Abstract
Chronic kidney disease (CKD) increases the risk of adverse outcomes in acute coronary syndrome (ACS). The optimal regimen of dual antiplatelet therapy (DAPT) post-percutaneous coronary intervention (PCI) in CKD poses a challenge due to the increased bleeding and clotting tendencies, particularly since patients with CKD were underrepresented in randomized controlled trials. We examined the practice patterns of DAPT prescription stratified by the presence of CKD. The multicentre prospective Canadian Observational Antiplatelet Study (COAPT) enrolled patients with ACS between December 2011 and May 2013. The present study is a subgroup analysis comparing type and duration of DAPT and associated outcomes among patients with and without CKD (eGFR < 60 ml/min/1.73 m2, calculated by CKD-EPI). Patients with CKD (275/1921, 14.3%) were prescribed prasugrel/ticagrelor less (18.5% vs 25.8%, p = 0.01) and had a shorter duration of DAPT therapy versus patients without CKD (median 382 vs 402 days, p = 0.003). CKD was associated with major adverse cardiovascular events (MACE) at 12 months (p < 0.001) but not bleeding when compared to patients without CKD. CKD was associated with MACE in both patients on prasugrel/ticagrelor (p = 0.017) and those on clopidogrel (p < 0.001) (p for heterogeneity = 0.70). CKD was associated with increased bleeding only among patients receiving prasugrel/ticagrelor (p = 0.007), but not among those receiving clopidogrel (p = 0.64) (p for heterogeneity = 0.036). Patients with CKD had a shorter DAPT duration and were less frequently prescribed potent P2Y12 inhibitors than patients without CKD. Overall, compared with patients without CKD, patients with CKD had higher rates of MACE and similar bleeding rates. However, among those prescribed more potent P2Y12 inhibitors, CKD was associated with more bleeding than those without CKD. Further studies are needed to better define the benefit/risk evaluation, and establish a more tailored and evidence-based DAPT regimen for this high-risk patient group.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The prevalence of chronic kidney disease (CKD) continues to increase globally [1] and these patients are at an increased risk of coronary artery disease (CAD), acute coronary syndrome (ACS), and cardiovascular death [2,3,4,5]. Dual antiplatelet therapy (DAPT) consisting of aspirin and a P2Y12 inhibitor is the standard of treatment post-percutaneous coronary intervention (PCI) in ACS. However, little is known regarding the efficacy of DAPT in patients with CKD as they are often underrepresented in randomized controlled trials [3].
Patients with CKD have increased platelet reactivity, heightened inflammation and oxidative stress, endothelial dysfunction, and associated comorbidities that predispose to thrombotic events [2, 6]. On the other hand, they also have increased bleeding risk due to dysregulated platelet function and alteration in drug metabolism [3, 4, 6]. This complex interplay of risk factors poses a unique challenge to clinicians in weighing the risk of thrombotic events against the risk of bleeding.
The current recommended duration of DAPT is 12 months post-ACS, with longer therapy recommended in the absence of increased bleeding risks [7, 8]. As patients with CKD have an increased risk of both thrombotic events and bleeding, it is unclear whether this patient group would derive the same benefit from this guideline-directed treatment based on randomized trials that enrolled only a minority of patients with CKD. This unique patient group may benefit from more individualized DAPT that may differ from that for the general ACS population.
The Canadian Observational Antiplatelet Study (COAPT) is a multicentre prospective cohort study of DAPT practices in a Canadian population. Using data from COAPT, we sought to examine the patterns of antiplatelet therapy after PCI for an ACS in relation to CKD status, and the associated major adverse cardiovascular events (MACE) and bleeding events.
Materials and methods
The methods of the Canadian Observational Antiplatelet Study (COAPT) have been previously described [9]. Briefly, COAPT was a prospective observational cohort study designed to describe the real-world patterns of P2Y12 inhibitor therapy duration after PCI in ACS in Canada. Of the 43 Canadian centres that performed PCI, 26 recruited patients into this study between December 2011 and May 2013, with the goal of enrolling 2200 patients to sufficiently describe DAPT patterns during the 15 month follow-up period.
Eligible patients included those over 18 years of age who were admitted for ST-elevation myocardial infarction (STEMI) or non-ST-elevation myocardial infarction (NSTEMI), and were prescribed antiplatelet therapy post-PCI. Patients with unstable angina were excluded, as were those who were participating in other clinical trials of antiplatelet or anticoagulant therapy. The present study included all participants who had a documented estimated glomerular filtration rate (eGFR). The research ethics board committees at participating institutions approved this study, and informed consent was obtained from all participants.
Participants were enrolled during their admission and followed thereafter with telephone interviews at 6 weeks, 6 months, 12 months, and 15 months. Information obtained included prescription of medications, major cardiovascular events, subsequent cardiac procedures, and bleeding based on patient reporting and verified by the local investigators based on additional relevant hospital medical records.
Primary outcomes included type of DAPT prescription and duration of DAPT prescription. Secondary outcomes included bleeding events or transfusion, and MACE. CKD was defined as an estimated glomerular filtration rate (eGFR) of < 60 ml/min/1.73 m2 as calculated by the CKD-EPI equation [10]. MACE included death, reinfarction, stroke, or urgent revascularization. Other secondary cardiac events included stent thrombosis, cardiogenic shock or new diagnosis or progression of heart failure. Bleeding events were classified as any bleeding event or transfusion which prompted presentation to the emergency department. MACE and bleeding events were analyzed individually. Events were reported by patients and verified by the local investigators, and there was no central adjudication. DAPT therapy comprised of aspirin and a P2Y12 inhibitor (clopidogrel, prasugrel or ticagrelor). Patients were classified by the type of P2Y12 inhibitor prescribed at index admission, and subsequent changes in P2Y12 inhibitor class were not accounted for in the present study. The more potent P2Y12 inhibitors prasugrel and ticagrelor were combined as one group for analysis in the present study.
Continuous variables were described with mean and standard deviation, or median and interquartile values, and compared using Mann–Whitney test. Categorical variables were reported as percentages and compared using chi-squared tests, and homogeneity across subgroups was evaluated using the Breslow–Day test. Multivariable logistic regression was utilized to assess the independent association of CKD with MACE and bleeding. Variables adjusted for when assessing the independent association between CKD and MACE included the GRACE risk score components of age, heart rate, systolic blood pressure, Killip class, cardiac arrest on presentation, and ST segment deviation [11]. Variables adjusted for when assessing the independent association between CKD and bleeding included age, previous GI bleeding event and gender [12] and type of ADPRi. We also tested for an interaction between CKD and type of ADRPi. Statistical analyses were completed using SPSS version 25 and statistical significance was defined as a two-sided P value at < 0.05.
Results
Of the 2179 participants enrolled, 1921 had available information regarding eGFR. Characteristics of the study population are outlined in Table 1. Of the 1921 participants, 275 (14%) had CKD. Overall, patients with CKD were older, more frequently female, and had higher prevalence of cerebrovascular disease, heart failure, hypertension, and previous MI/ACS. They were also more likely to have cardiac risk factors including diabetes, dyslipidemia, and current smoking, and previously documented gastrointestinal bleeding. Additionally, patients with CKD were more likely to have atrial fibrillation, and to be discharged on an oral anticoagulant and a proton pump inhibitor. Of the patients with CKD, 176 had an eGFR 45–59 (CKD Stage 3a), 68 had an eGFR 30–44 (stage 3b), 22 had an eGFR 15–29 (stage 4), and 9 had an eGFR of < 15 (Stage 5). At the 12 month follow-up period, data were missing in 35 participants for MACE and 134 for DAPT duration.
Patients with CKD were less likely than patients without CKD to be prescribed the more potent P2Y12 inhibitors prasugrel and ticagrelor [51/275 (18.5%) vs 425/1646 (25.8%), p = 0.01].
Duration of DAPT therapy was shorter in patients with CKD [median 382 days (IQR 239–459)] compared to patients without CKD [median 402 days (IQR 365–462), p = 0.003]. There was no difference in the duration of clopidogrel versus the more potent P2Y12 inhibitors in patients with CKD [386 days (IQR 233–460) versus 373 days (IQR 281–450), respectively).
Table 2 summarizes the reasons for discontinuation of DAPT in patients without CKD and with CKD. There was no significant difference in discontinuation due to adverse events, requirement for coronary artery bypass graft or surgery between patients with and without CKD. Discontinuation due to the need for an oral anticoagulant agent was more likely in patients with CKD. Patients with CKD more frequently were considered by their physicians to have an indication for ongoing DAPT therapy.
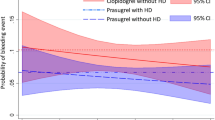
Figure 1 compares unadjusted MACE and bleeding events in patients discharged on each P2Y12 agent by CKD status. Patients with CKD more frequently had a MACE at 12 months versus patients without CKD (28.0% vs 16.0%, p < 0.001), and this remained significant after adjusting for other confounders (adjusted OR 1.73, 95% CI 1.17–2.56, p = 0.013). CKD was associated with MACE in both patients discharged on prasugrel/ticagrelor (13.1% vs 25.5%, p = 0.017) and those discharged on clopidogrel (17.0% vs 28.6%, p < 0.001) (p for heterogeneity = 0.70).
Overall, unadjusted bleeding event rates did not differ between patients with and without CKD (29.8% vs 25.6%, p = 0.16) and this remained unchanged after adjusting for confounders (adjusted OR 0.98, 95% CI 0.69–1.40 p = 0.93). CKD was associated with increased bleeding only among patients discharged on prasugrel/ticagrelor CKD in unadjusted analysis (49.0% vs 30.4%, p = 0.007), but not among those discharged on clopidogrel (24.0% vs 25.4%, p = 0.64) (p for heterogeneity = 0.036). In adjusted analysis, CKD was not independently associated with the outcome of bleeding when adjusting for P2Y12 inhibitor type, age, sex, and previous bleeding, but there was a significant interaction when assessing the outcome of bleeding with both CKD and potent P2Y12 inhibitors, with an increased risk of bleeding (p = 0.033).
Patients with CKD who were prescribed an oral anticoagulant [13] had a shorter duration of DAPT versus patients without CKD on an OAC [346 days (IQR 71–427) vs 393 (IQR 345–456), p = 0.001]. Of the patients prescribed an OAC, the use of more potent P2Y12 inhibitors was similar in the presence or absence of CKD [11/53 (20.8%) vs 58/216 (26.9%), p = 0.48, respectively]. Patients prescribed an OAC also had a similar bleeding rate between patients with and without CKD at 12 months [26/53 (49.1%) vs 89/216 (41.2%), p = 0.35, respectively].
Discussion
In this prospective multicentre Canadian observational study of the utilization of DAPT following PCI for ACS, patients with CKD were less likely to receive the more potent P2Y12 inhibitors than their non-CKD counterparts, and were prescribed dual antiplatelet therapy for a shorter duration. Patients with CKD were older, had more comorbid conditions and had a higher rate of ischemic events after PCI. Bleeding events were associated with CKD on the more potent P2Y12 agents compared with patients without CKD.
Subgroup analyses of the randomized controlled trials PLATO and TRITON-TIMI indicate that patients with CKD have a similar benefit to patients without CKD when prescribed ticagrelor and prasugrel post-PCI, with reduced MACE versus clopidogrel [14]. Higher platelet reactivity is a potential factor contributing to the decreased effectiveness of clopidogrel in patients with CKD [6, 15,16,17]. The benefits of decreased MACE with more potent P2Y12 inhibitors must be weighed against the increased bleeding risk in patients with CKD [18, 19]. Observational studies have shown an independent association between use of more potent P2Y12 agents and an increase in bleeding in patients with CKD [3, 20] and it has been postulated that patients with CKD have a 50% relative increase in bleeding risk with these potent P2Y12 inhibitors [19]. There is a lack of randomized controlled trial data to assess bleeding risk with these potent P2Y12 agents in CKD but subgroup analyses of PLATO indicate there is a similar bleeding risk in patients with and without CKD while on ticagrelor, which is not consistent with the increased bleeding risk seen in observational studies [14].
CKD was not associated with bleeding in the present study despite the established increased risk of bleeding in this patient population, particularly post-PCI [21]. This may be attributed to the relatively low number of patients in the present study with CKD and insufficient power to detect any difference. Additionally, bleeding risk tends to increase with CKD severity [22] and in the present study, there was a relatively low number of patients with advanced CKD. There was also likely an element of selection bias where patients with both CKD and those who were deemed to have a high-bleeding risk might not have been eligible for PCI and thus not enrolled in the present study. With the present multivariable logistic regression, there was an interaction when assessing the outcome of bleeding with both CKD and more potent P2Y12 inhibitor, indicating an increased risk of bleeding. Although patients with CKD had a similar rate of bleeding events when compared to patients without CKD while on prasugrel or ticagrelor, this might be partially attributed to the lower rate of potent P2Y12 prescription in patients with CKD. This might reflect physician selection of patients with CKD with a lower bleeding risk for these more potent P2Y12 inhibitors. The rationale for lower use of more potent P2Y12 inhibitors and other treatments to avoid bleeding events in CKD has been proposed in previous observational studies [3]. There were likely other confounding factors in the present observational study. Identifying these factors in future studies would assist in understanding bleeding risk in patients with CKD in clinical practice.
The recommended duration of DAPT post-PCI for ACS according to the current Canadian Cardiovascular Society and American College of Cardiology guidelines is a minimum 12 months of therapy, with continuation after 12 months based on an assessment of the risks of bleeding and ischemic events [7, 8]. Several tools exist to guide use of DAPT beyond 1 year [23, 24]. However, patients with CKD have higher risks for both MACE and bleeding which complicate the clinical decision making for DAPT duration. Previous meta-analyses have compared the duration of DAPT in patients with CKD and shown that a shorter duration of DAPT < 6 months may not confer an increased risk of MACE in patients with CKD [25, 26], and longer duration of DAPT is associated with an increased risk of bleeding [19, 21, 27], though the majority of these studies did not utilize the more potent P2Y12 inhibitors. Recently, there have been randomized controlled trials with inclusion of a small cohort with CKD which assessed the protective effects of shorter DAPT with ongoing monotherapy with a P2Y12 inhibitor rather than aspirin with decreased MACE and bleeding [28], though guidelines have not yet adopted P2Y12 monotherapy. In the present analysis, DAPT was stopped earlier in patients with CKD compared to patients without CKD.
A treatment-risk paradox describes how patients with higher cardiovascular risk have a decreased likelihood of receiving recommended therapy, such as PCI or pharmacologic treatment for ACS [29]. Despite the evidence showing that the more novel P2Y12 inhibitors may be more effective in patients with CKD, the PROMETHEUS registry of patients post-ACS demonstrated the decreased prescription of prasugrel in patients with CKD when compared to patients without CKD [3]. This is consistent with the findings in the present study, as we found decreased prescription of the more potent P2Y12 agents versus clopidogrel. There is a paucity of randomized controlled trial data assessing DAPT for patients with CKD post-PCI and ACS and it is unclear whether current guidelines should directly apply to patients with CKD, both in terms type of P2Y12 inhibitor recommended and duration of treatment. CKD severity is another additional factor that complicates the risks of MACE and bleeding [2, 20]. The planned randomized controlled trial to assess ticagrelor versus clopidogrel in patients with CKD post-PCI will be important in clarifying the cardiovascular protection and bleeding risks with ticagrelor in CKD stage > 2 [30] but further randomized controlled trials assessing DAPT duration, use of potent P2Y12 inhibitors, P2Y12 monotherapy in relation to CKD severity are warranted.
Previous observational studies that assessed DAPT prescription in patients with CKD showed a higher rate of disruption/discontinuation in patients with CKD, with a proposed reason for early discontinuation being an increased risk of bleeding [21]. In contrast, in the present study, there was no difference in the discontinuation of DAPT due to documented adverse events between patients with and without CKD. There was, however, an increased DAPT cessation in patients with CKD due to the prescription of an oral anticoagulant, likely related to a higher prevalence of atrial fibrillation. Although physicians more frequently assessed that patients with CKD had an ongoing indication for DAPT versus patients without CKD, patients with CKD had a shorter duration of DAPT. This discrepancy may be partially attributed to confounders such as other comorbidities and anticoagulant prescription. Future large registries will provide additional insight into the contemporary real-world use of DAPT in CKD patients.
This study needs to be considered in light of several limitations. The number of patients who had CKD and the number of patients on ticagrelor or prasugrel were relatively small. Patients were stratified by the presence/absence of CKD and not by CKD severity. With the observational nature of this study we also cannot infer causality between DAPT and outcomes. The present analysis did not account for changes between P2Y12 inhibitor type during the follow-up period, and adherence was not verified with additional sources such as prescription filling. Practice guidelines have been subsequently updated and the present study might not reflect current practice, but differences in prescription practices between patients with and without CKD are present nonetheless and this analysis may serve as a useful benchmark for future comparison. Despite these limitations, this observational study highlights the real-world practice of DAPT post-PCI, which is helpful in identifying how guidelines are applied in patients with CKD and how this differs from the treatment of non-CKD patients.
In conclusion, ACS patients with CKD had a higher rate of major adverse cardiovascular events post-PCI treatment, but were less likely to receive more potent P2Y12 inhibitors or prolonged DAPT. These findings reflect the uncertainties and complexities surrounding the optimal treatment for ACS patients with CKD, and further support the need for randomized controlled trials and large registries to address these important knowledge gaps and to develop specific guidelines for DAPT in this unique patient group.
Conflict of interest
Carol Anne Graham, no disclosures. Mary K. Tan, no disclosures. Derek P. Chew has received speaker/consulting honoraria and/or research grant support from AstraZeneca. Christopher P. Gale, no disclosures. Keith A. Fox has received speaker/consulting honoraria and/or research grant support from AstraZeneca, Bayer, Janssen, Regeneron, Verson and Sanofi. Akshay Bagai has received speaker/consulting honoraria and/or research grant support from Abbott Vascular, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb/Pfizer, HLS therapeutics, Servier. Mark A. Henderson, no disclosures. Ata ur Rehman Quraishi, no disclosures. Jean-Pierre Dery, no disclosures. Asim N. Cheema, no disclosures. Harold Fisher has received medical officer/salary support from Eli Lilly. David Breiger, no disclosures. Sohrab Lutchmedial, no disclosures. Shahar Lavi, no disclosures. Brian Y. Wong, no disclosures. Tomas Cieza has received speaker/consulting honoraria and/or research grant support from AstraZeneca and Eli Lilly. Shamir R. Mehta has received speaker/consulting honoraria and/or research grant support from AstraZeneca, Boston Scientific, Eli Lilly and Sanofi. Neil Brass, no disclosures. Shaun G. Goodman has received speaker/consulting honoraria and/or research grant support from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, CSL Behring, Daiichi Sankyo/American Regent, Eli Lilly, Esperion, Ferring Pharmaceuticals, GlaxoSmithKline, HLS Therapeutics, JAMP Pharma, Janssen/Johnson & Johnson, Merck, Novartis, Novo Nordisk A/C, Pendopharm, Pfizer, Regeneron, Sanofi, Servier, Valeo Pharma. Andrew T. Yan, research grant support and/or speaking consulting honoraria from AstraZeneca.
References
GBD Chronic Kidney Disease Collaboration (2020) Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 395(10225):709–733
Dumaine RL, Montalescot G, Steg PG, Ohman EM, Eagle K, Bhatt DL, REACH Registry Investigators (2009) Renal function, atherothrombosis extent, and outcomes in high-risk patients. Am Heart J 158(1):141–148.e1
Baber U, Chandrasekhar J, Sartori S, Aquino M, Kini AS, Kapadia S, Weintraub W, Muhlestein JB, Vogel B, Faggioni M (2017) Associations between chronic kidney disease and outcomes with use of prasugrel versus clopidogrel in patients with acute coronary syndrome undergoing percutaneous coronary intervention: a report from the PROMETHEUS study. JACC Cardiovasc Interv 10(20):2017–2025
Baber U, Mehran R, Kirtane AJ, Gurbel PA, Christodoulidis G, Maehara A, Witzenbichler B, Weisz G, Rinaldi MJ, Metzger DC (2015) Prevalence and impact of high platelet reactivity in chronic kidney disease: results from the assessment of dual antiplatelet therapy with drug-eluting stents registry. Circ Cardiovasc Interv 8(6):e001683
Tomaniak M, Chichareon P, Klimczak-Tomaniak D, Takahashi K, Kogame N, Modolo R, Wang R, Ono M, Hara H, Gao C (2020) Impact of renal function on clinical outcomes after PCI in ACS and stable CAD patients treated with ticagrelor: a prespecified analysis of the GLOBAL LEADERS randomized clinical trial. Clin Res Cardiol 109(7):930–943
Htun P, Fateh-Moghadam S, Bischofs C, Banya W, Müller K, Bigalke B, Stellos K, May AE, Flather M, Gawaz M (2011) Low responsiveness to clopidogrel increases risk among CKD patients undergoing coronary intervention. J Am Soc Nephrol 22(4):627–633
Mehta SR, Bainey KR, Cantor WJ, Lordkipanidzé M, Marquis-Gravel G, Robinson SD, Sibbald M, So DY, Wong GC, Abunassar JG (2018) 2018 Canadian Cardiovascular Society/Canadian association of interventional cardiology focused update of the guidelines for the use of antiplatelet therapy. Can J Cardiol 34(3):214–233
Levine GN, Bates ER, Bittl JA, Brindis RG, Fihn SD, Fleisher LA, Granger CB, Lange RA, Mack MJ, Mauri L (2016) 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 68(10):1082–1115
Sra S, Tan MK, Mehta SR, Fisher HN, Déry J-P, Welsh RC, Eisenberg MJ, Overgaard CB, Rose BF, Della Siega AJ (2016) Ischemic and bleeding events in patients with myocardial infarction undergoing percutaneous coronary intervention who require oral anticoagulation: Insights from the Canadian observational AntiPlatelet sTudy. Am Heart J 180:82–89
Levey AS, Stevens LA, Schmid CH, Zhang Y, Castro AF III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T (2009) A new equation to estimate glomerular filtration rate. Ann Intern Med 150(9):604–612
Fox KA, Dabbous OH, Goldberg RJ, Pieper KS, Eagle KA, Van de Werf F, Avezum Á, Goodman SG, Flather MD, Anderson FA Jr, Granger C (2006) Prediction of risk of death and myocardial infarction in the six months after presentation with acute coronary syndrome: prospective multinational observational study (GRACE). BMJ 333(7578):1091
Moscucci M, Fox KA, Cannon CP, Klein W, López-Sendón J, Montalescot G, White K, Goldberg RJ (2003) Predictors of major bleeding in acute coronary syndromes: the Global Registry of Acute Coronary Events (GRACE). Eur Heart J 24(20):1815–1823
Marx N, Noels H, Jankowski J, Floege J, Fliser D, Böhm M (2018) Mechanisms of cardiovascular complications in chronic kidney disease: research focus of the Transregional Research Consortium SFB TRR219 of the University Hospital Aachen (RWTH) and the Saarland University. Clin Res Cardiol 107(2):120–126
Bonello L, Angiolillo DJ, Aradi D, Sibbing D (2018) P2Y12-ADP receptor blockade in chronic kidney disease patients with acute coronary syndromes: review of the current evidence. Circulation 138(15):1582–1596
Angiolillo DJ, Bernardo E, Capodanno D, Vivas D, Sabaté M, Ferreiro JL, Ueno M, Jimenez-Quevedo P, Alfonso F, Bass TA (2010) Impact of chronic kidney disease on platelet function profiles in diabetes mellitus patients with coronary artery disease taking dual antiplatelet therapy. J Am Coll Cardiol 55(11):1139–1146
Morel O, El Ghannudi S, Jesel L, Radulescu B, Meyer N, Wiesel M-L, Caillard S, Campia U, Moulin B, Gachet C (2011) Cardiovascular mortality in chronic kidney disease patients undergoing percutaneous coronary intervention is mainly related to impaired P2Y12 inhibition by clopidogrel. J Am Coll Cardiol 57(4):399–408
Stone GW, Witzenbichler B, Weisz G, Rinaldi MJ, Neumann F-J, Metzger DC, Henry TD, Cox DA, Duffy PL, Mazzaferri E (2013) Platelet reactivity and clinical outcomes after coronary artery implantation of drug-eluting stents (ADAPT-DES): a prospective multicentre registry study. Lancet 382(9892):614–623
Baber U, Mehran R, Giustino G, Cohen DJ, Henry TD, Sartori S, Ariti C, Litherland C, Dangas G, Gibson CM (2016) Coronary thrombosis and major bleeding after PCI with drug-eluting stents: risk scores from PARIS. J Am Coll Cardiol 67(19):2224–2234
Palmer SC, Di Micco L, Razavian M, Craig JC, Perkovic V, Pellegrini F, Copetti M, Graziano G, Tognoni G, Jardine M (2012) Effects of antiplatelet therapy on mortality and cardiovascular and bleeding outcomes in persons with chronic kidney disease: a systematic review and meta-analysis. Ann Intern Med 156(6):445–459
Rymer JA, Kaltenbach LA, Doll JA, Messenger JC, Peterson ED, Wang TY (2019) Safety of dual‐antiplatelet therapy after myocardial infarction among patients with chronic kidney disease. J Am Heart Assoc 8(10):e012236
Baber U, Li SX, Pinnelas R, Pocock SJ, Krucoff MW, Ariti C, Gibson CM, Steg PG, Weisz G, Witzenbichler B (2018) Incidence, patterns, and impact of dual antiplatelet therapy cessation among patients with and without chronic kidney disease undergoing percutaneous coronary intervention: results from the PARIS Registry (Patterns of Non-Adherence to Anti-Platelet Regimens in Stented Patients). Circ: Cardiovasc Interv 11(3):e006144
Ocak G, Rookmaaker M, Algra A, De Borst G, Doevendans P, Kappelle L, Verhaar M, Visseren F, Group SS, van der Graaf Y (2018) Chronic kidney disease and bleeding risk in patients at high cardiovascular risk: a cohort study. J Thromb Haemost 16(1):65-73
Costa F, van Klaveren D, James S, Heg D, Räber L, Feres F, Pilgrim T, Hong M-K, Kim H-S, Colombo A (2017) Derivation and validation of the predicting bleeding complications in patients undergoing stent implantation and subsequent dual antiplatelet therapy (PRECISE-DAPT) score: a pooled analysis of individual-patient datasets from clinical trials. Lancet 389(10073):1025–1034
Yeh RW, Secemsky EA, Kereiakes DJ, Normand S-LT, Gershlick AH, Cohen DJ, Spertus JA, Steg PG, Cutlip DE, Rinaldi MJ (2016) Development and validation of a prediction rule for benefit and harm of dual antiplatelet therapy beyond 1 year after percutaneous coronary intervention. JAMA 315(16):1735–1749
Mavrakanas TA, Chatzizisis YS, Gariani K, Kereiakes DJ, Gargiulo G, Helft G, Gilard M, Feres F, Costa RA, Morice M-C (2019) Duration of dual antiplatelet therapy in patients with CKD and drug-eluting stents: a meta-analysis. J Am Soc Nephrol 14(6):810–822
Hwang D, Park KW, Lee JM, Rhee T-M, Hong M-K, Jang Y, Valgimigli M, Colombo A, Gilard M, Palmerini T (2018) Efficacy and safety of dual antiplatelet therapy after coronary stenting in patients with chronic kidney disease. Am Heart J 197:103–112
Montalescot G, Brieger D, Dalby AJ, Park S-J, Mehran R (2015) Duration of dual antiplatelet therapy after coronary stenting: a review of the evidence. J Am Coll Cardiol 66(7):832–847
O’Donoghue ML, Murphy SA, Sabatine MS (2020) The safety and efficacy of aspirin discontinuation on a background of a P2Y12 inhibitor in patients after percutaneous coronary intervention: a systematic review and meta-analysis. Circulation 142(6):538–545
Yan AT, Yan RT, Tan M, Huynh T, Soghrati K, Brunner LJ, DeYoung P, Fitchett DH, Langer A, Goodman SG (2007) Optimal medical therapy at discharge in patients with acute coronary syndromes: temporal changes, characteristics, and 1-year outcome. Am Heart J 154(6):1108–1115
Laine M, Lemesle G, Burley S, Cayla G, Range G, Quaino G, Canault M, Pankert M, Paganelli F, Puymirat E, Bonello L (2020) TicagRelor or Clopidogrel in severe or terminal chronic kidney patients undergoing PERcutaneous coronary intervention for acute coronary syndrome: the TROUPER trial. Am Heart J 225:19–26
Acknowledgements
The authors acknowledge editorial assistance by Sue Francis.
Funding
The COAPT study was sponsored by Eli Lilly and Daiichi Sankyo.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Graham, C.A., Tan, M.K., Chew, D.P. et al. Use and outcomes of dual antiplatelet therapy for acute coronary syndrome in patients with chronic kidney disease: insights from the Canadian Observational Antiplatelet Study (COAPT). Heart Vessels 37, 1291–1298 (2022). https://doi.org/10.1007/s00380-022-02029-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-022-02029-8