Abstract
The aim of this study was to assess the association between the spatial location of plaque rupture and remodeling pattern of culprit lesions in acute anterior myocardial infarction (MI). Positive remodeling suggests a potential surrogate marker of plaque vulnerability, whereas plaque rupture causes thrombus formation followed by coronary occlusion and MI. Intravascular ultrasound (IVUS) can determine the precise spatial orientation of coronary plaque formation. We studied 52 consecutive patients with acute anterior MI caused by plaque rupture of the culprit lesion as assessed by preintervention IVUS. The plaques were divided into those with and without positive remodeling. We divided the plaques into three categories according to the spatial orientation of plaque rupture site: myocardial (inner curve), epicardial (outer curve), and lateral quadrants (2 intermediate quadrants). Among 52 plaque ruptures in 52 lesions, 27 ruptures were oriented toward the epicardial side (52%), 18 toward the myocardial side (35%), and 7 in the 2 lateral quadrants (13%). Among 35 plaques with positive remodeling, plaque rupture was observed in 21 (52%) on the epicardial side, 12 (34%) on the myocardial side, and 2 (6%) on the lateral side. However, among 17 plaques without positive remodeling, plaque rupture was observed in 6 (35%), 6 (35%), and 5 (30%), respectively (p = 0.047). Atherosclerotic plaques with positive remodeling showed more frequent plaque rupture on the epicardial side of the coronary vessel wall in anterior MI than those without positive remodeling.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
There is increasing evidence that acute clinical manifestations of coronary atherosclerosis are caused by plaque rupture and subsequent thrombus formation resulting from exposure of plaque components to flowing blood [1–3]. Plaque rupture at culprit lesions is associated with larger infarcts and left ventricular dysfunction [4], and reduced left ventricular function is a major risk factor for sudden death after myocardial infarction (MI) [5]. Positive remodeling of atherosclerotic plaques suggests a potential surrogate marker of plaque vulnerability as an indicator of the risk of plaque rupture [6]. Intravascular ultrasound (IVUS) imaging technology allows direct visualization of the distribution and morphology of atherosclerotic plaques in vivo and has greatly contributed to our understanding of plaque characteristics [7–9]. It also helps to determine the inner and outer curve of the vessel precisely based on the vascular and perivascular landmarks, especially in the left anterior descending coronary artery [10]. Several studies using IVUS have identified eccentric morphology, a thin fibrous cap, large lipid core [11], expanding remodeling [12, 13], and spotty calcification [14] as indicators of plaque vulnerability. However, the interactions among these factors are not fully understood. Previous studies have demonstrated that high shear stress causes plaque rupture [15, 16]. An in vivo study showed that all coronary plaque ruptures were observed in the proximal portion of the plaque hill, suggesting that a localized elevation of shear stress might be related to coronary plaque rupture [17]. Therefore, we hypothesized that coronary plaque rupture was prone to be observed on the outer side of the atherosclerotic plaque where shear stress is relatively high. The purpose of this study was to assess the association between the spatial location of plaque rupture defined by IVUS and remodeling pattern in the setting of acute anterior MI.
Methods
Study patients
We included consecutive patients with a first ST-elevation acute anterior MI defined as the presence of continuous chest symptoms for more than 30 min, accompanied by ST-segment elevation of >0.2 mV in at least two contiguous electrocardiographic leads and by an increase in serum creatine kinase levels to more than twice the upper limit of normal. All patients received coronary reperfusion within 6 h after the onset of symptoms, and infarct-related arteries were confirmed by IVUS before any percutaneous coronary intervention (PCI). Patients with malignant disease, infectious disease, or inflammatory diseases such as collagen disease were excluded to avoid bias. We also excluded patients with cardiogenic shock because we performed reperfusion procedures as soon as possible without preintervention IVUS. Plaque rupture was assessed in 90 patients who met all inclusion criteria. Fifty-two patients showed plaque rupture at the culprit lesion detected by preintervention IVUS, and they were enrolled in the present study. The protocol for the study was approved by the ethics committee of the Yokohama City University Medical Center. We obtained written, informed consent from all participants before initial coronary angiography.
Study protocol
On admission, all patients received 200 mg aspirin, an intravenous bolus injection of 5000 IU heparin, and isosorbide dinitrate. In the catheterization laboratory, coronary angiography was performed via the femoral approach. Periprocedural intravenous heparin was given to maintain an activated clotting time ≥300 s. Intracoronary isosorbide dinitrate (2–2.5 mg) was administered before angiography to prevent coronary artery spasm. After careful manipulation of the guidewire and additional intracoronary isosorbide dinitrate (2–2.5 mg), the IVUS catheter was advanced distal to the culprit lesion.
All IVUS studies were performed with a commercially available system (Scimed; Boston Scientific, Boston, MA) before any balloon inflation or stent implantation. A 40-MHz IVUS catheter was advanced distal to the culprit lesion, and imaging was performed in a retrograde fashion to the aorto-ostial junction with an automatic pullback speed of 0.5 mm/s. While pulling back the catheter, we manually infused contrast medium or normal saline suitable for IVUS imaging, while carefully observing the lesion. This procedure enabled us to eliminate blood noise and to observe communication between the plaque and the coronary artery lumen [4]. The IVUS images were recorded on S-VHS videotape for off-line analysis.
Clinical histories, including cardiovascular risk factors and current medications, were obtained from detailed interviews and medical records. Blood samples were obtained from all subjects on admission and analyzed in the hospital laboratory.
Analysis of IVUS images
Morphological features on IVUS images were independently interpreted by 2 experienced observers blinded to the angiographic and clinical data. Images for which the 2 observers agreed on the diagnosis were included in the subsequent analysis. Evaluation of lesion morphology and other measurements during IVUS was done according to the American College of Cardiology Clinical Expert Consensus Document on Standards for Acquisition, Measurement, and Reporting of Intravascular Ultrasound Studies [18]. We defined lesions with plaque rupture on IVUS as follows: (1) lesions with fissure/dissection or (2) lesions without fissure/dissection but in which the injection of saline or contrast medium confirmed communication between the plaque and the coronary artery lumen [19] (Figs. 1, 2, 3). The eccentricity index was calculated as (maximum plaque plus media thickness minus minimum plaque plus media thickness) divided by maximum plaque plus media thickness. An eccentric plaque was defined by an eccentricity index of >0.5. Plaque composition was assessed visually and classified as echolucent when >75% of the plaque area was less bright than the adventitia; otherwise, plaque composition was classified as echodense.
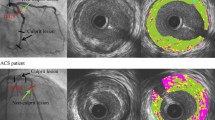
Representative case of plaque rupture on the myocardial side. Upper panel shows a preintervention angiographic image. i An intravascular image of plaque rupture. Plaque ulceration with fissure (white arrow) on the myocardial side and ruptured cavity (arrowheads) are observed. Lower panel shows the postintervention angiographic image and branching landmarks seen by intravascular ultrasound for spatial orientation in the coronary artery. a First diagonal branch (asterisk), b second diagonal branch (asterisk), c major septal branch (asterisk), d myocardial bridge (arrowheads) indicates that this side is epicardial
Representative case of plaque rupture on the epicardial side (ii). Plaque ulceration with fissure (white arrow) on the epicardial side and ruptured cavity (arrowheads) are observed. e–g Branching landmarks seen by intravascular ultrasound for spatial orientation in the coronary artery. e First major septal branch (asterisk), f first diagonal branch (asterisk), g second septal branch (asterisk), h this panel indicates that left side is epicardial
Representative case of plaque rupture on the lateral side (iii). Plaque ulceration with fissure (white arrow) on the lateral side and ruptured cavity (arrowheads) are observed. i, j Branching landmarks seen by intravascular ultrasound for spatial orientation in the coronary artery. i First major septal branch (asterisk), j first diagonal branch (asterisk), this panel indicates that left side is epicardial
Using the pericardium, side branches, coronary veins, and myocardium as landmarks, we divided the plaques into 3 categories according to the spatial orientation of the plaque rupture site (Figs. 1, 2, 3). Spatial orientation in the vessel was as follows: myocardial quadrant (inner curve of the vessel), epicardial quadrant (outer curve of the vessel), and lateral quadrants (2 quadrants intermediate).
External elastic membrane cross-sectional area (EEM CSA) and lumen CSA (in mm squared) at the lesion site and at the proximal and distal reference sites were analyzed using planimetry software (TapeMeasure, Indec Systems, Capitola, CA). The reference segments were the most normal-looking CSA within 5 mm proximal and distal to the lesion but before any side branch. Remodeling was considered positive when the lesion EEM CSA was greater than both the proximal and distal reference EEM CSAs [20]. The remodeling index was determined by dividing the EEM CSA at the lesion site by the mean value of the EEM CSA at the proximal and distal reference sites.
Statistical analysis
Statistical analysis was performed with StatView 5.0 software (SAS Institute, Cary, NC). Results are expressed as mean ± SD for continuous variables. Qualitative data are presented as numbers (%). Continuous variables were compared by means of Student’s t test, and categorical data were compared by the chi-square test or Fisher’s exact test between the groups. For all analyses, values of p < 0.05 were considered to indicate statistically significance.
Results
Coronary artery lesions were successfully observed before balloon inflation or stent implantation in all patients by IVUS, without procedural complications. Patients were classified according to the presence or absence of positive remodeling. Thirty-five patients showed positive remodeling (positive group), and 17 did not show positive remodeling (non-positive group). Baseline patient characteristics are summarized in Table 1. There were no statistically significant differences in age, gender, presence of preinfarction angina, risk factors, and laboratory results between the groups. The preintervention IVUS findings are summarized in Table 2. Plaques in the positive group showed a higher proportion of soft plaque morphology (94 vs. 65%, p = 0.0016) and had a larger vessel area at the culprit-lesion site (19.2 ± 4.4 vs. 15.2 ± 4.7 mm2, p = 0.0053) than plaques in the non-positive group. In 52 plaque ruptures, 27 were oriented toward the epicardial side (52%), 18 toward the myocardial side (35%), and 7 in the 2 lateral quadrants (13%). Among 35 plaques in the positive group, plaque rupture was observed in 21 (52%) on the epicardial side, 12 (34%) on the myocardial side, and 2 (6%) on the lateral side. Among 17 plaques in the non-positive group, plaque rupture was observed in 6 (35%) on the epicardial side, 6 (35%) on the myocardial side, and 5 (30%) on the lateral side. The orientations of plaque rupture were significantly different with an increased incidence of plaque rupture on the epicardial side in the positive group (p = 0.047) (Table 2). Among 33 plaques that showed eccentric plaque, the distribution of atherosclerotic plaque was not significantly different between the groups (data not shown).
Discussion
In this study, we examined the culprit plaque in acute MI and found that plaques with positive remodeling showed more frequent plaque rupture on the epicardial side of the coronary vessel wall than those without positive remodeling.
Positive remodeling is defined as a compensatory increase in local vessel size in response to increasing plaque burden [21]. Several studies have suggested that positive remodeling is a potential surrogate marker of plaque vulnerability [6, 22]. Previous studies demonstrated that positive remodeling and larger plaque areas were associated with acute coronary syndrome [12, 13] and suggested that positive arterial remodeling may be associated with an increased risk of plaque rupture [23]. In addition, recent optical coherence tomography studies demonstrated that the presence of microchannels in plaques correlated with a greater frequency of thin-cap fibroatheroma and positive remodeling [24, 25], suggesting an important role of vasa vasorum in plaque instability and coronary remodeling. Consistently, the biological implications of the relationship between coronary remodeling and plaque disruption suggest that large plaques with positive remodeling are more susceptible to mechanical forces that lead to plaque rupture. Imoto et al. [26] showed that positive remodeling led to a greater concentration of stress than did constrictive remodeling. In the present study, plaque rupture was observed equally in 3 categories (epicardial, myocardial, lateral sides) in patients without positive remodeling, whereas plaque rupture was more frequently on the epicardial side in patients with positive remodeling. These findings indicate that mechanical force such as high shear stress along the outer curve of the vessel wall, which corresponds to the epicardial side, may cause plaque rupture in plaques with positive remodeling.
Shear stress is one of the important physical factors in the process of atherosclerosis. Shear stress is defined as a stress produced by blood flow, which tends to cause vessel endothelium to slide or to be deformed. It has been recognized that regions of low shear stress are more susceptible to atheroma formation, and many studies have demonstrated an association between low shear stress and atherosclerotic deposits [27–30]. Furthermore, a recent study suggested that a localized elevation of shear stress might be related to coronary plaque rupture and showed that all coronary plaque ruptures were observed in the proximal of top portion of the plaque hill [17]. In addition to the process of atherosclerosis development and progression, several studies showed that lowered shear-stress lesions were associated with outward (positive, expansive) vascular remodeling [31, 32]. These studies indicate that low shear stress is relevant not only to the spatial orientation of atherosclerotic plaques, but also to the positive remodeling leading to increased plaque vulnerability.
Although the relationship between shear stress and plaque development is still evolving, there is little information in vivo on the relationship between plaque disruption and shear stress. A previous autopsy study showed differences in cell composition between upstream and downstream parts of plaques and suggested that high shear/high flow may be related to plaque instability [15]. Fukumoto et al. [17] demonstrated that a localized elevation of shear stress on the plaque surface was related to coronary plaque rupture in patients using a simplified computational analysis from three-dimensional IVUS plaque images. Our study showed that plaque rupture was observed more frequently on the epicardial side than myocardial and lateral sides in plaques with positive remodeling, further extending the previous findings regarding the association between shear stress and plaque rupture. Additionally, elucidation of the geometry of plaque rupture would contribute to future understanding of the coronary artery disease.
Atherosclerotic plaque is ubiquitous even in coronary arteries without angiographically significant stenosis [33]. Our findings suggest that coronary plaques located in the epicardial side of the coronary vessel wall with positive remodeling represent the high-risk for plaque rupture and may contribute to the management of the patients with coronary artery disease and prevention of coronary events. A recent study demonstrated that aggressive lipid-lowering therapy achieved significant regression of the coronary plaque volume with negative vessel remodeling in patients with acute coronary syndrome [34]. In a serial 3-vessel IVUS and optical coherence tomography study, Yamada et al. demonstrated that percent changes in fibrous cap thickness correlated negatively with the percent changes of EEM CSA during 6-month follow-up. These results provided evidence that administration of statins might reverse the remodeling process of atherosclerosis and reduce plaque vulnerability [35].
Our study had several limitations. This was a single-center, retrospective study with a relatively small number of patients. We enrolled only patients with anterior acute MI because arterial length and branching patterns differ among the left anterior descending, left circumflex, and right coronary arteries. Because of the differences in distortion among the three coronary arteries, the effect of endothelial shear stresses may differ. Therefore, our results cannot be extrapolated to left circumflex and right coronary arteries. Although we carefully examined lesions by flushing the surrounding region with saline or contrast medium, lesions with small ruptured plaques may have been misread as non-ruptured plaques. Finally, in the present study, we did not calculate the distribution of the shear stress on the plaque surface, so these results are largely conjectural.
Conclusion
In the setting of anterior MI, plaque rupture of atherosclerotic plaque with positive remodeling was located more frequently on the epicardial side of the coronary vessel wall.
References
Fuster V, Badimon L, Badimon JJ, Chesebro JH (1992) The pathogenesis of coronary artery disease and the acute coronary syndromes (1). N Engl J Med 326:242–250
Fuster V, Badimon L, Badimon JJ, Chesebro JH (1992) The pathogenesis of coronary artery disease and the acute coronary syndromes (2). N Engl J Med 326:310–318
Setianto BY, Hartopo AB, Gharini PP, Anggrahini DW, Irawan B (2010) Circulating soluble CD40 ligand mediates the interaction between neutrophils and platelets in acute coronary syndrome. Heart Vessels 25:282–287
Kusama I, Hibi K, Kosuge M, Nozawa N, Ozaki H, Yano H, Sumita S, Tsukahara K, Okuda J, Ebina T, Umemura S, Kimura K (2007) Impact of plaque rupture on infarct size in ST-segment elevation anterior acute myocardial infarction. J Am Coll Cardiol 50:1230–1237
Mukharji J, Rude RE, Poole WK, Gustafson N, Thomas LJ Jr, Strauss HW, Jaffe AS, Muller JE, Roberts R, Raabe DS Jr (1984) Risk factors for sudden death after acute myocardial infarction: two-year follow-up. Am J Cardiol 54:31–36
Varnava AM, Mills PG, Davies MJ (2002) Relationship between coronary artery remodeling and plaque vulnerability. Circulation 105:939–943
Yock PG, Linker DT (1990) Intravascular ultrasound. Looking below the surface of vascular disease. Circulation 81:1715–1718
Nissen SE, Gurley JC, Grines CL, Booth DC, McClure R, Berk M, Fischer C, de Maria AN (1991) Intravascular ultrasound assessment of lumen size and wall morphology in normal subjects and patients with coronary artery disease. Circulation 84:1087–1099
Tobis JM, Mallery J, Mahon D, Lehmann K, Zalesky P, Griffith J, Gessert J, Moriuchi M, McRae M, Dwyer ML (1991) Intravascular ultrasound imaging of human coronary arteries in vivo. Analysis of tissue characterizations with comparison to in vitro histological specimens. Circulation 83:913–926
Fitzgerald PJ, Yock C, Yock PG (1998) Orientation of intracoronary ultrasonography: looking beyond the artery. J Am Soc Echocardiogr 11:13–19
Nissen SE, Yock P (2001) Intravascular ultrasound: novel pathophysiological insights and current clinical applications. Circulation 103:604–616
von Birgelen C, Klinkhart W, Mintz GS, Papatheodorou A, Herrmann J, Baumgart D, Haude M, Wieneke H, Ge J, Erbel R (2001) Plaque distribution and vascular remodeling of ruptured and nonruptured coronary plaques in the same vessel: an intravascular ultrasound study in vivo. J Am Coll Cardiol 37:1864–1870
Schoenhagen P, Ziada KM, Kapadia SR, Crowe TD, Nissen SE, Tuzcu EM (2000) Extent and direction of arterial remodeling in stable versus unstable coronary syndromes: an intravascular ultrasound study. Circulation 101:598–603
Ehara S, Kobayashi Y, Yoshiyama M, Shimada K, Shimada Y, Fukuda D, Nakamura Y, Yamashita H, Yamagishi H, Takeuchi K, Naruko T, Haze K, Becker AE, Yoshikawa J, Ueda M (2004) Spotty calcification typifies the culprit plaque in patients with acute myocardial infarction: an intravascular ultrasound study. Circulation 110:3424–3429
Dirksen MT, van der Wal AC, van den Berg FM, van der Loos CM, Becker AE (1998) Distribution of inflammatory cells in atherosclerotic plaques relates to the direction of flow. Circulation 98:2000–2003
Cheng GC, Loree HM, Kamm RD, Fishbein MC, Lee RT (1993) Distribution of circumferential stress in ruptured and stable atherosclerotic lesions. A structural analysis with histopathological correlation. Circulation 87:1179–1187
Fukumoto Y, Hiro T, Fujii T, Hashimoto G, Fujimura T, Yamada J, Okamura T, Matsuzaki M (2008) Localized elevation of shear stress is related to coronary plaque rupture: a 3-dimensional intravascular ultrasound study with in vivo color mapping of shear stress distribution. J Am Coll Cardiol 51:645–650
Mintz GS, Nissen SE, Anderson WD, Bailey SR, Erbel R, Fitzgerald PJ, Pinto FJ, Rosenfield K, Siegel RJ, Tuzcu EM, Yock PG (2001) American College of Cardiology Clinical Expert Consensus Document on Standards for Acquisition, Measurement and Reporting of Intravascular Ultrasound Studies (IVUS). A report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol 37:1478–1492
Sano T, Tanaka A, Namba M, Nishibori Y, Nishida Y, Kawarabayashi T, Fukuda D, Shimada K, Yoshikawa J (2003) C-reactive protein and lesion morphology in patients with acute myocardial infarction. Circulation 108:282–285
Hibi K, Ward MR, Honda Y, Suzuki T, Jeremias A, Okura H, Hassan AH, Maehara A, Yeung AC, Pasterkamp G, Fitzgerald PJ, Yock PG (2005) Impact of different definitions on the interpretation of coronary remodeling determined by intravascular ultrasound. Catheter Cardiovasc Interv 65:233–239
Losordo DW, Rosenfield K, Kaufman J, Pieczek A, Isner JM (1994) Focal compensatory enlargement of human arteries in response to progressive atherosclerosis. In vivo documentation using intravascular ultrasound. Circulation 89:2570–2577
Smits PC, Pasterkamp G, de Jaegere PP, de Feyter PJ, Borst C (1999) Angioscopic complex lesions are predominantly compensatory enlarged: an angioscopy and intracoronary ultrasound study. Cardiovasc Res 41:458–464
Pasterkamp G, Schoneveld AH, van der Wal AC, Haudenschild CC, Clarijs RJ, Becker AE, Hillen B, Borst C (1998) Relation of arterial geometry to luminal narrowing and histologic markers for plaque vulnerability: the remodeling paradox. J Am Coll Cardiol 32:655–662
Kitabata H, Tanaka A, Kubo T, Takarada S, Kashiwagi M, Tsujioka H, Ikejima H, Kuroi A, Kataiwa H, Ishibashi K, Komukai K, Tanimoto T, Ino Y, Hirata K, Nakamura N, Mizukoshi M, Imanishi T, Akasaka T (2010) Relation of microchannel structure identified by optical coherence tomography to plaque vulnerability in patients with coronary artery disease. Am J Cardiol 105:1673–1678
Kashiwagi M, Tanaka A, Kitabata H, Tsujioka H, Matsumoto H, Arita Y, Ookochi K, Kuroi A, Kataiwa H, Tanimoto T, Ikejima H, Takarada S, Kubo T, Hirata K, Nakamura N, Mizukoshi M, Imanishi T, Akasaka T (2009) Relationship between coronary arterial remodeling, fibrous cap thickness and high-sensitivity C-reactive protein levels in patients with acute coronary syndrome. Circ J 73:1291–1295
Imoto K, Hiro T, Fujii T, Murashige A, Fukumoto Y, Hashimoto G, Okamura T, Yamada J, Mori K, Matsuzaki M (2005) Longitudinal structural determinants of atherosclerotic plaque vulnerability: a computational analysis of stress distribution using vessel models and three-dimensional intravascular ultrasound imaging. J Am Coll Cardiol 46:1507–1515
Friedman MH, Bargeron CB, Deters OJ, Hutchins GM, Mark FF (1987) Correlation between wall shear and intimal thickness at a coronary artery branch. Atherosclerosis 68:27–33
Krams R, Wentzel JJ, Oomen JA, Vinke R, Schuurbiers JC, de Feyter PJ, Serruys PW, Slager CJ (1997) Evaluation of endothelial shear stress and 3D geometry as factors determining the development of atherosclerosis and remodeling in human coronary arteries in vivo. Combining 3D reconstruction from angiography and IVUS (ANGUS) with computational fluid dynamics. Arterioscler Thromb Vasc Biol 17:2061–2065
Jeremias A, Huegel H, Lee DP, Hassan A, Wolf A, Yeung AC, Yock PG, Fitzgerald PJ (2000) Spatial orientation of atherosclerotic plaque in non-branching coronary artery segments. Atherosclerosis 152:209–215
Wentzel JJ, Janssen E, Vos J, Schuurbiers JC, Krams R, Serruys PW, de Feyter PJ, Slager CJ (2003) Extension of increased atherosclerotic wall thickness into high shear stress regions is associated with loss of compensatory remodeling. Circulation 108:17–23
Cheng C, Tempel D, van Haperen R, van der Baan A, Grosveld F, Daemen MJ, Krams R, de Crom R (2006) Atherosclerotic lesion size and vulnerability are determined by patterns of fluid shear stress. Circulation 113:2744–2753
Chatzizisis YS, Jonas M, Coskun AU, Beigel R, Stone BV, Maynard C, Gerrity RG, Daley W, Rogers C, Edelman ER, Feldman CL, Stone PH (2008) Prediction of the localization of high-risk coronary atherosclerotic plaques on the basis of low endothelial shear stress: an intravascular ultrasound and histopathology natural history study. Circulation 117:993–1002
Ishio N, Kobayashi Y, Iwata Y, Kitahara H, Fukushima K, Asano T, Nakayama T, Kuroda N, Komuro I (2010) Ubiquitous atherosclerosis in coronary arteries without angiographically significant stenosis. Heart Vessels 25:35–40
Hiro T, Kimura T, Morimoto T, Miyauchi K, Nakagawa Y, Yamagishi M, Ozaki Y, Kimura K, Saito S, Yamaguchi T, Daida H, Matsuzaki M (2009) Effect of intensive statin therapy on regression of coronary atherosclerosis in patients with acute coronary syndrome: a multicenter randomized trial evaluated by volumetric intravascular ultrasound using pitavastatin versus atorvastatin (JAPAN-ACS [Japan assessment of pitavastatin and atorvastatin in acute coronary syndrome] study). J Am Coll Cardiol 54:293–302
Yamada R, Okura H, Kume T, Saito K, Miyamoto Y, Imai K, Tsuchiya T, Maehama T, Okahashi N, Obase K, Hayashida A, Neishi Y, Kawamoto T, Yoshida K (2010) Relationship between arterial and fibrous cap remodeling: a serial three-vessel intravascular ultrasound and optical coherence tomography study. Circ Cardiovasc Interv 3:484–490
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kusama, I., Hibi, K., Kosuge, M. et al. Intravascular ultrasound assessment of the association between spatial orientation of ruptured coronary plaques and remodeling morphology of culprit plaques in ST-elevation acute myocardial infarction. Heart Vessels 27, 541–547 (2012). https://doi.org/10.1007/s00380-011-0184-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-011-0184-7