild-type mice. However, the majority of this effect was attributed to the lower central venous pressure in AQP1 knockouts due to systemic effects in kidneys [36, 37].
Longatti and colleagues, Mobasheri et al., and also Praetorius et al. confirmed the presence of AQP1 at the apical side of the choroid plexus in humans [19, 21, 27]. Interestingly, some reports indicate that AQP1 expression is downregulated in hydrocephalus. Smith et al. report a case of 15-month-old girl with choroid plexus hyperplasia, a rare case of nonobstructive hydrocephalus related to CSF hyperproduction by choroid plexuses [38]. The choroid plexus was excised, and immunohistochemistry revealed a dramatic decrease in immunoreactivity of AQP1 of the specimen compared with controls, suggesting an adaptive process that downregulated AQP1 in order to lower the CSF production. Longatti et al. reported a series of nine patients with choroid plexus tumors. The observed expression levels of AQP1 were not homogenous in cases with a choroid plexus tumor and hydrocephalus. Aquaporin-1 showed a strong expression in four cases of choroid plexus papillomas with hydrocephalus (obstructive in three and communicating in one) while weak expression was observed in one case of choroid plexus carcinoma complicated by obstructive hydrocephalus. The small number of human cases reported, the heterogeneous expression of AQP1 in these cases, and the use of immunohistochemistry (a qualitative method) not accompanied by quantitative methods like immunofluorescence, Western blot, or RT-PCR protocols cannot reveal the real patterns of expression of AQP1 in humans with hydrocephalus at present [38, 39]. Paul and colleagues studied the expression of AQP1 and AQP4 in congenital hydrocephalic H-Tx rats with RT-PCR, Western blot, immunohistochemistry, and ELISA [40]. They also found that AQP1 expression was significantly decreased in choroid plexus of H-Tx rats while AQP4 expression was increased in the whole brain and cortex and slightly decreased in choroid plexus.
Although further research should be conducted, these preliminary data indicate that possibly a feedback mechanism exists which associates the expression of AQP1 in choroid plexus and the presence of hydrocephalus. Adaptive feedback mechanisms exist and regulate the expression of AQP1 in choroid plexus under specific circumstances, e.g., low gravity reduces the amount of AQP1 in choroid plexus [41]. In addition, AQP1 expression is downregulated in cases of compromised cerebral perfusion [41–43], while Silverberg et al. demonstrated that CSF production is reduced in patients with chronic hydrocephalus [44]. Hydrocephalus seems to be associated with AQP1 downregulation in choroid plexus, which could be an adaptive protective mechanism in order to lower the production of CSF and reduce the increased intracranial pressure.
AQP4 and hydrocephalus
Bloch and colleagues used a kaolin injection model in cisterna magna of mice in order to reproduce obstructive hydrocephalus in wild-type mice and AQP4-knockout mice [45]. The kaolin injection model is a well-established model of progressive acute obstructive hydrocephalus in rodents [46]. The authors assessed parameters of ICP, ventriculomegaly, brain parenchymal water content, and 5-day survival rate between mice groups over a specific time frame [45]. The AQP4-knockout mice demonstrated significant ventriculomegaly; ICP was increased and brain water content was also increased by 2–3% [45]. The increased brain water content in AQP4-knockout mice indicated the existence of “hydrocephalic edema.” In 5 days, 84% of the wild-type mice survived compared to 66% of AQP4 knockouts, indicating that hydrocephalus induced by kaolin injection was much more severe in mice that do not express AQP4. The authors constructed a mathematical computational model of kaolin-induced hydrocephalus using the data obtained from these experiments. The mathematical model reconfirmed the observations obtained and predicted that the severity of hydrocephalus would be much more reduced when AQP4 expression increased. A protective role of AQP4 concerning hydrocephalus was suggested in the article based on the above observations. Since AQP4 is expressed at brain–CSF (ependyma and glia limitans under the subarachnoid space) and blood–brain barriers the authors proposed a significant role for AQP4-mediated transparenchymal CSF absorption in cerebral vasculature in cases of hydrocephalus [45]. The significant role of aquaporin-4 in the clearance of excess brain water and specifically vasogenic brain edema has been documented in multiple studies [7, 13, 24, 47–57]. Feng and colleagues published an interesting observation in AQP4-knockout mice [58]. In a series of 612 AQP4 knockouts, a percentage of 9.6% demonstrated obstructive hydrocephalus. The site of obstruction was identified and was located at the level of cerebral aqueduct leading to aqueductal stenosis. Histological studies at the site of stenosis revealed marked ependymal disorganization. The authors attributed the presence of sporadic obstructive hydrocephalus in a subset of AQP4 knockouts to AQP4 polymorphisms that could contribute to the development of aqueductal stenosis [9, 58]. The kaolin injection model has also been used in rats to study the expression of AQP4 after these animals develop obstructive hydrocephalus [59–61].
Mao and colleagues also used a kaolin injection hydrocephalus model in order to study AQP4 changes in normal rat brains that finally developed severe hydrocephalus [62]. In this study, only rats with severe hydrocephalus were included (ventricle to brain ratio > 0.25). Aquaporin-4 expression was upregulated 3–4 weeks after kaolin injection. Areas of intense expression were identified in perivascular areas, parietal cerebrum and hippocampus, ependymal lining, and glia limitans. Interestingly, AQP4 levels do not increase significantly until rats become 7 weeks old while elevated expression can be observed for up to 9 months, indicating a time-sensitive and pressure- or ECF-fluid-adaptive (due to transparenchymal flow of CSF in hydrocephalus) mechanism. The white matter demonstrated significant edema and showed evidence of fragmentation in the youngest group of hydrocephalic rats while it was atrophic in the older group. Hydrocephalus-induced AQP4 expression revealed in this study indicates that this water channel plays a distinct role in the pathophysiological process of hydrocephalus evolution [62]. In another study of a communicating inflammatory lysophosphatidylcholine-induced hydrocephalus by Tourdias et al., AQP4 was also upregulated in hydrocephalic rat brain in relation to controls at blood–CSF and blood–brain barrier interfaces [63]. Magnetic resonance studies in these rats revealed a significantly bigger apparent diffusion coefficient (APC) and larger CSF volumes, which were correlated with elevated expression of AQP4.
Shen et al. and Paul et al. studied the expression of AQP4 in another animal model of hydrocephalus, the congenitally hydrocephalic rat (H-Tx rat) [40, 64]. Aquaporin-4 was highly expressed at the ependymal lining, the feet processes of pericapillary astrocytes, and the subpial zone in H-Tx rats and indicated an adaptive mechanism. Shen et al. further argued about the role of AQP4 in the pathophysiology of a subset H-Tx rat group with “arrested hydrocephalus” [64]. This interpretation and the definition of “arrested hydrocephalus” were mainly based on the higher survival of animals expressing more AQP4 without providing any evidence about the severity of ventriculomegaly [65].
Questions to be answered and suggestions
The association between aquaporins and hydrocephalus is demonstrated in studies (Table 1) based mainly in animal models like AQP4-knockout mice, kaolin, or LPS-induced hydrocephalus in rats and congenitally hydrocephalic rats (H-Tx rats, Table 1). Aquaporin-1 is downregulated in hydrocephalus, and aquaporin-4 expression is upregulated in the blood–CSF and blood–brain barriers, suggesting the presence of an adaptive feedback mechanism that lowers the production of ICP and increases the clearance of CSF in cases where transparenchymal flow and “hydrocephalic edema” are observed. The specific molecular pathway that triggers these adaptive responses in animal models is currently unknown and should be identified. The threshold for a successful adaptation process via an AQP1- and AQP4-mediated water movement regulation in hydrocephalus has not been currently established. This effort could shed more light in the pathophysiology of cases of “compensated” hydrocephalus and identify the role of AQPs in the compensation process.
Currently, there are few reports about the expression of AQPs in cases of hydrocephalus in humans [38, 39], which can only indicate potential research targets and underline the necessity for further studies in this scientific area (Table 1). The confirmation of the results obtained in animal models in humans could be possible only after an extended research targeted towards the various types of hydrocephalus in humans.
If the results in larger human studies confirm the experimental results obtained from animal models, then a potential pharmacological treatment of hydrocephalus would seem likely. Aquaporin-1 inhibitors and aquaporin-4 upregulators could form a new category of drugs for the treatment of hydrocephalus, the aquaporin regulators [66–68]. Various candidate drugs or substances have been tested and proposed like acetazolamide [69], AgNO3, sulfadiazine [70], arylsulfonamide [56], antiepileptics [71], vinpocetine [40], or thrombin [72]. One should be very cautious about interpreting the results in studies concerning AQPs modulators. Many inhibitors are toxic (e.g., AgNO3); some results were not confirmed in other studies; the candidate drug should demonstrate an adequate central nervous system penetration while the design of drugs that increase the expression of a protein is quite challenging [66–68].
One of the basic characteristics of the next critical step in hydrocephalus treatment research would be a shunt-free treatment for hydrocephalus. Aquaporins possess the potential to regulate the turnover of cerebrospinal fluid at the blood–CSF and BBB tissue interfaces. Animal studies and few human reports indicate that a protective role of aquaporin expression in hydrocephalus emerged via adaptation processes. They represent one of the most promising scientific fields in the research of hydrocephalus and probably would lead us to a new era of candidate drugs targeting its pathophysiology.
References
Kimelberg HK (2004) Water homeostasis in the brain: basic concepts. Neuroscience 129:851–860
Brown PD, Davies SL, Speake T, Millar ID (2004) Molecular mechanisms of cerebrospinal fluid production. Neuroscience 129:957–970
Macaulay N, Zeuthen T (2009) Water transport between CNS compartments: contributions of aquaporins and cotransporters. Neuroscience 168:941–956
Praetorius J (2007) Water and solute secretion by the choroid plexus. Pflugers Arch 454:1–18
Agre P, Sasaki S, Chrispeels MJ (1993) Aquaporins: a family of water channel proteins. Am J Physiol 265:F461
Agre P, King LS, Yasui M, Guggino WB, Ottersen OP, Fujiyoshi Y, Engel A, Nielsen S (2002) Aquaporin water channels—from atomic structure to clinical medicine. J Physiol (Lond) 542:3–16
Badaut J, Lasbennes F, Magistretti PJ, Regli L (2002) Aquaporins in brain: distribution, physiology, and pathophysiology. J Cereb Blood Flow Metab 22:367–378
Tait MJ, Saadoun S, Bell BA, Papadopoulos MC (2008) Water movements in the brain: role of aquaporins. Trends Neurosci 31:37–43
Verkman AS (2009) Aquaporins: translating bench research to human disease. J Exp Biol 212:1707–1715
Badaut J, Regli L (2004) Distribution and possible roles of aquaporin 9 in the brain. Neuroscience 129:971–981
Gorelick DA, Praetorius J, Tsunenari T, Nielsen S, Agre P (2006) Aquaporin-11: a channel protein lacking apparent transport function expressed in brain. BMC Biochem 7:14
Shin I, Kim HJ, Lee JE, Gye MC (2006) Aquaporin7 expression during perinatal development of mouse brain. Neurosci Lett 409:106–111
Marmarou A (2007) A review of progress in understanding the pathophysiology and treatment of brain edema. Neurosurg Focus 22:E1
Pudenz RH (1981) The surgical treatment of hydrocephalus—an historical review. Surg Neurol 15:15–26
Rekate HL (2008) The definition and classification of hydrocephalus: a personal recommendation to stimulate debate. Cerebrospinal Fluid Research 5:2
Rekate HL (2009) A contemporary definition and classification of hydrocephalus. Semin Pediatr Neurol 16:9–15
Cserr HF (1971) Physiology of the choroid plexus. Physiol Rev 51:273–311
McComb JG (1983) Recent research into the nature of cerebrospinal fluid formation and absorption. J Neurosurg 59:369–383
Mobasheri A, Marples D (2004) Expression of the AQP-1 water channel in normal human tissues: a semiquantitative study using tissue microarray technology. Am J Physiol Cell Physiol 286:C529–C537
Mobasheri A, Marples D, Young IS, Floyd RV, Moskaluk CA, Frigeri A (2007) Distribution of the AQP4 water channel in normal human tissues: protein and tissue microarrays reveal expression in several new anatomical locations, including the prostate gland and seminal vesicles. Channels Austin 1:29–38
Praetorius J, Nielsen S (2006) Distribution of sodium transporters and aquaporin-1 in the human choroid plexus. Am J Physiol Cell Physiol 291:C59–C67
Yool AJ (2007) Aquaporins: multiple roles in the central nervous system. Neuroscientist 13:470–485
Klatzo I (1994) Evolution of brain edema concepts. Acta Neurochir Suppl 60:3–6
Papadopoulos MC, Manley GT, Krishna S, Verkman AS (2004) Aquaporin-4 facilitates reabsorption of excess fluid in vasogenic brain edema. FASEB J 18:1291–1293
Verkman AS, Binder DK, Bloch O, Auguste K, Papadopoulos MC (2006) Three distinct roles of aquaporin-4 in brain function revealed by knockout mice. Biochim Biophys Acta 1758:1085–1093
Badaut J, Hirt L, Granziera C, Bogousslavsky J, Magistretti PJ, Regli L (2001) Astrocyte-specific expression of aquaporin-9 in mouse brain is increased after transient focal cerebral ischemia. J Cereb Blood Flow Metab 21:477–482
Longatti PL, Basaldella L, Orvieto E, Fiorindi A, Carteri A (2004) Choroid plexus and aquaporin-1: a novel explanation of cerebrospinal fluid production. Pediatr Neurosurg 40:277–283
Nielsen S, Smith BL, Christensen EI, Agre P (1993) Distribution of the aquaporin CHIP in secretory and resorptive epithelia and capillary endothelia. Proc Natl Acad Sci U S A 90:7275–7279
Speake T, Freeman LJ, Brown PD (2003) Expression of aquaporin 1 and aquaporin 4 water channels in rat choroid plexus. Biochim Biophys Acta 1609:80–86
Johansson PA, Dziegielewska KM, Ek CJ, Habgood MD, Møllgård K, Potter A, Schuliga M, Saunders NR (2005) Aquaporin-1 in the choroid plexuses of developing mammalian brain. Cell Tissue Res 322:353–364
Furukawa M, Shimoda H, Kajiwara T, Kato S, Yanagisawa S (2008) Topographic study on nerve-associated lymphatic vessels in the murine craniofacial region by immunohistochemistry and electron microscopy. Biomed Res 29:289–296
Ohtani O, Ohtani Y (2008) Structure and function of rat lymph nodes. Arch Histol Cytol 71:69–76
Johnston M, Zakharov A, Papaiconomou C, Salmasi G, Armstrong D (2004) Evidence of connections between cerebrospinal fluid and nasal lymphatic vessels in humans, non-human primates and other mammalian species. Cerebrospinal Fluid Res 1:2
Kida S, Pantazis A, Weller RO (1993) CSF drains directly from the subarachnoid space into nasal lymphatics in the rat. Anatomy, histology and immunological significance. Neuropathol Appl Neurobiol 19:480–488
Verkman AS, Mitra AK (2000) Structure and function of aquaporin water channels. Am J Physiol Ren Physiol 278:F13–F28
Oshio K, Song Y, Verkman AS, Manley GT (2003) Aquaporin-1 deletion reduces osmotic water permeability and cerebrospinal fluid production. Acta Neurochir Suppl 86:525–528
Oshio K, Watanabe H, Song Y, Verkman AS, Manley GT (2005) Reduced cerebrospinal fluid production and intracranial pressure in mice lacking choroid plexus water channel aquaporin-1. FASEB J 19:76–78
Smith ZA, Moftakhar P, Malkasian D, Xiong Z, Vinters HV, Lazareff JA (2007) Choroid plexus hyperplasia: surgical treatment and immunohistochemical results. Case report. J Neurosurg 107:255–262
Longatti P, Basaldella L, Orvieto E, Dei Tos A, Martinuzzi A (2006) Aquaporin(s) expression in choroid plexus tumours. Pediatr Neurosurg 42:228–233
Paul L, Madan M, Rammling M, Behman B, Pattisapu JV (2009) The altered expression of aquaporin 1 and 4 in choroid plexus of congenital hydrocephalus. Cerebrospinal Fluid Res 6:S7
Masseguin C, Corcoran M, Carcenac C, Daunton NG, Güell A, Verkman AS, Gabrion J (2000) Altered gravity downregulates aquaporin-1 protein expression in choroid plexus. J Appl Physiol 88:843–850
Edwards RJ, Dombrowski SM, Luciano MG, Pople IK (2004) Chronic hydrocephalus in adults. Brain Pathol 14:325–336
Masseguin C, Mani-Ponset L, Herbuté S, Tixier-Vidal A, Gabrion J (2001) Persistence of tight junctions and changes in apical structures and protein expression in choroid plexus epithelium of rats after short-term head-down tilt. J Neurocytol 30:365–377
Silverberg GD, Huhn S, Jaffe RA, Chang SD, Saul T, Heit G, Von Essen A, Rubenstein E (2002) Downregulation of cerebrospinal fluid production in patients with chronic hydrocephalus. J Neurosurg 97:1271–1275
Bloch O, Auguste KI, Manley GT, Verkman AS (2006) Accelerated progression of kaolin-induced hydrocephalus in aquaporin-4-deficient mice. J Cereb Blood Flow Metab 26:1527–1537
Collins P (1979) Experimental obstructive hydrocephalus in the rat: a scanning electron microscopic study. Neuropathol Appl Neurobiol 5:457–468
Agre P, Kozono D (2003) Aquaporin water channels: molecular mechanisms for human diseases. FEBS Lett 555:72–78
Badaut J, Brunet JF, Grollimund L, Hamou MF, Magistretti PJ, Villemure JG, Regli L (2003) Aquaporin 1 and aquaporin 4 expression in human brain after subarachnoid hemorrhage and in peritumoral tissue. Acta Neurochir Suppl 86:495–498
Hirt L, Ternon B, Price M, Mastour N, Brunet J-F, Badaut J (2009) Protective role of early aquaporin 4 induction against postischemic edema formation. J Cereb Blood Flow Metab 29:423–433
Manley GT, Binder DK, Papadopoulos MC, Verkman AS (2004) New insights into water transport and edema in the central nervous system from phenotype analysis of aquaporin-4 null mice. Neuroscience 129:983–991
Nico B, Mangieri D, Tamma R, Longo V, Annese T, Crivellato E, Pollo B, Maderna E, Ribatti D, Salmaggi A (2009) Aquaporin-4 contributes to the resolution of peritumoural brain oedema in human glioblastoma multiforme after combined chemotherapy and radiotherapy. Eur J Cancer 45:3315–3325
Papadopoulos MC, Saadoun S, Binder DK, Manley GT, Krishna S, Verkman AS (2004) Molecular mechanisms of brain tumor edema. Neuroscience 129:1011–1020
Papadopoulos MC, Verkman AS (2007) Aquaporin-4 and brain edema. Pediatr Nephrol 22:778–784
Saadoun S, Papadopoulos M, Bell B, Krishna S, Davies D (2002) The aquaporin-4 water channel and brain tumour oedema. J Anat 200:528
Tait MJ, Saadoun S, Bell BA, Verkman AS, Papadopoulos MC (2010) Increased brain edema in aqp4-null mice in an experimental model of subarachnoid hemorrhage. Neuroscience 167:60–67
Yool A, Brown E, Flynn G (2009) Roles for novel pharmacological blockers of aquaporins in the treatment of brain oedema and cancer. Clin Exp Pharmacol Physiol 37:403–409
Zador Z, Bloch O, Yao X, Manley GT (2007) Aquaporins: role in cerebral edema and brain water balance. Prog Brain Res 161:185–194
Feng X, Papadopoulos MC, Liu J, Li L, Zhang D, Zhang H, Verkman AS, Ma T (2009) Sporadic obstructive hydrocephalus in Aqp4 null mice. J Neurosci Res 87:1150–1155
Skjolding A, Rowland I, Soegaard L, Praetorius J, Nielsen S, Penkowa M, Juhler M (2008) Spatiotemporal regulation of aquaporin-4 in the kaolin induced hydrocephalic rat brain—experimental study using in vivo MRI and immunological methods. Hydrocephalus 2008, Hannover, Germany, pp S1–45
Skjolding A, Rowland I, Soegaard L, Praetorius J, Nielsen S, Penkowa M, Juhler M (2009) Aquaporins and histopathology in experimental hydrocephalus. Hydrocephalus 2009, Baltimore, USA
Skjolding A, Rowland I, Soegaard L, Praetorius J, Nielsen S, Penkowa M, Juhler M (2010) Experimental hydrocephalus induces changes in constitutive aquaporin-4 expression and changes morphology of aquaporin-4 positive ependyma. International Hydrocephalus Workshop 2010, Crete, Greece
Mao X, Enno TL, Del Bigio MR (2006) Aquaporin 4 changes in rat brain with severe hydrocephalus. Eur J Neurosci 23:2929–2936
Tourdias T, Dragonu I, Fushimi Y, Deloire MSA, Boiziau C, Brochet B, Moonen C, Petry KG, Dousset V (2009) Aquaporin 4 correlates with apparent diffusion coefficient and hydrocephalus severity in the rat brain: a combined MRI-histological study. Neuroimage 47:659–666
Shen XQ, Miyajima M, Ogino I, Arai H (2006) Expression of the water-channel protein aquaporin 4 in the H-Tx rat: possible compensatory role in spontaneously arrested hydrocephalus. J Neurosurg 105:459–464
Mcallister JP, Miller JM (2006) Aquaporin 4 and hydrocephalus. J Neurosurg 105:457–458, discussion 458
Frigeri A, Nicchia GP, Svelto M (2007) Aquaporins as targets for drug discovery. Curr Pharm Des 13:2421–2427
Gunnarson E, Zelenina M, Aperia A (2004) Regulation of brain aquaporins. Neuroscience 129:947–955
Papadopoulos MC, Verkman AS (2008) Potential utility of aquaporin modulators for therapy of brain disorders. Prog Brain Res 170:589–601
Tanimura Y, Hiroaki Y, Fujiyoshi Y (2009) Acetazolamide reversibly inhibits water conduction by aquaporin-4. J Struct Biol 166:16–21
Niemietz CM, Tyerman SD (2002) New potent inhibitors of aquaporins: silver and gold compounds inhibit aquaporins of plant and human origin. FEBS Lett 531:443–447
Huber VJ, Tsujita M, Kwee IL, Nakada T (2009) Inhibition of aquaporin 4 by antiepileptic drugs. Bioorg Med Chem 17:418–424
Tang Y, Cai D, Chen Y (2007) Thrombin inhibits aquaporin 4 expression through protein kinase C-dependent pathway in cultured astrocytes. J Mol Neurosci 31:83–93
Competing Interests
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Filippidis, A.S., Kalani, M.Y.S. & Rekate, H.L. Hydrocephalus and aquaporins: lessons learned from the bench. Childs Nerv Syst 27, 27–33 (2011). https://doi.org/10.1007/s00381-010-1227-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-010-1227-6