Abstract
No-reflow is one of the major complications of primary percutaneous coronary artery intervention (pPCI) in the treatment of acute ST-segment elevation myocardial infarction (STEMI). Fibrinogen-to-albumin ratio (FAR) has currently emerged as a novel inflammatory marker to predict inflammation in chronic diseases. This study aimed to investigate whether admission FAR values predicts angiographic no-reflow and short-term prognosis in all STEMI patients. A total of 510 consecutive STEMI patients who underwent successful pPCI between September 2016 and May 2018 were included in this study. Patients were divided into groups based on thrombolysis in myocardial infarction (TIMI) flow grades after pPCI. No-reflow was defined as a post-PCI TIMI flow grade of 0, 1, or 2. Angiographic success was defined as TIMI flow grade 3. Fibrinogen, hs-CRP, and admission FAR values were significantly higher among patients with no-reflow. On multivariate analysis, admission FAR was an independent predictor of angiographic no-reflow (p < 0.001). Receiver-operating characteristics analysis revealed the cut-off value of admission FAR was a predictor of no-reflow with a sensitivity of 79.59% and a specificity of 69.42%. In multivariable Cox regression models adjusted for potential confounders, admission FAR values, and LVEF, hs-CRP was independently and positively associated with the 30-day all-cause mortality. Admission FAR was associated independently and significantly with angiographic no-reflow and short-term mortality in patients with STEMI undergoing pPCI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
ST-segment elevation myocardial infarction (STEMI) is a common form of acute myocardial infarction (AMI). Percutaneous coronary intervention (PCI) is performed as soon as possible within 12 h of onset to open the infarct-related coronary artery, which has been recommended as the preferred method of reperfusion [1]. Slow coronary flow or no-reflow is a serious complication of reperfusion in patients with STEMI, which lead to poor prognosis and increased mortality [2, 3]. Some experimental models have found that neutrophil accumulation, reactive oxygen species, and the coagulation cascade via endothelial dysfunction and microvascular constriction are associated with slow or no-reflow [4]. However, the pathophysiological mechanisms of coronary no-reflow are not fully understood. Inflammation plays an important role in the development, progression, and evolution of atherosclerosis. Previous studies have shown a relationship between no-reflow phenomenon and increased inflammatory activity, for instance, neutrophil-to-lymphocyte ratio (NLR) and C-reactive protein (CRP) have been reported as independent predictors of no-reflow among STEMI patients treated with primary PCI [5, 6].
Recently, fibrinogen-to-albumin ratio (FAR) has been reported as a new inflammatory marker closely related to a variety of diseases, including cardiovascular diseases. For example, FAR has been shown to provide a reliable inflammatory index to be used in predicting the severity of coronary artery disease in patients with STEMI. However, there are no data regarding the prognostic value of FAR in the prediction of postprocedural no-reflow among STEMI patients undergoing primary PCI. Therefore, the purpose of this study was to explore the relationship between FAR and no-reflow phenomenon and short-term prognosis in patients with acute STEMI undergoing direct PCI.
Methods
This retrospective analytic-cross sectional study used the data of 510 consecutive patients from September 2016 to May 2018 who were admitted to our cardiovascular center with a diagnosis of acute STEMI and underwent primary PCI within 12 h from symptom onset. The definition of STEMI was based on the criteria of the classic symptoms of coronary ischemia and detection of a 1-mm ST-segment elevation in the inferior lead, or a 2-mm ST-segment elevation in the anterior chest lead occurring in two contiguous leads, or on the presence of a new (or presumably new) left bundle branch block. Patients with culprit lesion in left main coronary artery, left main stenosis over 50%, previous coronary artery bypass surgery, cardiogenic shock, pain to balloon time over 12 h, treatment with fibrinolytic, active infectious or inflammatory diseases, presence of any chronic inflammatory–autoimmune disease including rheumatologic disorders, hematologic diseases, end-stage liver and renal failures, and known malignancy were excluded from the current study. Patients on the following medications were also excluded from the study: glycoprotein IIb/IIIa receptor blockers, corticosteroids, cytotoxic drugs, and diuretics. The study protocol was approved by the Second Affiliated Hospital of Zhengzhou University Ethics Committee. Written informed consent was obtained from all patients.
All participants that underwent coronary angiography performed in multiple orthogonal projections were using Judkins technique. Patients who were candidates for primary PCI received 300 mg of aspirin and a single loading dose of 600 mg clopidogrel or 180 mg ticagrelor at the time of diagnosis of STEMI coronary angiography was performed using standard technique. Immediately after the decision of coronary intervention, 50–70 unit/kg of intravenous bolus dose of unfractionated heparin was administered to the patients who were not treated with enoxaparin before the coronary angiography. The TIMI flow grades were analyzed by two interventional cardiologists blinded to patient clinical data and the frame rate of cine images was 30 frames per seconds. Analysis of cineangiography was performed using an Axiom (Siemens Medical Solution, Erlangen, Germany) workstation. TIMI flow grade three for the treated coronary vessel with a residual stenosis under 20% was considered as a successful pPCI. The no-reflow phenomenon was identified in patients with anterograde flow less than or equal to second level for TIMI and in the absence of dissection, thrombus, spasm, or distal embolization in the final angiogram [7]. Accordingly, the patients were subdivided into normal-reflow group and no-reflow group. Multivessel disease was defined as the presence of more than or equal to one lesion with over 50% stenosis in more than or equal to one major epicardial coronary artery or its major branches remote from the IRA.
All patients received venous blood samples from the antecubital veins after admission, which was performed prior to coronary angiography. Plasma levels of albumin were measured using an automated chemistry analyzer (AU5400, OLYMPUS, JAPAN). Plasma fibrinogen levels were measured using an automatic coagulation analyzer (STA Compact Max, STAGO, FRANCE). F/A ratios were calculated by loading all the data to the statistical program used. Standard methods were used to measure the serum level of glucose, glycated hemoglobin, hemoglobin, uric acid, hs-CRP, and lipid profile. Cardiac troponin T (cTnT), creatine kinase (CK), and CK-MB were measured using a Hitachi modular E-170 (Roche Diagnostics GmbH, Mannheim, Germany). All echocardiographic measurements were made immediately after obtaining the blood samples, using a GE ViVidE7 ultrasound machine (GE Healthcare, Piscataway, USA) with a 3.5-MHz transducer. Left ventricular ejection fraction (LVEF) was measured using the Simpson method according to the recommendations of the American Society of Echocardiography.
Statistical analysis was performed using the SPSS 22.0 Statistical Package Programmed for Windows (SPSS, Inc., Chicago, Illinois). A two-sided p value of < 0.05 was considered significant. Distribution of continuous variables was assessed with the one-sample Kolmogorov–Smirnov test. Continuous variables were expressed as mean ± standard deviation for normally distributed variables and as the median (25th–75th percentiles) for non-normally distributed variables. The differences between groups were tested by independent samples t test or Mann–Whitney U test. Categorical variables were summarized as percentages and compared with the χ2 test. Multivariant stepwise logistic regression, including covariates found to have a significant association with no-reflow in univariate analysis, was used to identify independent predictors of no-reflow. The receiver-operating characteristic (ROC) curve was used to determine the cut-off value of FAR values to predict the no-reflow. A multivariate Cox proportional-hazards regression model was used to find the independent predictors of the primary endpoint. Factors entered into the multivariate model included those with p value < 0.05 from the univariate analysis and variables with known prognostic value. The 30-day survival curves for FAR groups were analyzed using the Kaplan–Meier method, and statistical assessment was performed using the log-rank test.
Results
Among the 510 patients of STEMI (mean age 61.14 ± 11.15 years, 78.9% male), the incidence of angiographic no-reflow was 19.2%. Study participants were divided into two groups according to TIMI flow grades after pPCI. No-reflow group with flow grades 0 to 2 (n = 98, 67 men, mean age 64.09 ± 11.53 years) and the normal-reflow group with flow TIMI grade 3 (n = 412, 325 men, mean age 60.44 ± 10.96 years). Demographic, clinical, laboratory, and procedural characteristics in individual groups are listed in Table 1.
Compared to normal-reflow group, patients in the no-reflow group were older (64.09 ± 11.53 vs. 60.44 ± 10.96 years, p = 0.004, Table 1) and prevalence of diabetes mellitus was significantly higher in them (37.8% vs. 27.4%, p = 0.044, Table 1). Besides, more men were in the reflow group (78.9% vs. 62.4%, p = 0.026, Table 1).
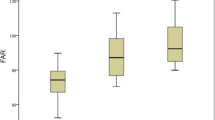
The comparison of admission hematological parameters of both groups is presented in Table 2. The patients in the no-reflow group had significantly higher fibrinogen, FAR and hs-CRP levels when compared to those in reflow patients. The comparison of angiographic and echocardiographic characteristics of the two groups showed no statistically significant differences apart from chest pain to balloon time, initial TIMI flow, and the presence of multivessel disease (Table 2). Pain to balloon time in the no-reflow group was longer than that of the patients in the reflow group, as presented in Table 2 (5.0 h vs. 4.0 h, p = 0.018). There were more patients with multivessel disease in the no-reflow group compared to that of the reflow group (48.8% vs. 65.3%, p < 0.001). In both study groups, the left anterior descending coronary artery is the most common IRA. The percentage of stent implantation, stent length, and diameter in the two groups were similar (Table 2).
Effects of the variables with an unadjusted p < 0.05 in logistic regression analysis and clinically important variables on the no-flow were analyzed using univariate and multivariate logistic regression analyses. Male (OR 0.45; 95% confidence interval [CI] 0.23–0.87; p = 0.017, Table 3), Initial TIMI flow(OR 0.56; 95% CI 0.37–0.85; p = 0.007, Table 3), FAR (OR: 1.80; 95% CI 1.57–2.07; p < 0.001, Table 3), multivessel disease (OR 0.46; 95% CI 0.26–0.79; p = 0.005, Table 3), hs-CRP (OR 1.08; 95% CI 1.00–1.17; p = 0.041, Table 3) were observed to be independent predictors for the development of no-reflow phenomenon.
ROC statistical analyses showed that FAR above 10.89 had 79.59% sensitivity and 69.42% specificity for the development of no-reflow status (95% CI 0.786–0.852, p < 0.001, Fig. 1).
In the multivariate Cox regression model, procedural success, FAR, LVEF, and hs-CRP at admission were independent predictors of 30-day mortality after pPCI (Table 4). Moreover, Kaplan–Meier curves between patients with FAR < 10.89 and ≥ 10.89 for 30-day mortality revealed worse outcomes in patients with high FAR (p < 0.001) (Fig. 2).
Discussion
To our knowledge, this is the first study to report the relationship among the FAR, no-reflow and short-term mortality in patients with STEMI undergoing pPCI. The results of this study showed that FAR, fibrinogen, and hs-CRP values independent predictors of no-reflow after pPCI on multivariate analysis in this study. Furthermore, FAR was an independent factor for 30-day mortality in these patients.
Primary PCI is the preferred revascularization method for most patients with STEMI, but the performance of primary PCI fails to normalize the coronary flow and myocardial perfusion in some of these patients [8]. This phenomenon described as no-reflow is significantly associated with poor outcome and increased mortality after pPCI [9]. Therefore, early identification of patients with high risk of coronary angiography no-reflow can enable physicians to choose the best treatment strategy.
Previous studies have found that no-reflow is associated with a variety of pathological factors, including endothelial dysfunction, reactive oxygen species, ischemia–reperfusion injury, platelet aggregation, micro-thrombosis, distal embolization, and vasomotor dysfunction, but the exact mechanism of no-reflow is still unclear [4, 10, 11]. Celik et al. [12] suggested that distal embolization plays an important role in the mechanism of no-reflow after pPCI. Several high-risk factors, such as high thrombotic load in the coronary artery, right coronary artery lesions, and women (parallel structure), have been found to predict the risk of distal coronary thrombosis in STEMI patients [12,13,14,15]. Recent studies have shown that higher inflammation status play an important role in the occurrence of the no-reflow phenomenon in STEMI patients after pPCI [13]. Akpek et al. [14] reported that neutrophil-to-lymphocyte ratio and CRP had a positive correlation with no-reflow in patients with STEMI undergoing pPCI. Oduncu et al. [15] showed that patients with no-reflow who underwent PCI have higher baseline CRP levels.
Serum albumin is the major protein in human serum, maintains the permeability of the capillary membrane, and participates in the acute and chronic inflammatory response [16, 17]. In addition, serum albumin is an important inhibitor of platelet activation and aggregation and is an important mediator of platelet induction [18, 19]. Recent studies have shown that the serum albumin levels are closely related to the occurrence, development and prognosis of coronary heart disease. It has been suggested that serum albumin levels lower than 3.5 g/dl on admission were one of the independent predictors of new heart failure and in-hospital mortality in patients with acute coronary syndrome [20]. Kurtul et al. [21] showed that albumin was associated with coronary artery SYNTAX scores and the incidence of adverse events during hospitalization in patients with ACS. Meanwhile, fibrinogen is also a biomarker of chronic inflammation, which is known to be a precursor to fibrin and accelerates platelet aggregation [22]. In the Atherosclerosis Risk in Communities Study, elevated fibrinogen levels have been shown to be one of the risk factors for coronary heart disease. However, in healthy adults, the role of predicting coronary events is weaker than traditional risk factors [23]. Gao et al. [24] showed that fibrinogen levels were associated with coronary artery Gensini scores in men under 35 years of age. Kurtul et al. [25] showed that fibrinogen levels in patients with the acute coronary syndrome were positively correlated with coronary SYNTAX scores.
Based on the previous research results, both higher fibrinogen levels and lower serum albumin levels were reported to be associated with adverse cardiovascular outcomes in the setting of STEMI. Its relation with inflammation has been investigated some previous clinical studies. It has been found that there is a positive correlation between FAR and coronary SYNTAX score in patients with STEMI, and FAR can be used as an independent predictor of higher SYNTAX Score in patients with STEMI [26]. Karahan et al. [27] showed that FAR levels are related to the clinical classification and severity of chronic venous insufficiency. Yang et al. [28] have found that the albumin-to-fibrinogen ratio (AFR) is associated with disease activity in patients with rheumatoid arthritis (RA). AFR may serve as two novel inflammatory markers for monitoring disease activity in RA patients. Furthermore, several studies have shown that the FAR has been used frequently to predict outcomes in several types of human cancers [29,30,31,32]. Our results showed that FAR levels were significantly higher in the no-reflow group compared with the normal-reflow group. We also found an optimal cut-off point for FAR of at least 10.89. It predicted the no-reflow with good sensitivity and specificity. In multivariate Cox regression, FAR, LVEF, and hs-CRP at admission were independent predictors of 30-day mortality after pPCI. One of the mechanisms by which higher levels of FAR cause no-reflow may be that low albumin levels promotes fibrinolysis, thereby inhibiting the physiological fibrinolytic system and reducing the spontaneous dissolution of the thrombus [33]. In addition, the bioavailability of prostacyclin (PGI2), an effective platelet aggregation inhibitor, also depends on serum albumin levels [16, 34]. On the other hand, serum albumin inhibits the expression of vascular cell adhesion molecule-1(VCAM-1) induced by tumor necrosis factor A(TNF-a), thereby reducing apoptosis of endothelial cells [35]. The deposition of fibrinogen can change the permeability of vascular endothelium, promote the aggregation and oxidation of low-density lipoprotein under the endothelium, further stimulate the proliferation and migration of vascular smooth muscle cells to the intima, and eventually lead to the formation of atherosclerotic plaque [36].
In summary, we believe that FAR, as a readily available and cheaper marker, may help clinicians in predicting the development of no-reflow and short-term mortality after pPCI. Therefore, FAR is considered to be a new marker of inflammation in STEMI patients. It may be used as a component in accurate risk stratification when a patient is a candidate for pPCI. However, the occurrence of no-reflow appears to involve many mechanisms, of which only a few may have been illuminated. Therefore, further clinical research is needed in the future to better understand the specific mechanism of this phenomenon. Our study has some limitations. First, the sample size is very small, and the results shown in this study should be verified in larger clinical trials. Second, there are multiple risk factors for the no-reflow that we did not assess them and it might have affected our multivariate analysis. Finally, FAR has confined clinical utility, because when performing pPCI, the surgeon usually does not know the results of fibrinogen and albumin.
References
O’Gara PT, Kushner FG, Ascheim DD, Casey DJ, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, Granger CB, Krumholz HM, Linderbaum JA, Morrow DA, Newby LK, Ornato JP, Ou N, Radford MJ, Tamis-Holland JE, Tommaso CL, Tracy CM, Woo YJ, Zhao DX, Anderson JL, Jacobs AK, Halperin JL, Albert NM, Brindis RG, Creager MA, DeMets D, Guyton RA, Hochman JS, Kovacs RJ, Kushner FG, Ohman EM, Stevenson WG, Yancy CW (2013) 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 127:e362–e425
Jaffe R, Charron T, Puley G, Dick A, Strauss BH (2008) Microvascular obstruction and the no-reflow phenomenon after percutaneous coronary intervention. Circulation 117:3152–3156
Galasso G, Schiekofer S, D’Anna C, Gioia GD, Piccolo R, Niglio T, Rosa RD, Strisciuglio T, Cirillo P, Piscione F, Trimarco B (2014) No-reflow phenomenon: pathophysiology, diagnosis, prevention, and treatment. A review of the current literature and future perspectives. Angiology 65:180–189
Schwartz BG, Kloner RA (2012) Coronary no reflow. J Mol Cell Cardiol 52:873–882
Celik T, Iyisoy A, Yuksel UC, Jata B, Ozkan M (2009) The impact of admission C-reactive protein levels on the development of no-reflow phenomenon after primary PCI in patients with acute myocardial infarction: the role of inflammation. Int J Cardiol 86–88
Kurtul A, Yarlioglues M, Celik IE, Duran M, Elcik D, Kilic A, Oksuz F, Murat SN (2015) Association of lymphocyte-to-monocyte ratio with the no-reflow phenomenon in patients who underwent a primary percutaneous coronary intervention for ST-elevation myocardial infarction. Coron Artery Dis 26:706–712
Niccoli G, Burzotta F, Galiuto L, Crea F (2009) Myocardial no-reflow in humans. J Am Coll Cardiol 54:281–292
Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, Caforio A, Crea F, Goudevenos JA, Halvorsen S, Hindricks G, Kastrati A, Lenzen MJ, Prescott E, Roffi M, Valgimigli M, Varenhorst C, Vranckx P, Widimsky P (2018) 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 39:119–177
Durante A, Camici PG (2015) Novel insights into an “old” phenomenon: the no reflow. Int J Cardiol 187:273–280
Jaffe R, Charron T, Puley G, Dick A, Strauss BH (2008) Microvascular obstruction and the no-reflow phenomenon after percutaneous coronary intervention. Circulation 117:3152–3156
Rezkalla SH, Kloner RA (2008) Coronary no-reflow phenomenon: from the experimental laboratory to the cardiac catheterization laboratory. Catheter Cardiovasc Interv 72:950–957
Celik T, Balta S, Ozturk C, Kaya MG, Aparci M, Yildirim OA, Demir M, Unlu M, Demirkol S, Kilic S, Iyisoy A (2015) Predictors of no-reflow phenomenon in young patients with acute ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Angiology 67:683–689
Kurtul A, Murat SN, Yarlioglues M, Duran M, Celik IE, Kilic A (2015) Mild to moderate renal impairment is associated with no-reflow phenomenon after primary percutaneous coronary intervention in acute myocardial infarction. Angiology 66:644–651
Akpek M, Kaya MG, Lam YY, Sahin O, Elcik D, Celik T, Ergin A, Gibson CM (2012) Relation of neutrophil/lymphocyte ratio to coronary flow to in-hospital major adverse cardiac events in patients with ST-elevated myocardial infarction undergoing primary coronary intervention. Am J Cardiol 110:621–627
Oduncu V, Tanalp AC, Erkol A, Sirma D, Dundar C, Akgun T, Turkyilmaz E, Kilicgedik A, Gozubuyuk G, Tigen K, Izgi A, Kirma C (2011) Impact of chronic pre-treatment of statins on the level of systemic inflammation and myocardial perfusion in patients undergoing primary angioplasty. Am J Cardiol 107:179–185
Nicholson JP, Wolmarans MR, Park GR (2000) The role of albumin in critical illness. Br J Anaesth 85:599–610
Quinlan GJ, Martin GS, Evans TW (2005) Albumin: biochemical properties and therapeutic potential. Hepatology 41:1211–1219
Chojkier M (2005) Inhibition of albumin synthesis in chronic diseases: molecular mechanisms. J Clin Gastroenterol 39:S143–S146
Gresele P, Deckmyn H, Huybrechts E, Vermylen J (1984) Serum albumin enhances the impairment of platelet aggregation with thromboxane synthase inhibition by increasing the formation of prostaglandin D2. Biochem Pharmacol 33:2083–2088
Gonzalez-Pacheco H, Amezcua-Guerra LM, Sandoval J, Martinez-Sanchez C, Ortiz-Leon XA, Pena-Cabral MA, Bojalil R (2017) Prognostic implications of serum albumin levels in patients with acute coronary syndromes. Am J Cardiol 119:951–958
Kurtul A, Murat SN, Yarlioglues M, Duran M, Ocek AH, Koseoglu C, Celik IE, Kilic A, Aksoy O (2016) Usefulness of SYNTAX Score and in-hospital mortality in patients with acute coronary syndrome. Angiology 67:34–40
Nguyen XM, Lane J, Smith BR, Nguyen NT (2009) Changes in inflammatory biomarkers across weight classes in a representative US population: a link between obesity and inflammation. J Gastrointest Surg 13:1205–1212
Folsom AR, Aleksic N, Park E, Salomaa V, Juneja H, Wu KK (2001) Prospective study of fibrinolytic factors and incident coronary heart disease: the Atherosclerosis Risk in Communities (ARIC) Study. Arterioscler Thromb Vasc Biol 21:611–617
Gao XY, Zhou BY, Zhang MZ, Zhao X, Qing P, Zhu CG, Wu NQ, Guo YL, Gao Y, Li XL, Wang Y, Liu G, Dong Q, Guo LH, Li JJ (2017) Association between fibrinogen level and the severity of coronary stenosis in 418 male patients with myocardial infarction younger than 35 years old. Oncotarget 8:81361–81368
Kurtul A, Yarlioglues M, Murat SN, Duran M, Oksuz F, Koseoglu C, Celik IE, Kilic A, Aksoy O (2016) The association of plasma fibrinogen with the extent and complexity of coronary lesions in patients with acute coronary syndrome. Kardiol Pol 74:338–345
Karahan O, Acet H, Ertas F, Tezcan O, Caliskan A, Demir M, Kaya AF, Demirtas S, Cevik MU, Yavuz C (2016) The relationship between fibrinogen to albumin ratio and severity of coronary artery disease in patients with STEMI. Am J Emerg Med 34:1037–1042
Karahan O, Yavuz C, Kankilic N, Demirtas S, Tezcan O, Caliskan A, Mavitas B (2016) Simple blood tests as predictive markers of disease severity and clinical condition in patients with venous insufficiency. Blood Coagul Fibrinol 27:684–690
Yang WM, Zhang WH, Ying HQ, Xu YM, Zhang J, Min QH, Huang B, Lin J, Chen JJ, Wang XZ (2018) Two new inflammatory markers associated with disease activity score-28 in patients with rheumatoid arthritis: albumin to fibrinogen ratio and C-reactive protein to albumin ratio. Int Immunopharmacol 62:293–298
Tan Z, Zhang M, Han Q, Wen J, Luo K, Lin P, Zhang L, Yang H, Fu J (2017) A novel blood tool of cancer prognosis in esophageal squamous cell carcinoma: the fibrinogen/albumin ratio. J Cancer 8:1025–1029
Li SQ, Jiang YH, Lin J, Zhang J, Sun F, Gao QF, Zhang L, Chen QG, Wang XZ, Ying HQ (2018) Albumin-to-fibrinogen ratio as a promising biomarker to predict clinical outcome of non-small cell lung cancer individuals. Cancer Med 7(4):1221–1231
Hwang KT, Chung JK, Roh EY, Kim J, Oh S, Kim YA, Rhu J, Kim S (2017) Prognostic influence of preoperative fibrinogen to albumin ratio for breast cancer. J Breast Cancer 20:254–263
Xu Q, Yan Y, Gu S, Mao K, Zhang J, Huang P, Zhou Z, Chen Z, Zheng S, Liang J, Lin Z, Wang J, Yan J, Xiao Z (2018) A Novel Inflammation-Based Prognostic Score: the fibrinogen/albumin ratio predicts prognoses of patients after curative resection for hepatocellular carcinoma. J Immunol Res 2018:4925498
de Sain-van der Velden MGM, Smolders HC, van Rijn HJM, Voorbij HAM (2000) Does albumin play a role in fibrinolysis by its inhibition of plasminogen activation? Fibrinolysis Proteolysis 14(4):242–246
Garcia-Martinez R, Caraceni P, Bernardi M, Gines P, Arroyo V, Jalan R (2013) Albumin: pathophysiologic basis of its role in the treatment of cirrhosis and its complications. Hepatology 58:1836–1846
Albert MA, Glynn RJ, Buring JE, Ridker PM (2007) Relation between soluble intercellular adhesion molecule-1, homocysteine, and fibrinogen levels and race/ethnicity in women without cardiovascular disease. Am J Cardiol 99:1246–1251
Guo YH, Hernandez I, Isermann B, Kang TB, Medved L, Sood R, Kerschen EJ, Holyst T, Mosesson MW, Weiler H (2009) Caveolin-1-dependent apoptosis induced by fibrin degradation products. Blood 113:4431–4439
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
There are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhao, Y., Yang, J., Ji, Y. et al. Usefulness of fibrinogen-to-albumin ratio to predict no-reflow and short-term prognosis in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Heart Vessels 34, 1600–1607 (2019). https://doi.org/10.1007/s00380-019-01399-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-019-01399-w