Abstract
As highly sensitive and specific markers of myocardial damage, cardiac troponins were demonstrated to correlate with clinical parameters of patients with hypertrophic cardiomyopathy. However, the relationship between cardiac troponins and presence of non-sustained ventricular tachycardia (NSVT) in hypertrophic cardiomyopathy remains unclear. The aim of our study was to explore the association between serum cardiac troponin I (cTNI) and presence of NSVT in patients with hypertrophic obstructive cardiomyopathy (HOCM). A total of 309 HOCM patients were enrolled in our study. All participants underwent clinical evaluations, including collections of medical history, blood tests, 24-h Holter monitoring, echocardiography, and cardiac magnetic resonance imaging. There were 53 (17.2%) patients with NSVT and 256 patients without it. Compared to patients without NSVT, serum cTNI (P < 0.001) and plasma NT-proBNP (P = 0.042) were significantly higher in patients with NSVT. Moreover, cTNI and NT-proBNP were positively correlated with left atrial diameter, maximum wall thickness (MWT), left ventricular volume index and left ventricular mass index. In multivariable logistic analysis, log cTNI [odds ratio (OR) = 2.408, 95% confidence interval (CI) 1.108–5.325, P = 0.027], left ventricular end-diastole diameter (OR = 0.922, 95%CI 0.856–0.994, P = 0.034), MWT (OR = 1.131, 95%CI 1.035–1.235, P = 0.006) and left ventricular end-systole volume index (OR = 1.060, 95%CI 1.025–1.096, P = 0.001) were independent determinants of NSVT occurrence after adjustment for potential cofounders. Serum cTNI level was elevated in patients with NSVT. And it was independently associated with NSVT in patients with HOCM. Our results suggest that it may be more reasonable for HOCM patients with elevated serum cTNI to extend the time of Holter monitoring.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hypertrophic cardiomyopathy (HCM) is the most common genetic heart disease with a prevalence of 1:500 in general population [1,2,3]. Approximately, two-thirds of patients with HCM are manifested with obstruction of the left ventricular outflow tract (LVOT) resulted from asymmetric septal hypertrophy, which is referred to as hypertrophic obstructive cardiomyopathy (HOCM) [4]. Moreover, HCM is also a frequent cause of sudden cardiac death (SCD) in young individuals [3,4,5]. With a prevalence of 20–30%, non-sustained ventricular tachycardia (NSVT) was an important risk factor for SCD and death from cardiovascular causes in patients with HCM [5,6,7,8,9]. Therefore, it is crucial for longitudinal surveillance and management strategies in HCM patients to early recognize the predisposing factors to NSVT.
Cardiac troponins, including cardiac troponin I (cTNI) and cardiac troponin T (cTNT), are highly sensitive and specific markers of myocardial injury [10, 11]. Various studies demonstrated that serum levels of cardiac troponins were elevated in patients with HCM, and associated with left ventricular mass index (LVMI), maximum wall thickness (MWT), left ventricular (LV) diastolic and systolic dysfunction, left atrial size and age [12,13,14,15,16]. Furthermore, cardiac troponins were valuable prognostic markers of adverse cardiovascular events in HCM [10, 17]. However, it is unclear whether cardiac troponins were associated with NSVT in patients with HCM. Hence, we sought to elucidate the association between cTNI and NSVT in a relatively large series of consecutive patients with HOCM.
Methods
Our study was approved by the Ethics Committee of Fuwai Hospital (Beijing, China) and conducted in accordance with the Declaration of Helsinki. The informed consents were written by all patients.
Study population
Consecutive patients with HOCM who referred to Fuwai Hospital from January 2014 to June 2016 were enrolled in our study. The diagnostic criteria of HOCM complied with the recommendations of 2011 American Heart Association/American College of Cardiology [18] and 2014 European Society of Cardiology [6], which mainly include an otherwise unexplained increase in septal thickness (≥ 15 or ≥ 13 mm with an unequivocal family history of HCM). The obstruction of LVOT was defined as a peak instantaneous LVOTG pressure gradient (LVOTPG) ≥ 30 mmHg at rest or during physiological provocation, such as standing, exercise, and Valsalva manoeuver. All participants underwent 24-h Holter monitoring and cardiac magnetic resonance (CMR) imaging. Exclusion criteria included cardiac valve disease, acute coronary syndrome, old myocardial infarction, coronary stent implantation, LV ejection fraction (LVEF) < 50%, infection, connective tissue disease and pregnancy. Additionally, patients with a history of septal ablation, septal myectomy or permanent mechanical device implantation were excluded. Eventually, 152 patients ruled out, and 309 patients were recruited into our study, including 256 HOCM patients without NSVT and 53 HOCM patients with NSVT (Fig. 1). Non-sustained ventricular tachycardia (NSVT) was defined as three or more ventricular extrasystoles at a rate of ≥ 120 beats per minute, lasting < 30 s (Fig. 2) [19].
Representative electrocardiography images of SVT (a) and NSVT (b) in patients with HOCM. SVT supraventricular tachycardia; other abbreviations as in Fig. 1
Echocardiography
As the recommendations of the American Society of Echocardiography [20], echocardiography was performed by an experienced cardio-sonographer with an iE33 Color Doppler Ultrasound System (Philips Healthcare, Andover, MA, USA). Two dimensional, M-mode images and Doppler tracings were obtained in a standard manner. Resting LVOTPG of all participants was evaluated with continuous-wave Doppler echocardiography, while provoked LVOTPG was only determined in patients with a resting LVOTPG < 50 mmHg. Complying with the criteria of European Association of Echocardiography, the severity of mitral regurgitation, graded from mild to severe, was assessed semi-quantitatively with color Doppler flow imaging [21].
CMR imaging
CMR imaging examinations were performed on a 1.5-T scanner (Magnetom Avanto, Siemens Medical Solutions, Erlangen, Germany), as described previously [12]. Briefly, a true fast imaging with steady-state precession (TrueFisp) sequence of heart were applied in LVOT view, LV short-axis views, and LV two-chamber and four-chamber long-axis views. CMR imaging parameters as follow: field of view (FOV) 360 × 315 mm2, image matrix 192 × 162, flip angle 70°, slice thickness 6–8 mm, Time-to-repetition (TR) 2.7 ms, Time-to-echo (TE) 1.2 ms, temporal resolution 45 ms. Imaging analysis was performed by an experienced radiologist with dedicated software (version VE36A, ARGUS, Siemens, Germany). LV end-diastole volume (LVEDV), LV end-systole volume (LVESV), stroke volume, cardiac output, LVEF, and LV mass (LVM) were calculated in a standard fashion. LVM was the product of the LV myocardial volume (determined at end-diastole) multiplied by the specific gravity of myocardium (1.05 g/mL). All those parameters were indexed to body surface area, except LVEF. The LV end-diastole diameter (LVEDD) and MWT were determined on the short-axis views at end-diastole.
Laboratory tests
Fasting venous blood samples of all participants were collected within 2 days of echocardiography and 1 week of CMR imaging. All blood samples were immediately analyzed by experienced medical technologists unaware of studied patients in the clinical laboratory of Fuwai Hospital. Serum levels of cTNI were determined with immunochemiluminometric assays (Access AccuTnI, Beckman Coulter, CA, USA). Electrochemiluminescent immunoassays (Elecsys proBNP II assay, Roche Diagnostics, Mannheim, Germany) were used to measure the plasma levels of N-terminal pro-B-type natriuretic peptide (NT-proBNP).
Statistical analysis
Data were expressed as mean ± standard deviation or median (interquartile range) for continuous variables and counts (percentages) for categorical variables. Logarithmic transformation was performed to acquire normal distribution for cTNI and NT-proBNP. The unpaired student’s t test or Mann–Whitney U test was used to compare continuous variables between NSVT and non-NSVT as appropriate, and categorical variables were compared using χ2 test or Fisher’s exact test. Pearson correlation analysis was performed to estimate the correlations between log cTNI or log NT-proBNP and other continuous variables. Univariable and multivariable binary logistic regression analyses were used to determine factors associated with NSVT. Variables with a P value < 0.10 in univariable analysis or those published on previous literature were included in multivariable analysis. Eventually, log cTNI, LVEDD, MWT, and LVESV index were entered into multivariable logistic regression model. A two-tailed P value < 0.05 was considered as statistically significant. Statistical analyses were performed using SPSS (version 25.0; SPSS Inc, Chicago, IL, USA).
Results
A total of 309 HOCM patients were recruited in our study, including 175 males (56.6%). Mean age and resting LVOTG were 49.1 ± 11.7 years and 72.6 ± 36.1 mmHg, respectively. All patients underwent 24-h Holter monitoring, and 53 patients (17.2%) affected with NSVT. The demographic and clinical characteristic of HOCM patients with or without NSVT are depicted in Table 1. Patients with NSVT had higher prevalence of family history of HCM than those without NSVT (P = 0.023) (Fig. 3a). Compared to patients without NSVT, patients with NSVT have significantly greater serum cTNI (P < 0.001) (Fig. 3b) and plasma NT-proBNP (P = 0.042) (Fig. 3c). Additionally, a trend toward higher heart rate in patients with NSVT was observed (P = 0.066). However, there were no significant differences with the respect of age, body mass index (BMI), blood pressure, syncope, atrial fibrillation, family history of SCD, cardiovascular risk factor, and medications.
Prevalence of HCM family history (a), serum levels of cTNI (b), plasma levels of NT-proBNP (c), maximum wall thickness (d), LVESV index (e), and LVM index (f) in HOCM patients with or without NSVT. cTNI cardiac troponin I, NT-proBNP N-termimal pro-B-type natriuretic peptide, LVESV left ventricular end-systole volume, LVM left ventricular mass; other abbreviations as in Fig. 1
Echocardiographic and CMR parameters of patients with or without NSVT are represented in Table 2. Patients with NSVT were more likely to have greater MWT (25.4 ± 4.7 mm vs 22.9 ± 4.2 mm, P < 0.001) (Fig. 3d), LVESV index (28.6 ± 16.8 mL/m2 vs 20.9 ± 7.8 mL/m2, P = 0.003) (Fig. 3e) and LVMI (99.2 ± 34.3 g/m2 vs 86.2 ± 28.6 g/m2, P = 0.007) (Fig. 3f), compared to patients without NSVT. Moreover, patients with NSVT appear to have greater LVEDV index (P = 0.054). Nevertheless, LVEF was significantly lower in patients with NSVT than those without NSVT (P = 0.007). In the terms of systolic anterior motion, moderate or severe mitral regurgitation, resting LVOTPG, left atrial diameter, stroke volume index, and cardiac index, there were no differences between patients with NSVT and patients without NSVT. The representative CMR images of HOCM patients with and without NSVT are shown in Fig. 4.
Representative CMR images of HOCM patients with and without NSVT. End-diastole 4-chamber and short-axis view cine images of a 32-year-old female HOCM patient without NSVT (a–d), and her serum cTNI level was 0.003 ng/mL. The similar view cine images of a 48-year-old female patient with NSVT (e–h) and her serum cTNI level was significantly elevated at 0.142 ng/mL. CMR, cardiac magnetic resonance; other abbreviations as in Fig. 1
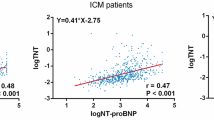
Univariable analysis of correlation between log cTNI or log NT-proBNP and other continuous variables is reported in Table 3. Log cTNI was negatively correlated with Age and LVEF (r = − 0.183, P = 0.003), while positively with log NT-proBNP (r = 0.420, P < 0.001), left atrial diameter, MWT (r = 0.342, P < 0.001), LVESV index (r = 0.249, P < 0.001), LVEDV index (r = 0.227, P < 0.001), and LVMI (r = 0.444, P < 0.001). Log NT-proBNP was positively correlated with resting LVOTPG, left atrial diameter, MWT, LVEDV index, LVESV index, cardiac index, and LVMI, whereas, negatively with BMI, blood pressure.
To clarify the association between cTNI and NSVT in HOCM patients, univariable and multivariable binary logistic regression analyses were performed. As shown in Table 4, family history of HCM (P = 0.025), log cTNI, log NT-proBNP (P = 0.027), MWT, LVEF (P = 0.001), LVEDV index (P = 0.013), LVESV index, and LVMI (P = 0.008) were significantly associated with NSVT in univariable logistic regression model. Nevertheless, age, sex, BMI, hypertension, diabetes mellitus, coronary heart disease, family history of SCD, syncope, atrial fibrillation, beta-blockers, non-dihydropyridine CCB, left atrial diameter, and LVEDD were not related with NSVT. However, after adjustment for potential cofounders, log cTNI was a strongest predictor in multivariable logistic analysis model (OR = 2.408, 95%CI 1.108–5.235, P = 0.027). Moreover, LVEDD (odds ratio (OR) = 0.922, 95% confidence interval (CI), 0.856–0.994, P = 0.034), MWT (OR = 1.131 95%CI 1.035–1.235, P = 0.006) and LVESV index (OR = 1.060, 95%CI 1.025–1.096, P = 0.001) were independent determinants of NSVT occurrence.
Discussion
NSVT is a high-risk factor of SCD in patients with HCM [6,7,8]. Thus, it is beneficial for patients with HCM to early identify predisposing factors of NSVT. Our study demonstrated the relationship between cTNI and NSVT in patients with HOCM. In the present study, 17.2% of HOCM patients underwent 24-h Holter monitoring affected with NSVT. Patients with NSVT have higher serum cTNI and plasma NT-proBNP than those without NSVT. Log cTNI and log NT-proBNP were positively correlated with left atrial diameter, MWT, LVESV index, LVEDV index, and LVMI, whereas, log cTNI was negatively associated with LVEF. Moreover, log NT-proBNP was positively related with LVOTPG at rest. After adjusting for other potential confounders, log cTNI, LVEDD, MWT and LVESV index were independent determinants of the presence of NSVT. These data suggest that elevated serum cTNI was independently associated with the presence of NSVT.
As specific and sensitive markers of myocyte injury, cardiac troponins are well-established diagnostic and prognostic biomarkers of patients with acute coronary syndrome [10, 11]. Except for acute myocardial infarction, chronic myocardial damage could also contribute to troponins release [11]. Cardiac troponins were elevated in a significant proportion of patients with HCM. Elevated serum cTNT and cTNI were proved to be associated with MWT, LVMI, LV diastolic and systolic dysfunction, LV volume, age and left atrial diameter [12,13,14,15,16]. Moreover, elevated cardiac troponins were useful for predicting adverse outcomes in HCM [10, 17]. Similarly, our study demonstrated that serum cTNI was positively correlated with left atrial diameter, MWT, LVMI, left ventricular volume index, and NT-proBNP, whereas negatively with LVEF.
Electrocardiography-Holter monitoring is contributed to the detection of ventricular tachyarrhythmias and risk stratification of asymptomatic or symptomatic patients with HCM, which can identify patients at higher risk for SCD [6, 8, 18, 19]. Studies about the relationship between cardiac troponins and NSVT in HCM were limited. Moreno et al. [13] showed that there was no significant association between elevated high-sensitive cTNT (hs-cTNT) levels and NSVT in 95 hemodynamically stable HCM patients. Jenab et al. [15] performed univariable and multivariable logistic analyses for 98 patients with HCM, which demonstrated that hs-cTNT was significantly related with NSVT in univariable analysis, but not in multivariable analysis model. However, Einvik et al. [22] found that premature ventricular complex was more prevalent and related with cTNT in acute exacerbated chronic obstructive pulmonary disease. Incidental significant arrhythmia correlated with high-sensitive cTNI in scleroderma [23]. Our study indicated that serum cTNI was associated with NSVT in univariable and multivariable logistical analyses. This discrepancy may mainly be account for sample size and methodology.
The pathophysiological mechanisms underlying the association between serum cTNI and NSVT in HOCM have not been clarified in our present study. Several mechanisms might be involved in this process. First and foremost, myocardial fibrosis has been verified as an independent predictor of NSVT in patients with HCM [19]. Chronic myocardial ischemia resulted in myocyte injury and apoptosis, and myocardial fibrosis [11]. An imbalance between inappropriate hypertrophy of the myocardium and microvascular dysfunction resulted in relative myocardial ischemia and mismatch between oxygen and supply in HCM [10, 24, 25]. Moreno et al. [13] reported that hs-cTNT levels were elevated in HCM patients with the presence of myocardial fibrosis indicated by late gadolinium-enhanced CMR. Progressive myocyte loss and fibrosis may contribute to the increased incidence of NSVT in older patients with HCM [7]. Secondly, reduced regional longitudinal myocardial deformation and temporal nonuniformity of relaxation and contraction were induced by regional differences in the distribution of left ventricular hypertrophy, which was related with NSVT in HCM [26]. Last but not least, cardiac cellular damage and alternation in myocardial excitability resulted from extensive hypertrophied and disorganized myocytes in the condition of relative myocardial ischemia, which may lead to cardiac repolarization abnormalities and elevated susceptibility to the incidence of ventricular tachyarrhythmias in HCM [27]. Herein, the precise pathophysiological mechanism of the relationship between cTNI and NSVT should be further clarified.
BNP is primarily produced and released by stretched ventricular myocytes [28]. Substantial studies have suggested that circulating BNP and NT-proBNP were elevated in patients with HCM, and significantly related with exercise capacity, MWT, LVMI, left atrial size, resting LVOTPG, and diastolic function [12, 28,29,30]. Additionally, BNP and NT-proBNP could predict long-term adverse events of patients with HCM [17, 31]. In line with these studies, our study indicated that plasma NT-proBNP was positively correlated with MWT, LVMI, left atrial diameter, left ventricular volume index, and resting LVOTPG. Nevertheless, different from serum cTNI, plasma NT-proBNP was not independently associated with NSVT in multivariable logistic analysis. This divergence may be attributed to differences in the clinical significance of serum cTNI and plasma NT-proBNP. Serum cardiac troponins indicated ongoing myocardial injury and ventricular remodeling, while plasma NT-proBNP reflected cardiac overload [12, 14, 32].
Limitations
There are some limitations in our study. Firstly, the sample size of HOCM patients with NSVT is relatively small. Secondly, the prevalence of NSVT is lower in our study than that in previous literature. It may be reasonable for HOCM patients to prolong the time of Holter monitoring. Thirdly, genetic testing was not performed in this study. Fourthly, the data of late gadolinium enhancement was lacked in our study. In addition, we did not have follow-up data of these participants. Finally, this is a single-center and observational study with inherent drawbacks which could not confirm cause and effect. Further prospective cohort studies with a long-term follow-up are required to establish the casual relationship between serum cTNI and NSVT in HOCM.
Conclusions
Serum cTNI level was elevated in patients with NSVT. And it was independently associated with the presence of NSVT in patients with HOCM. Our results suggest that it may be more reasonable for HOCM patients with elevated serum cTNI to extend the time of Holter monitoring. Further studies are necessary to verify our conclusions.
References
Maron BJ, Ommen SR, Semsarian C, Spirito P, Olivotto I, Maron MS (2014) Hypertrophic cardiomyopathy: present and future, with translation into contemporary cardiovascular medicine. J Am Coll Cardiol 64:83–99
Maron BJ, Maron MS (2013) Hypertrophic cardiomyopathy. Lancet 381:242–255
Guo L, Zhang M, Hu M, Wang B, Wang J, Zuo L, Yang W, Liu B, Liu L (2019) Prevalence of subcutaneous implantable cardioverter-defibrillator based on template ECG screening and ineligible surface ECG predicting factors in patients with hypertrophic cardiomyopathy in China. Heart Vessels 34:851–859
Veselka J, Anavekar NS, Charron P (2017) Hypertrophic obstructive cardiomyopathy. Lancet 389:1253–1267
Marian AJ, Braunwald E (2017) Hypertrophic cardiomyopathy: genetics, pathogenesis, clinical manifestations, diagnosis, and therapy. Circ Res 121:749–770
Authors/Task Force m, Elliott PM, Anastasakis A, Borger MA, Borggrefe M, Cecchi F, Charron P, Hagege AA, Lafont A, Limongelli G, Mahrholdt H, McKenna WJ, Mogensen J, Nihoyannopoulos P, Nistri S, Pieper PG, Pieske B, Rapezzi C, Rutten FH, Tillmanns C, Watkins H (2014) 2014 ESC guidelines on diagnosis and management of hypertrophic cardiomyopathy: the task force for the diagnosis and management of hypertrophic cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J 35:2733–2779
Monserrat L, Elliott PM, Gimeno JR, Sharma S, Penas-Lado M, McKenna WJ (2003) Non-sustained ventricular tachycardia in hypertrophic cardiomyopathy. J Am Coll Cardiol 42:873–879
Dimitrow PP, Chojnowska L, Rudzinski T, Piotrowski W, Ziolkowska L, Wojtarowicz A, Wycisk A, Dabrowska-Kugacka A, Nowalany-Kozielska E, Sobkowicz B, Wrobel W, Aleszewicz-Baranowska J, Rynkiewicz A, Loboz-Grudzien K, Marchel M, Wysokinski A (2010) Sudden death in hypertrophic cardiomyopathy: old risk factors re-assessed in a new model of maximalized follow-up. Eur Heart J 31:3084–3093
Hen Y, Tsugu-Yagawa M, Iguchi N, Utanohara Y, Takada K, Machida H, Takara A, Teraoka K, Inoue K, Takamisawa I, Takayama M, Yoshikawa T (2018) Prognostic value of cardiovascular magnetic resonance imaging for life-threatening arrhythmia detected by implantable cardioverter-defibrillator in Japanese patients with hypertrophic cardiomyopathy. Heart Vessels 33:49–57
Kubo T, Kitaoka H, Yamanaka S, Hirota T, Baba Y, Hayashi K, Iiyama T, Kumagai N, Tanioka K, Yamasaki N, Matsumura Y, Furuno T, Sugiura T, Doi YL (2013) Significance of high-sensitivity cardiac troponin T in hypertrophic cardiomyopathy. J Am Coll Cardiol 62:1252–1259
Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, Joint ESCAAHAWHFTFfUDoMI, Authors/Task Force Members C, Thygesen K, Alpert JS, White HD, Biomarker S, Jaffe AS, Katus HA, Apple FS, Lindahl B, Morrow DA, Subcommittee ECG, Chaitman BR, Clemmensen PM, Johanson P, Hod H, Imaging S, Underwood R, Bax JJ, Bonow JJ, Pinto F, Gibbons RJ, Classification S, Fox KA, Atar D, Newby LK, Galvani M, Hamm CW, Intervention S, Uretsky BF, Steg PG, Wijns W, Bassand JP, Menasche P, Ravkilde J, Trials, Registries S, Ohman EM, Antman EM, Wallentin LC, Armstrong PW, Simoons ML, Trials, Registries S, Januzzi JL, Nieminen MS, Gheorghiade M, Filippatos G, Trials, Registries S, Luepker RV, Fortmann SP, Rosamond WD, Levy D, Wood D, Trials, Registries S, Smith SC, Hu D, Lopez-Sendon JL, Robertson RM, Weaver D, Tendera M, Bove AA, Parkhomenko AN, Vasilieva EJ, Mendis S, Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck-Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, Document R, Morais J, Aguiar C, Almahmeed W, Arnar DO, Barili F, Bloch KD, Bolger AF, Botker HE, Bozkurt B, Bugiardini R, Cannon C, de Lemos J, Eberli FR, Escobar E, Hlatky M, James S, Kern KB, Moliterno DJ, Mueller C, Neskovic AN, Pieske BM, Schulman SP, Storey RF, Taubert KA, Vranckx P, Wagner DR (2012) Third universal definition of myocardial infarction. J Am Coll Cardiol 60:1581–1598
Zhang C, Liu R, Yuan J, Cui J, Hu F, Yang W, Zhang Y, Yang C, Qiao S (2015) Significance and determinants of cardiac troponin i in patients with obstructive hypertrophic cardiomyopathy. Am J Cardiol 116:1744–1751
Moreno V, Hernandez-Romero D, Vilchez JA, Garcia-Honrubia A, Cambronero F, Casas T, Gonzalez J, Martinez P, Climent V, de la Morena G, Valdes M, Marin F (2010) Serum levels of high-sensitivity troponin T: a novel marker for cardiac remodeling in hypertrophic cardiomyopathy. J Card Fail 16:950–956
Kubo T, Kitaoka H, Okawa M, Yamanaka S, Hirota T, Hoshikawa E, Hayato K, Yamasaki N, Matsumura Y, Yasuda N, Sugiura T (2010) Serum cardiac troponin I is related to increased left ventricular wall thickness, left ventricular dysfunction, and male gender in hypertrophic cardiomyopathy. Clin Cardiol 33:E1–7
Jenab Y, Pourjafari M, Darabi F, Boroumand MA, Zoroufian A, Jalali A (2014) Prevalence and determinants of elevated high-sensitivity cardiac troponin T in hypertrophic cardiomyopathy. J Cardiol 63:140–144
Cramer G, Bakker J, Gommans F, Brouwer M, Kurvers M, Fouraux M, Verheugt F, Kofflard M (2014) Relation of highly sensitive cardiac troponin T in hypertrophic cardiomyopathy to left ventricular mass and cardiovascular risk. Am J Cardiol 113:1240–1245
Kubo T, Kitaoka H, Okawa M, Yamanaka S, Hirota T, Baba Y, Hayato K, Yamasaki N, Matsumura Y, Yasuda N, Sugiura T, Doi YL (2011) Combined measurements of cardiac troponin I and brain natriuretic peptide are useful for predicting adverse outcomes in hypertrophic cardiomyopathy. Circ J 75:919–926
Gersh BJ, Maron BJ, Bonow RO, Dearani JA, Fifer MA, Link MS, Naidu SS, Nishimura RA, Ommen SR, Rakowski H, Seidman CE, Towbin JA, Udelson JE, Yancy CW, American College of Cardiology Foundation/American Heart Association Task Force on Practice G, American Association for Thoracic S, American Society of E, American Society of Nuclear C, Heart Failure Society of A, Heart Rhythm S, Society for Cardiovascular A, Interventions, Society of Thoracic S (2011) 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 124:2761–2796
Katritsis DG, Zareba W, Camm AJ (2012) Nonsustained ventricular tachycardia. J Am Coll Cardiol 60:1993–2004
Nagueh SF, Bierig SM, Budoff MJ, Desai M, Dilsizian V, Eidem B, Goldstein SA, Hung J, Maron MS, Ommen SR, Woo A (2011) American Society of Echocardiography clinical recommendations for multimodality cardiovascular imaging of patients with hypertrophic cardiomyopathy: endorsed by the American Society of Nuclear Cardiology, Society for Cardiovascular Magnetic Resonance, and Society of Cardiovascular Computed Tomography. J Am Soc Echocardiogr 24:473–498
Lancellotti P, Moura L, Pierard LA, Agricola E, Popescu BA, Tribouilloy C, Hagendorff A, Monin JL, Badano L, Zamorano JL, European Association of E (2010) European Association of Echocardiography recommendations for the assessment of valvular regurgitation. Part 2: mitral and tricuspid regurgitation (native valve disease). Eur J Echocardiogr 11:307–332
Einvik G, Bhatnagar R, Holmedahl NH, Neukamm A, Omland T, Soyseth V (2017) Premature ventricular complex is more prevalent during acute exacerbated than stable states of chronic obstructive pulmonary disease, and is related to cardiac troponin T. COPD 14:318–323
Bissell LA, Dumitru RB, Erhayiem B, Abignano G, Fent G, Kidambi A, Donica H, Burska A, Del Galdo F, Biglands J, Buckley DL, Greenwood JP, Plein S, Graham L, Buch MH (2019) Incidental significant arrhythmia in scleroderma associates with cardiac magnetic resonance measure of fibrosis and hs-TnI and NT-proBNP. Rheumatology (Oxford) 58:1221–1226
Bravo PE, Zimmerman SL, Luo HC, Pozios I, Rajaram M, Pinheiro A, Steenbergen C, Kamel IR, Wahl RL, Bluemke DA, Bengel FM, Abraham MR, Abraham TP (2013) Relationship of delayed enhancement by magnetic resonance to myocardial perfusion by positron emission tomography in hypertrophic cardiomyopathy. Circ Cardiovasc Imaging 6:210–217
Frey N, Luedde M, Katus HA (2011) Mechanisms of disease: hypertrophic cardiomyopathy. Nat Rev Cardiol 9:91–100
Di Salvo G, Pacileo G, Limongelli G, Baldini L, Rea A, Verrengia M, D'Andrea A, Russo MG, Calabro R (2010) Non sustained ventricular tachycardia in hypertrophic cardiomyopathy and new ultrasonic derived parameters. J Am Soc Echocardiogr 23:581–590
Maron BJ (2010) Contemporary insights and strategies for risk stratification and prevention of sudden death in hypertrophic cardiomyopathy. Circulation 121:445–456
Maron BJ, Tholakanahalli VN, Zenovich AG, Casey SA, Duprez D, Aeppli DM, Cohn JN (2004) Usefulness of B-type natriuretic peptide assay in the assessment of symptomatic state in hypertrophic cardiomyopathy. Circulation 109:984–989
Arteaga E, Araujo AQ, Buck P, Ianni BM, Rabello R, Mady C (2005) Plasma amino-terminal pro-B-type natriuretic peptide quantification in hypertrophic cardiomyopathy. Am Heart J 150:1228–1232
Park JR, Choi JO, Han HJ, Chang SA, Park SJ, Lee SC, Choe YH, Park SW, Oh JK (2012) Degree and distribution of left ventricular hypertrophy as a determining factor for elevated natriuretic peptide levels in patients with hypertrophic cardiomyopathy: insights from cardiac magnetic resonance imaging. Int J Cardiovasc Imaging 28:763–772
Geske JB, McKie PM, Ommen SR, Sorajja P (2013) B-type natriuretic peptide and survival in hypertrophic cardiomyopathy. J Am Coll Cardiol 61:2456–2460
Kawasaki T, Sakai C, Harimoto K, Yamano M, Miki S, Kamitani T (2013) Usefulness of high-sensitivity cardiac troponin T and brain natriuretic peptide as biomarkers of myocardial fibrosis in patients with hypertrophic cardiomyopathy. Am J Cardiol 112:867–872
Funding
None.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
All authors have no conflicts of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhou, Y., Yu, M., Yuan, J. et al. Cardiac troponin I is associated with non-sustained ventricular tachycardia in patients with hypertrophic obstructive cardiomyopathy. Heart Vessels 35, 876–885 (2020). https://doi.org/10.1007/s00380-019-01549-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-019-01549-0