Abstract
Despite the positive impact of percutaneous coronary intervention (PCI) on reducing mortality, a small percentage of patients experience poor myocardial reperfusion following PCI. However, factors associated with no-reflow remain unclear. We investigated clinical factors associated with no-reflow following PCI for coronary artery disease (CAD). We retrospectively analyzed 1622 consecutive CAD patients who underwent PCI over a 5-year period at our institution. Patients were divided into two groups according to the presence (n = 31) or absence (n = 1591) of no-reflow, defined as Thrombolysis in Myocardial Infarction flow grade <3 after PCI. No significant differences in patient characteristics or PCI strategy were seen between the no-reflow and normal flow groups. The incidence of no-reflow was significantly lower in the left circumflex artery (LCx) than in the left anterior descending artery (LAD) (P = 0.0015), with no differences in characteristics or PCI strategy between these two target vessels. Multivariate analysis revealed that involvement of the LCx was an independent protective factor against no-reflow (odds ratio 0.14, 95 % confidence interval 0.02–0.98, P = 0.044). In conclusion, LCx as the target vessel was protective against no-reflow compared with LAD following PCI for CAD. Our results suggest that embolic protection devices may be unnecessary in CAD patients with involvement of LCx.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Although percutaneous coronary intervention (PCI) has dramatically improved survival rates after acute myocardial infarction (AMI), a small proportion of patients with coronary artery disease (CAD) experience poor myocardial reperfusion following PCI, a phenomenon that is termed “no-reflow” [1, 2]. No-reflow, whose reported incidence ranges between 3 % and 5 % for all types of PCI [3–5], is independently associated with increased in-hospital mortality, cardiac dysfunction and failure, and poor long-term prognosis [6–9]. Although a number of intervention strategies to improve reperfusion in AMI patients before and after PCI have been implemented [10], no significant change in the incidence of no-reflow has been obtained, and factors underlying the increased susceptibility to this phenomenon are poorly understood.
The main approaches to preventing or treating no-reflow following PCI include the use of vasodilators [11–13], antiplatelet therapy [14], calcium-channel blockers [15, 16], nicorandil [17, 18], and embolic protection devices [19]. Although these various approaches have led to improvements in mortality outcomes, the frequency of no-reflow has not significantly improved, and no standardized treatment for this phenomenon has been established. In general, when plaque instability is identified in CAD patients by intravascular ultrasonography (IVUS), embolic protection devices are used as a preventative measure against no-reflow. However, three major trials [20–22] found that the use of such devices did not lead to significant differences in myocardial reperfusion or microvascular flow. These findings indicate that the optimum use of these protection devices awaits a better understanding of the underlying clinical factors associated with the development of no-reflow following PCI.
In our clinical experience, the incidence of no-reflow is lower in patients who receive PCI for CAD involving the left circumflex artery (LCx). Although in Japan embolic protection devices are often implanted in response to the detection of lesions without distinction of the type of vessel, outcome benefits based on target vessel have not been conclusively demonstrated. Recently, Ndrepepa et al. [23] reported that the incidences of no-reflow among patients with ST-elevation myocardial infarction (STEMI) treated with PCI varied considerably among target vessels, albeit without statistical significance. It therefore remains unclear as to whether the target vessel influences the development of no-reflow in the setting of PCI for CAD.
Here, we retrospectively analyzed patients with CAD who had undergone PCI to determine the incidence of no-reflow with respect to target vessel.
Patients and methods
Patients
In total, 1622 consecutive CAD patients who underwent PCI for either stable CAD (n = 749) or acute coronary syndrome (ACS) (n = 873) at our institution between January 2006 and December 2010 were retrospectively analyzed. Data were obtained from clinical records, which included clinical history, and all patients provided written informed consent to undergo the procedure.
Angiographic analyses
Patients were divided into two groups according to the presence or absence of no-reflow, defined as Thrombolysis in Myocardial Infarction (TIMI) flow grade <3 after PCI. All coronary angiograms were analyzed using quantitative coronary analysis (QCA) software (CAAS V; Pie Medical Imaging, Maastricht, The Netherlands) and plaque areas were calculated. For each lesion, an end-diastolic frame from the angiogram was selected with identical angulations that best showed the stenosis at its greatest severity with minimal foreshortening and branch overlap.
Clinical data collection and biochemical measurements
We collected the following data: age, sex, coronary risk factors (smoking and hypertension, as defined by the Joint National Committee VII [24, 25], diabetes mellitus, as defined by the World Health Organization study group [26], and dyslipidemia), and cardiovascular medications before PCI. Serum creatinine before PCI was measured by the creatinase–sarcosine oxidase–peroxidase method. The estimated glomerular filtration rate (eGFR) was calculated using the Modification of Diet in Renal Disease Study equation, as follows: eGFR = 175 × serum creatinine level (mg/dl)−1.154 × age (years)−0.203 (if female, ×0.742) [27]. The ethnicity factor used in this equation for the Japanese population was 0.741 [28].
Medications and follow-up
All patients in this study underwent PCI. Aspirin (100 mg for stable CAD patients; 162–200 mg for ACS patients) was administered before PCI. Intravenous heparin (10,000 IU) was administered once arterial access had been obtained to achieve an activated clotting time of 200–300 s. Postprocedural antithrombotic therapy consisted of aspirin (81–100 mg/day) and ticlopidine (100 mg twice daily) or clopidogrel (75 mg daily). We followed the patients for 30 days after PCI, observing for in-hospital cardiac deaths.
Statistical analyses
For continuous variables, comparisons between the no-reflow and normal flow, and LAD and LCx groups were performed using Student’s t test and Fisher’s exact test. Continuous data are expressed as the mean ± standard deviation (SD). Categorical variables are reported as frequencies with percentages, and were compared between the two groups using the Chi-squared test. Comparisons between the incidences of no-reflow based on the target vessel of PCI were performed using the Chi-squared and post hoc Bonferroni multiple-comparison tests. Kaplan–Meier curves were drawn to compare mortality outcomes between the no-reflow and normal-flow patient groups. Univariate analysis of variance was performed to assess the effects of various factors on the development of no-reflow, with variables with a P value of less than 0.10 in the univariate analysis included in the multivariate logistic regression analysis. All statistical analyses were performed using SPSS statistical software (SPSS, Chicago, IL, USA), with P values of less than 0.05 considered statistically significant.
Results
Patients’ baseline characteristics and incidence of no-reflow
Baseline characteristics of the 1622 study patients are summarized in Table 1. The patients were predominantly male (77 %) and had a mean age of 67 ± 11 years. Over 80 % of all patients received aspirin prior to PCI; however, nearly half were also treated with statins (51 %). Among the 1622 patients, 31 (1.9 %) experienced no-reflow following PCI. No significant differences were detected between patients with normal (n = 1591) and no-reflow in any of the examined variables.
The incidence of no-reflow based on PCI strategy is summarized in Table 2. Drug-eluting stents (DES) were the most common PCI procedure (85 %). No significant differences in the rates of no-reflow were detected for DES, bare-metal stents, or plain balloon angioplasty. In addition, the incidences of no-reflow and normal flow did not significantly differ based on the implantation of an embolic protection device, or the use of aspiration.
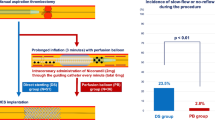
Kaplan–Meier estimates of in-hospital mortality during the 30 days after PCI were 6.5 % in patients with no-reflow and 0.6 % in patients with normal flow (P < 0.001; Fig. 1).
Incidence of no-reflow by target vessel of PCI
We also examined the incidence of no-reflow based on the target vessel of PCI (Table 3). For the LAD, a significantly higher incidence of no-reflow was detected in comparison with all patients with normal flow. The inverse association was detected for LCx, with patients displaying a markedly lower incidence of no-reflow. Only a single case of no-reflow was found among the 351 patients who underwent PCI for the LCx, in contrast to the 20 cases detected when the LAD was the target vessel. We directly compared the relationship between the development of no-reflow following PCI for each target vessel using the Chi-squared and post hoc Bonferroni multiple-comparison tests (Fig. 2). A significant difference in the incidence of no-reflow was detected only between the LCx and LAD (P = 0.0015), with no-reflow occurring less frequently in patients with LCx as the target vessel (<0.5 %).
Incidence of no-reflow based on the target vessel of percutaneous coronary intervention (PCI). The percentage of total patients with coronary artery disease experiencing no-reflow after PCI involving the left anterior descending artery (LAD), left circumflex artery (LCx), left main trunk coronary artery (LMT), right coronary artery (RCA), or saphenous vein graft (SVG) are shown. The Chi-squared and post hoc Bonferroni multiple-comparison tests were used to evaluate statistical significance. ns not significant
Protective factors against no-reflow phenomenon
Univariate analysis was performed to identify potentially protective factors against no-reflow (Table 4). Three variables with a P value of less than 0.10 were identified: a history of hypertension (P = 0.05), and either the LCx (P = 0.03) or the LAD as a target vessel (P = 0.02). To identify factors that were independently associated with the development of no-reflow, multivariate logistic regression analysis was conducted (Table 4). The analysis revealed that only LCx was independently associated with a reduced incidence of no-reflow following PCI.
LCx-patient and LAD-patient groups
Following the identification of the LCx and LAD as being significantly associated with lower and higher incidences of no-reflow, respectively, we further examined the background characteristics of CAD patients with involvement of either the LCx (n = 351) or LAD (n = 711) (Table 5). There were no significant differences in background characteristics, including plaque burden calculated by the QCA method and collateral source contributions, between the two patient groups. Of note, the two groups had similar rates of ACS including STEMI, non-STEMI and unstable angina, and the rates of medication use were nearly identical before PCI. Furthermore, we confirmed that there were no significant differences in PCI strategies between these two groups (Table 6).
Discussion
In the present study of consecutive CAD patients who underwent PCI for either stable CAD or ACS, the incidence of no-reflow was significantly lower when the LCx was the target vessel, compared with that associated with the LAD. This is the first report to identify a relationship between the development of no-reflow and the target vessel of PCI in the setting of stable CAD and ACS. In addition, no differences in baseline characteristics or PCI strategies were detected between the LCx and LAD patient groups. Our findings suggest that the use of embolic protection devices may not be warranted in PCI treatment of the LCx in CAD.
It is noteworthy that the LAD and LCx patient groups had similar baseline characteristics, particularly with regard to the incidence of ACS, which tends to be associated with the development of no-reflow [29, 30], and furthermore did not differ with respect to PCI strategy. Although it is possible that the placement of the embolic protection device itself may influence the no-reflow phenomenon, we found no difference in the incidence of no-reflow between patients who underwent direct stenting, embolic protection, or aspiration. The overall incidence of no-reflow among our consecutive CAD patients was 1.9 %; however, when the LAD was the target vessel, the rate increased to 2.8 %, which was 10-fold higher than that associated with the LCx. Although the reason for this finding is presently unclear, we speculate that the large difference may be related to factors associated with vessel morphology, such as the degree of septal branching and propensity to form atheromatous coronary lesions [31]. In the present study, we analyzed plaque burden by the QCA method and found no significant differences between the LCx and LAD patient groups. However, the QCA method is unsatisfactory for evaluating actual plaque characteristics and amount. For this purpose, IVUS is preferable to QCA. A full understanding of this difference awaits further prospective clinical studies, employing IVUS analyses, in both Western and Japanese CAD patients.
A number of studies have examined predictors of the no-reflow phenomenon [23, 32–34]; however, our present study is the first to identify an association between the development of no-reflow and type of target vessel. Recently, Ndrepepa et al. [23] found no significant association between the incidence of no-reflow and target vessel, which included the LAD (9.3 %) and LCx (7.4 %), among 1140 STEMI patients treated with PCI. Although the overall incidence of no-reflow observed here was markedly lower for all examined target vessels than for those of Ndrepepa et al. [23], a direct comparison between the two study populations is not possible because of differences in ethnic composition, type of CAD, and the potential influence of country-based differences in PCI techniques and treatment strategies. Of note, our study included CAD patients with either stable CAD or ACS, while that of Ndrepepa et al. [23] consisted exclusively of STEMI patients.
Although our findings, together with the clinical evidence reported to date [20, 21], generally support the routine use of embolic protective devices during PCI, their use may not be warranted in patients with CAD associated with the LCx. Approaches for predicting patients at risk of developing no-reflow, such as examining lesion morphology [35] using IVUS [36] and angioscopy [37], although often unreliable, may also provide useful information. Our findings should aid physicians when deciding a PCI strategy for CAD patients, particularly concerning the use of embolic protective devices for involvement of the LCx. However, further prospective studies examining revascularization outcomes after insertion of embolic protection devices in such patients are needed to confirm the present results.
Our study has several strengths. First, this represents the first identification of the LCx being resistant to the no-reflow phenomenon, a finding that has important clinical implications. Second, our study population consisted of clinical cases that were continuous over a 5-year period at a single facility. Thus, the quality and type of PCI procedure and patient care remained relatively consistent throughout the study period, thereby limiting the potential impact of this confounding factor on the study results. Finally, our study population was relatively large and consisted of CAD patients with either stable CAD or ACS, thus increasing the generalizability of our findings.
A few limitations of the present study also warrant mention. First, the number of patients in the no-reflow group was small, thus limiting the significance of the results. Given that the overall incidence of no-reflow is low [38], pooled analysis may provide further insights into the factors associated with this phenomenon. Second, as the CAD patients analyzed in this study included those with either stable CAD or ACS, it is possible that thrombus formation and the properties of plaques may have differed between the patients. Third, our study population was enrolled at a single institution, and even after 5 years the incidence of the no-reflow phenomenon was relatively low. Finally, as only a limited number of patients underwent IVUS, data relating to vessel diameter and plaque characteristics were not available for all patients.
In conclusion, our analysis of CAD patients has revealed that the incidence of no-reflow was lower when the LCx was the target vessel for PCI than when the LAD was the target. Our findings suggest that the use of embolic protection devices may be unnecessary in CAD patients with involvement of the LCx. However, further prospective studies examining reperfusion outcomes after insertion of embolic protection devices in such patients are needed to confirm the present findings.
References
Jaffe R, Charron T, Puley G, Dick A, Strauss BH (2008) Microvascular obstruction and the no-reflow phenomenon after percutaneous coronary intervention. Circulation 117:3152–3156
Rezkalla SH, Kloner RA (2002) No-reflow phenomenon. Circulation 105:656–662
Butler M, Ajani A, Andrianopoulos N, Clarke D, Reid C, Shaw J, Brennan A, Lew R, Dart A, Duffy S (2008) Predictors and outcomes of the no-reflow phenomenon. Heart Lung Circ 17(Supplement 3):S176
Piana RN, Paik GY, Moscucci M, Cohen DJ, Gibson CM, Kugelmass AD, Carrozza JP Jr, Kuntz RE, Baim DS (1994) Incidence and treatment of ‘no-reflow’ after percutaneous coronary intervention. Circulation 89:2514–2518
Schwartz BG, Kloner RA (2012) Coronary no reflow. J Mol Cell Cardiol 52:873–882
Abbo KM, Dooris M, Glazier S, O’Neill WW, Byrd D, Grines CL, Safian RD (1995) Features and outcome of no-reflow after percutaneous coronary intervention. Am J Cardiol 75:778–782
Resnic FS, Wainstein M, Lee MKY, Behrendt D, Wainstein RV, Ohno-Machado L, Kirshenbaum JM, Rogers CDK, Popma JJ, Piana R (2003) No-reflow is an independent predictor of death and myocardial infarction after percutaneous coronary intervention. Am Heart J 145:42–46
Ndrepepa G, Tiroch K, Fusaro M, Keta D, Seyfarth M, Byrne RA, Pache J, Alger P, Mehilli J, Schomig A, Kastrati A (2010) 5-year prognostic value of no-reflow phenomenon after percutaneous coronary intervention in patients with acute myocardial infarction. J Am Coll Cardiol 55:2383–2389
Mehta RH, Harjai KJ, Boura J, Cox D, Stone GW, O’Neill W, Grines CL (2003) Prognostic significance of transient no-reflow during primary percutaneous coronary intervention for ST-elevation acute myocardial infarction. Am J Cardiol 92:1445–1447
Butler MJ, Chan W, Taylor AJ, Dart AM, Duffy SJ (2011) Management of the no-reflow phenomenon. Pharmacol Ther 132:72–85
Hillegass WB, Dean NA, Liao L, Rhinehart RG, Myers PR (2001) Treatment of no-reflow and impaired flow with the nitric oxide donor nitroprusside following percutaneous coronary interventions: initial human clinical experience. J Am Coll Cardiol 37:1335–1343
Pasceri V, Pristipino C, Pelliccia F, Granatelli A, Speciale G, Roncella A, Pironi B, Capasso M, Richichi G (2005) Effects of the nitric oxide donor nitroprusside on no-reflow phenomenon during coronary interventions for acute myocardial infarction. Am J Cardiol 95:1358–1361
Kobatake R, Sato T, Fujiwara Y, Sunami H, Yoshioka R, Ikeda T, Saito H, Ujihira T (2011) Comparison of the effects of nitroprusside versus nicorandil on the slow/no-reflow phenomenon during coronary interventions for acute myocardial infarction. Heart Vessels 26:379–384
Investigators E (1998) Randomised placebo-controlled and balloon-angioplasty-controlled trial to assess safety of coronary stenting with use of platelet glycoprotein-IIb/IIIa blockade. Lancet 352:87–92
Michaels AD, Appleby M, Otten MH, Dauterman K, Ports TA, Chou TM, Gibson CM (2002) Pretreatment with intragraft verapamil prior to percutaneous coronary intervention of saphenous vein graft lesions: results of the randomized, controlled vasodilator prevention on no-reflow (vapor) trial. J Invasive Cardiol 14:299–302
Kaplan BM, Benzuly KH, Kinn JW, Bowers TR, Tilli FV, Grines CL, O’Neill WW, Safian RD (1996) Treatment of no-reflow in degenerated saphenous vein graft interventions: comparison of intracoronary verapamil and nitroglycerin. Cathet Cardiovasc Diagn 39:113–118
Ono H, Osanai T, Ishizaka H, Hanada H, Kamada T, Onodera H, Fujita N, Sasaki S, Matsunaga T, Okumura K (2004) Nicorandil improves cardiac function and clinical outcome in patients with acute myocardial infarction undergoing primary percutaneous coronary intervention: role of inhibitory effect on reactive oxygen species formation. Am Heart J 148:E15
Ishii H, Ichimiya S, Kanashiro M, Amano T, Matsubara T, Murohara T (2006) Effects of intravenous nicorandil before reperfusion for acute myocardial infarction in patients with stress hyperglycemia. Diabetes Care 29:202–206
Jaffe R, Dick A, Strauss BH (2010) Prevention and treatment of microvascular obstruction-related myocardial injury and coronary no-reflow following percutaneous coronary intervention: a systematic approach. JACC Cardiovasc Interv 3:695–704
Stone GW, Webb J, Cox DA, Brodie BR, Qureshi M, Kalynych A, Turco M, Schultheiss HP, Dulas D, Rutherford BD, Antoniucci D, Krucoff MW, Gibbons RJ, Jones D, Lansky AJ, Mehran R (2005) Distal microcirculatory protection during percutaneous coronary intervention in acute ST-segment elevation myocardial infarction: a randomized controlled trial. JAMA 293:1063–1072
Gick M, Jander N, Bestehorn HP, Kienzle RP, Ferenc M, Werner K, Comberg T, Peitz K, Zohlnhofer D, Bassignana V, Buettner HJ, Neumann FJ (2005) Randomized evaluation of the effects of filter-based distal protection on myocardial perfusion and infarct size after primary percutaneous catheter intervention in myocardial infarction with and without ST-segment elevation. Circulation 112:1462–1469
Haeck JD, Koch KT, Bilodeau L, Van der Schaaf RJ, Henriques JP, Vis MM, Baan J Jr, Van der Wal AC, Piek JJ, Tijssen JG, Krucoff MW, De Winter RJ (2009) Randomized comparison of primary percutaneous coronary intervention with combined proximal embolic protection and thrombus aspiration versus primary percutaneous coronary intervention alone in ST-segment elevation myocardial infarction: the PREPARE (Proximal Embolic Protection in Acute Myocardial Infarction and Resolution of ST-Elevation) study. JACC Cardiovasc Interv 2:934–943
Ndrepepa G, Tiroch K, Keta D, Fusaro M, Seyfarth M, Pache J, Mehilli J, Schomig A, Kastrati A (2010) Predictive factors and impact of no reflow after primary percutaneous coronary intervention in patients with acute myocardial infarction. Circ Cardiovasc Interv 3:27–33
Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, Jones DW, Materson BJ, Oparil S, Wright JT Jr, Roccella EJ (2003) Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension 42:1206–1252
Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, Jones DW, Materson BJ, Oparil S, Wright JT Jr, Roccella EJ (2003) The seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA 289:2560–2572
Alberti KG, Zimmet PZ (1998) Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 15:539–553
Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F (2006) Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 145:247–254
Imai E, Horio M, Nitta K, Yamagata K, Iseki K, Hara S, Ura N, Kiyohara Y, Hirakata H, Watanabe T, Moriyama T, Ando Y, Inaguma D, Narita I, Iso H, Wakai K, Yasuda Y, Tsukamoto Y, Ito S, Makino H, Hishida A, Matsuo S (2007) Estimation of glomerular filtration rate by the MDRD study equation modified for Japanese patients with chronic kidney disease. Clin Exp Nephrol 11:41–50
Ito H (2006) No-reflow phenomenon and prognosis in patients with acute myocardial infarction. Nat Clin Pract Cardiovasc Med 3:499–506
Kondo M, Nakano A, Saito D, Shimono Y (1998) Assessment of “Microvascular no-reflow phenomenon” Using technetium-99m macroaggregated albumin scintigraphy in patients with acute myocardial infarction. J Am Coll Cardiol 32:898–903
Kotani J, Nanto S, Mintz GS, Kitakaze M, Ohara T, Morozumi T, Nagata S, Hori M (2002) Plaque gruel of atheromatous coronary lesion may contribute to the no-reflow phenomenon in patients with acute coronary syndrome. Circulation 106:1672–1677
Seong MM Jr, Kim KS, Kim B-k, Son j-y, Hong S-p, Lee YS, Lee JB, Ryu JK, Choi JY, Chang S-G (2011) Predictors of no-reflow phenomenon during primary PCI in STEMI patients. J Am Coll Cardiol 57:E942
Hong YJ, Jeong MH, Choi YH, Ko JS, Lee MG, Kang WY, Lee SE, Kim SH, Park KH, Sim DS, Yoon NS, Youn HJ, Kim KH, Park HW, Kim JH, Ahn Y, Cho JG, Park JC, Kang JC (2009) Predictors of no-reflow after percutaneous coronary intervention for culprit lesion with plaque rupture in infarct-related artery in patients with acute myocardial infarction. J Cardiol 54:36–44
Chen Y, Wang C, Yang X, Wang L, Sun Z, Liu H, Chen L (2012) Independent no-reflow predictors in female patients with ST-elevation acute myocardial infarction treated with primary percutaneous coronary intervention. Heart Vessels 27:243–249
Tanaka A, Kawarabayashi T, Nishibori Y, Sano T, Nishida Y, Fukuda D, Shimada K, Yoshikawa J (2002) No-reflow phenomenon and lesion morphology in patients with acute myocardial infarction. Circulation 105:2148–2152
Kotani J, Mintz GS, Pregowski J, Kalinczuk L, Pichard AD, Satler LF, Suddath WO, Waksman R, Weissman NJ (2003) Volumetric intravascular ultrasound evidence that distal embolization during acute infarct intervention contributes to inadequate myocardial perfusion grade. Am J Cardiol 92:728–732
Mizote I, Ueda Y, Ohtani T, Shimizu M, Takeda Y, Oka T, Tsujimoto M, Hirayama A, Hori M, Kodama K (2005) Distal protection improved reperfusion and reduced left ventricular dysfunction in patients with acute myocardial infarction who had angioscopically defined ruptured plaque. Circulation 112:1001–1007
Niccoli G, Burzotta F, Galiuto L, Crea F (2009) Myocardial no-reflow in humans. J Am Coll Cardiol 54:281–292
Acknowledgments
We thank our medical assistants (NHO Saitama Hospital) for collecting the clinical data and Eiichi Sekizuka (Head of NHO Saitama Hospital) for supporting our research.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nagai, T., Hirano, T., Tsunoda, M. et al. Left circumflex coronary artery is protected against no-reflow phenomenon following percutaneous coronary intervention for coronary artery disease. Heart Vessels 28, 559–565 (2013). https://doi.org/10.1007/s00380-012-0281-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-012-0281-2