Abstract
Purpose
To evaluate the clinical features and treatment results of the primary paravertebral malignant tumors (PMTs) in our department.
Methods
Medical records of 28 children with primary PMTs treated between 1988–2007 were analyzed retrospectively.
Results
Primary PMTs constituted 4.8% of the cancer cases in our department. Tumor diagnoses were mostly neuroblastoma (46.4%) and soft tissue sarcomas (35.7%). These cases presented with pain (64.3%), motor dysfunction (42.8%), sphincter dysfunction (35.7%), palpable mass (32.1%), and sensory deficits (7.1%). All tumors were extradural. Physical examination revealed motor deficits (53.6%), deep tendon reflex alterations (53.6%), sphincter dysfunction (35.7%), pathologic reflexes (25%), abnormal cutaneous reflexes (25%), and sensory deficits (17.8%). Sixteen had cord compression (CC; 13 clinical, three radiological CC). Eleven of them presented with advanced disease. Seven were managed by surgical departments by primary surgery (three unresponsive). Others were managed by pediatric oncology: five with corticosteroids ± chemotherapy (one unresponsive), one with radiotherapy (RT), and two with surgery for the clinical CC. Surgery was tumor excision in nine, laminectomy in nine, laminotomy in one, and delayed surgery after chemotherapy in two cases. In chemotherapy and surgery groups, there were neurologic sequela associated with the advanced disease at diagnosis in 38% and 37%, respectively. At 3-year median follow-up, nine patients died, 17 are alive (four with neurologic sequela), and two are lost of follow-up.
Conclusion
Majority of cases presented with advanced disease. Late referral is the major cause of morbidity and mortality. The CC caused by PMTs should be initially managed with corticosteroids ± chemotherapy to avoid the adverse late effects of RT and surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Paravertebral malignant or benign tumors seen in children and adolescents may show extension to spinal canal through intervertebral foramens. These patients commonly present with radicular or back pain and neurological deficits related to spinal cord compression (CC). In any child presenting with these symptoms, paravertebral malignant tumors (PMTs) such as neuroblastoma, ganglioneuroblastoma, soft tissue sarcomas (STS), Ewing sarcoma, primitive neuroectodermal tumor, rhabdomyosarcoma, Langerhans cell histiocytosis (LCH), and more uncommonly vertebral osteosarcoma should be considered. Spinal cord compression is one of the oncologic emergencies, so the diagnosis and appropriate treatment should be made urgently. Here, we reported our clinical experience with paravertebral malignant tumors in children.
Materials and methods
We reviewed the medical records of 575 children and adolescents with malignant tumors (lymphomas and solid tumors) who had been diagnosed and treated in our Pediatric Oncology Department between Jan 1, 1988 and Dec 31, 2007. All patients were under 19 years old. Patients with primary PMTs were enrolled and medical records were retrospectively evaluated regarding to age, gender, and complaints at admission, clinical findings, primary tumor site and extension, diagnostic and therapeutic interventions, histopathological diagnosis, treatment results, and sequelae. Patients with leukemia were not included in this study, because of leukemias were treated in Pediatric Hematology Department in our center. Patients with primary brain or spinal tumors, patients with spinal seeding or relapsed disease, and patients having paravertebral benign tumors were excluded.
Statistical analysis
The Statistical Package for the Social Sciences 11.0 program was used for data analysis. Patient characteristics were summarized using descriptive statistics.
Results
The hospital records of 575 children with lymphomas and solid tumors seen in our department between Jan 1, 1988 and Dec 31, 2007 were evaluated. Of these, 28 (4.8%) children had primary PMTs. The median age of diagnosis was 5.6 years (2 months–17 years), male to female ratio was 1:1. Table 1 shows some clinical features of these patients at admission and the distribution of primary PMT sites.
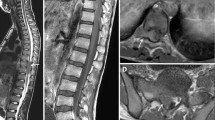
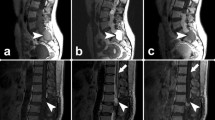
Radiological evaluation was performed by magnetic resonance imaging (MRI) in 24 (86%), by computerized tomography (CT) in 24 (86%), and by direct radiography in 22 (76%). Combined radiography, MRI, and CT evaluation was performed in 21 (75%) children. All primary PMTs were extradural lesions in our patients. Sixteen patients had radiological findings of CC, and 13 (81%) of them also had clinical findings of CC (Table 2). Pathologic findings by neurological examination were as follows: motor deficits in 15 (53.6%), alterations of deep tendon reflexes (DTRs) in 15 (53.6%), bladder sphincter and/or intestinal dysfunction in ten (35.7%), pathologic plantar reflexes in seven (25%), superficial skin reflexes deficits in seven (25%), and sensory deficits in five (17.8%; Table 3).
Sixteen of these patients were primarily admitted to our Pediatric Oncology Department. Seven of these cases had clinical signs of CC and five of them were put on corticosteroid therapy immediately after diagnosis. Twelve patients were initially admitted to surgical departments, and six of them had clinical findings of CC at admission; however, no data was available in medical records regarding the initial corticosteroid administration for these cases (Table 4). Bone marrow examination (BME) was performed in 26 patients, and tumoral involvement was documented in five (19%). The tumor diagnosis was made by histopathologic examination of the tumor after primary surgery in 53.6% patients (Table 4). The diagnoses of patients and the median age at diagnosis are shown in Table 5.
Sixteen (57%) had cord compression (13 clinical, three radiological CC). Eleven of them presented with advanced disease. Primary treatment for patients with radiological ± clinical CC (n = 16) was decompression surgery in ten (complete resection in three, partial resection in seven), chemotherapy in five, and radiotherapy in one (Table 6). Seven were managed by surgical departments by primary surgery (three unresponsive). Others were managed by pediatric oncology; five with corticosteroids ± chemotherapy (one unresponsive), one with radiotherapy, and two with surgery for the clinical CC. One of these two patients who underwent decompressive surgery was initially diagnosed with ganglioneuroma by Trucut biopsy. Following surgical excision, a diagnosis of ganglioneuroblastoma was made and chemotherapy was given to the patient.
Primary treatment for patients without CC (n = 12) were primary surgery in nine (complete resection in five, partial resection in four) and chemotherapy in three (Table 6). Tracheal compression and respiratory distress was the indication for urgent chemotherapy in one infant with cervical ganglioneuroblastoma. Surgery was tumor excision in nine, laminectomy in nine, laminoplasty in one, and delayed surgery after chemotherapy in two cases.
For patients with CC initially, 11 (69%) cases had advanced disease at admission and seven cases (44%) had neurologic sequela despite the treatment. Initial treatment was surgery in four and chemotherapy in three of these seven patients, and three of them also received radiotherapy. Other nine patients in this group were treated without sequela. For patients without CC initially (n = 12), three cases (25%) had neurologic sequela, one related with progression of disease + surgery, one related with surgery, and one related with surgery + radiotherapy.
In chemotherapy and surgery groups, neurological sequelae associated with advanced disease at diagnosis were present in 38% and 37%, respectively. At 3 years of median follow-up, nine patients died, 17 are alive (four with neurological sequela), and two are lost of follow-up.
Discussion
Paravertebral masses may occur as a consequence of benign or malignant tumors. Benign tumors include plexiform neurofibroma, hemangioma, angiolipoma, and osteoblastoma. Also an infectious process, especially paravertebral Mycobacterium tuberculosis abscess (Pott’s disease) should be kept in mind in developing countries. The majority of childhood primary paravertebral tumors are chemosensitive malignant tumors that might cause CC. Cord compression may be the presenting symptom or develop at any time during the course of malignant tumors in children.
Majority of our patients presented with advanced disease and had radicular and/or back pain (64%), motor deficits (42.8%), and bladder and/or bowel dysfunction (35.7%) at admission. The rate of noted sensory deficits was low (7%), which could be related to the difficulties in expressing and/or assessing the sensory deficits experienced by these children. In children, complaints of back pain and/or radicular pain should be taken seriously and spinal CC should be ruled out since these complaints can be the first sign of a paravertebral mass. Local or radicular pain is the most frequent (80%) and early sign of CC [1]. Neurological deficits may appear during progression of the process and may cause irreversible sequela. In patients presented with radicular/back pain and/or neurologic dysfunction, detailed neurologic examination is essential and if indicated, imaging studies should be performed immediately.
Half of our patients had either motor deficits and/or DTR alterations, one third with sphincter dysfunction, one fourth with abnormal cutaneous reflexes, and nearly one fifth with sensory deficits. Clinical cord compression was detected in 13 patients. Radiologic evaluation was performed for all patients. Computerized tomography and MRI studies were the preferred imaging modality for bone and soft tissue involvement, respectively. In 17 patients, both of these imaging modalities were performed. Sixteen (57%) had radiological cord compression and 11 of these patients presented with advanced disease.
All primary PMTs were extradural lesions and sites were T10–L2 vertebral level in 50%, above T10 level in 39.3%, and cauda equina in 10.7%. The level of spinal cord involvement can be determined by clinical examination; however, the presence of a paravertebral mass cannot be excluded by the absence of neurological dysfunction as it had been the case in three of our patients who had only radiological findings of CC.
In nearly half of our cases with primary PMTs, the tumor diagnosis was sympathetic nervous system (SNS) tumors (46.4%). The others had STS (35.7%), LCH (14.3%), and Hodgkin’s lymphoma (3.6%). The diagnosis of 16 patients with CC was SNS tumors in six, STS in six, LCH in three, and Hodgkin’s lymphoma in one. Our results were consistent with the previous reports [1, 2]. Klein et al. [2] reported 113 children with spinal CC and solid malignant tumors including neuroblastoma (n = 32), Ewing’s sarcoma (n = 30), osteogenic sarcoma (n = 16), rhabdomyosarcoma (n = 14), Hodgkin’s disease (n = 8), STS (n = 4), and germ cell tumor (n = 2), hepatoma (n = 1). Pollono et al. [3] reported 70 children with spinal CC and solid malignant tumors including central nervous system tumors (n = 18), STS (n = 17), neuroblastoma (n = 10), LCH (n = 5), and Hodgkin’s lymphoma (n = 2). Bouffet et al. [4] reported 17 children with spinal CC and solid malignant tumors including neuroblastoma (n = 10), Ewing’s sarcoma (n = 4), lymphoma (n = 2), and osteosarcoma (n = 1). Aysun et al. [5] reported 12 children with spinal CC and solid malignant tumors including neuroblastoma (n = 7), non-Hodgkin lymphoma (n = 3), rhabdomyosarcoma (n = 1), and Ewing’s sarcoma (n = 1).
The age of our patients varied with the histological type of the tumor. The age was <2 years in seven of 13 patients with sympathetic nervous system tumors, >10 years in seven of ten patients with soft tissue sarcoma, and >6 years in three of four patients with Langerhans cell histiocytosis. These results were consistent with the common occurrence of these specific childhood tumor types.
Spinal cord compression is one of the oncological emergencies that can be seen at the time of presentation or during oncological treatment because of progressing refractory disease or during relapse. Immediate recognition and appropriate urgent intervention may prevent morbidity. Late referral or delayed diagnosis is the major cause of morbidity and mortality. The tumor may cause CC by infiltrating through intervertebral foraminas or by involving vertebral body. Clinical neurological improvement can be achieved by dexamethasone and/or chemotherapy. Bone marrow examination is necessary and should be performed in children with primary PMTs with a diagnostic aim before corticosteroid therapy since bone marrow findings may change with corticosteroids. The diagnosis of leukemia, lymphoma, and bone marrow metastatic neuroblastoma can be revealed by BM (bone marrow) examination and this prevents unnecessary surgical intervention [6]. Tumoral involvement of BM was detected in five patients and the diagnosis of neuroblastoma was made by bone marrow involvement, radiologic findings, and elevated vanillylmandelic acid level in 24-h urine in one patient. Sixteen patients were initially admitted to our Pediatric Oncology Department, and eight of them received corticosteroid therapy after BM aspiration.
In our cases that were initially managed by surgeons, primary surgery was performed in 100% (12/12) of patients. On the other hand, 44% (seven of 16) of the patients who were initially managed in Oncology Department with equal eventual sequela underwent primary surgery. In chemotherapy and surgery groups, the incidence of neurological sequela associated with advanced disease at diagnosis was high (38% and 37%, respectively). Radiotherapy was given to one patient with LCH at the vertebral body who had a risk for pathologic fracture. There is a difference in the initial management of children with PMTs with or without CC by surgery and oncology departments. However, it is hard to compare the absolute outcomes of chemotherapy, surgery, and radiotherapy. Cord compression caused by PMTs should initially be managed with corticosteroids with an aim to rescue the cord compression. If no response can be obtained with corticosteroids, anticancer chemotherapy is another option to avoid the adverse late effects of radiotherapy and surgery since childhood PMTs are mostly chemosensitive tumors. Leukemia, lymphoma, and stage 4 neuroblastoma can be diagnosed with BM aspiration, and specific chemotherapy can be started immediately [1, 7–11]. Katzenstein et al. [7] reported lower rates of orthopedic sequela in chemotherapy group rather than laminectomy group although similar rates of neurologic recovery for both groups in patients with neuroblastoma. De Bernardi et al. [8] reported that patients with neuroblastoma treated with chemotherapy usually did not require additional therapy. Chemotherapy should be considered as the first choice of treatment for spinal cord compression particularly caused by leukemias and malignant lymphomas [6, 9–11]. In cases that underwent biopsy for a PMT causing clinical cord compression, corticosteroids, or appropriate chemotherapy should be continued while waiting for the histopathological diagnosis. If BM aspiration reveals no diagnosis and if a trucut or open biopsy seems to cause potential morbidity, the most probable tumor diagnosis can be predicted according to the patient’s age, clinical and radiological findings, and chemotherapy can be given.
Late referral or advanced disease-related serious neuropathological findings may require surgical interventions. In children, decompressive laminectomy (posterior approach) is considered as an effective surgical approach for PMTs that causes CC [1, 2, 12]. But since laminectomy can cause spinal instability and growth problems in children, the rationale of surgical management and alternative treatment modalities should be discussed by multidisciplinary teams. In experience of our center, laminectomy was performed in nine patients, but in recent years, laminoplasty was preferred in three cases (primary surgery (one), delayed surgery (two)). The surgical procedure related spinal deformity risk was reported in lower rates by laminoplasty rather than laminotomy [13–15].
Neurologic sequelae might be related with advanced disease and treatment modalities. The neurological status at admission was the main factor determining morbidity. For patients with CC initially, 11 (69%) cases had advanced disease at admission and seven cases (44%) had sequela despite the treatment. Initial treatment was surgery in four and chemotherapy in three of these seven patients. Three of them also received radiotherapy. Surgery- and/or radiotherapy-related neurologic sequela developed in three patients who had no CC at admission, due to treatments (primary surgery (n = 3) and radiotherapy (n = 2)). One of them also had progression of disease. If there are no clinical CC findings and there is no risk of vertebral body collapse, chemotherapy should be considered as an initial treatment for chemosensitive primary PMTs of childhood.
Conclusion
The majority of childhood primary paravertebral tumors are chemosensitive malignant tumors that can cause CC. Neurologic, radiologic evaluation, and oncologic diagnosis should be made immediately. Late referral is the major cause of morbidity and mortality. The PMTs with/without CC should be initially managed with corticosteroids ± chemotherapy to avoid the adverse late effects of RT and surgery. Pediatric patients with paravertebral tumors should be managed by multidisciplinary approach by expert teams consisting of pediatric oncologists, neurosurgeons, radiation oncologists, radiologists, pathologists, and physiotherapists. Treatment should be tailored for each patient and the aim of treatment should include improving quality of life besides cure for children with primary PMTs.
References
Rheingold SR, Lange BJ (2006) Oncologic emergencies. In: Pizzo PA, Poplack DG (eds) Principles and practice of pediatric oncology, 5th edn. Lippincott-Roven, Philedelphia, pp 1202–1230
Klein SL, Sanford RA, Muhlbauer MS (1991) Pediatric spinal epidural metastases. J Neurosurg 74(1):70–75
Pollono D, Tomarchia S, Drut R et al (2003) Spinal cord compression: a review of 70 pediatric patients. Pediatr Hematol Oncol 20:457–466
Bouffet E, Marec-Berard P, Thiesse P et al (1997) Spinal cord compression by secondary epi- and intradural metastases in childhood. Child’s Nerv Syst 13:383–387
Aysun S, Topcu M, Gunay M, Topaloglu H (1994) Neurologic features as initial presentations of childhood malignancies. Pediatr Neurol 10:40–43
Buyukavci M, Karacan M, Olgun H, Tan H (2003) Significance of bone marrow examination in the diagnostic process of paraspinal mass in children: a case report. J Pediatr Hematol Oncol 25(10):822–823
Katzenstein HM, Kent PM, London WB, Cohn SL (2001) Treatment and outcome of 83 children with intraspinal neuroblastoma: the Pediatric Oncology Group experience. J Clin Oncol 19(4):1047–1055
De Bernardi B, Pianca C, Pistamiglio P et al (2001) Neuroblastoma with symptomatic spinal cord compression at diagnosis: treatment and results with 76 cases. J Clin Oncol 19(1):183–190
Matsubara H, Watanabe K, Sakai H et al (2004) Rapid improvement of paraplegia caused by epidural involvements of Burkitt’s lymphoma with chemotherapy. Spine 29(1):E4–E6
Daley MF, Partington MD, Kadan-Lottick N, Odom LF (2003) Primary epidural Burkitt lymphoma in a child: case presentation and literature review. Pediatr Hematol Oncol 20(4):333–338
Ses E, N’dri Oka D, Varlet G et al (2001) Medullary compression by Burkitt lymphoma. Analysis of 7 cases. Neurochirurgie 47(6):552–556
Raffel C, Neave VC, Lavine S, McComb JG (1991) Treatment of spinal cord compression by epidural malignancy in childhood. Neurosurgery 28(3):349–352
McGirt MJ, Chaichana KL, Atiba A et al (2008) Incidence of spinal deformity after resection of intramedullary spinal cord tumors in children who underwent laminectomy compared with laminoplasty. J Neurosurg Pediatrics 1(1):57–62
Ghanem I, Zeller R, Dubousset J (1996) Extra osseous tumors of the spine in children and adolescents. Spinal complications. Rev Chir Orthop Repar Appar Mot 82(4):313–320
Papagelopoulos PJ, Peterson HA, Ebersold MJ, Emmanuel PR, Choudhury SN, Quast LM (1997) Spinal column deformity and instability after lumbar or thoracolumbar laminectomy for intraspinal tumors in children and young adults. Spine 22(4):442–451
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gunes, D., Uysal, K.M., Cetinkaya, H. et al. Paravertebral malignant tumors of childhood: analysis of 28 pediatric patients. Childs Nerv Syst 25, 63–69 (2009). https://doi.org/10.1007/s00381-008-0717-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-008-0717-2