Abstract
Use of inorganic fertilizers in smallholder cropping systems in Africa is often becoming inefficient due to increasing unresponsiveness to fertilizer application. A study was conducted for 2 years (four seasons) to assess the effects of biochar made from Prosopis juliflora (Sw.) DC. biomass on nutrients, fauna abundance and subsequent influence on maize planted in a nitisol. There were 12 amendments comprising: (i) biochar applied alone at a rate of 5 and 10 Mg ha−1; (ii) three fertilizer types applied separately (di-ammonium phosphate (18:46:0), urea (46:0:0) and composite NPK (23:23:0)); (iii) six fertilizer + biochar blends of the three fertilizer types and two biochar rates (0.05 and 0.1 Mg ha−1); and (iv) a control with no inputs. Treatments were replicated four times in a randomized complete block design. The amendments were applied in the first two seasons, while the last two were used to assess residual effects. At the end of the first two seasons, total C and N were higher in soils where biochar or fertilizer + biochar was applied, with more than 15.0 g C and 1.9 g N kg−1, compared to 10.4 g C and 1.0 g N kg−1 in control plots. Available P and exchangeable K were over 200% and 100% higher in biochar or fertilizer + biochar amended than control soils, respectively. Application of biochar had no effects on macrofauna such as beetles, centipedes, millipedes, termites and ants, but attracted earthworms. Soil that received 10 Mg biochar ha−1 recorded twice the number of earthworms (207 individuals m−2) compared to soil with 5 Mg biochar ha−1 (105 individuals m−2) and control (97 individuals m−2). Soils which received biochar, with or without fertilizer, had higher taxonomic richness (7.0 species) compared to soils which received DAP (2.8) or NPK (3.8). Nematodes, particularly bacterivorous groups, decreased by more than eight times with biochar application. In the first and second seasons, 5.6 Mg maize grain yield ha−1 was obtained from plots amended with biochar (without fertilizer), which was about six times higher than that harvested from unfertilised control at 0.9 Mg ha−1. Yield differences in plots where fertilizer was applied with or without biochar were not significant. Yield in the third and fourth seasons declined to 3.2 and 1.5 Mg ha−1, irrespective of fertilizer type or biochar amounts.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sustaining crop productivity in Africa is a major challenge given that the cropping systems rely on low external organic or inorganic inputs despite continuous cultivation, resulting in degradation of the soils (Mbau et al. 2015). Thus, continual use of only inorganic fertilizers is becoming inefficient and unsustainable, especially in smallholder farming systems, due to high cost of fertilizers and increased unresponsiveness of the soils to inorganic fertilizer application, which is mainly driven by soil degradation and insufficient organic residue inputs (Vanlauwe et al. 2011). One of the major drivers of soil degradation is the declining levels of soil organic matter (SOM) and efforts are being made across tropical agricultural systems to improve such soils by restoring SOM content (Ayuke et al. 2011). Biochar has been suggested as a promising soil amendment to maintain high SOM even under humid tropic conditions due to its persistence (Chan et al. 2008; Lehmann et al. 2006), significantly contribute to cation retention (Liang et al. 2006; Mao et al. 2012) and reduce decomposition of native SOM (Ventura et al. 2019). In addition, it has been suggested as a potential amendment for improving soil fertility by directly adding elements such as P, K, Ca as well as microelements (Glaser et al. 2002). However, the properties of biochar and thus, the potential of biochar to improve supply of these nutrients, is greatly affected by production process and type of feedstock (Chan et al. 2008; Lehmann et al. 2011; Schimmelpfennig and Glaser 2012). For instance, under similar production conditions, biochar made from woody materials is expected to be of low nutrient value compared to that derived from manure due to the low nutrient contents in the wood (Kamau et al. 2017; Waters et al. 2011). Nonetheless, use of manure in biochar production in Africa may be viable only in large-scale farming systems e.g. pig farmers who are often faced with a challenge of disposing the waste due to high loads of pathogens. Thus, when woody materials are the main feedstock, there may be a need to supplement the biochar with either high-quality organic or inorganic fertilizer. Besides chemical properties, biochar has been shown to cause significant shifts in soil biota, which could be associated with alteration in soil biotic and abiotic conditions (Chen et al. 2018; Cheng et al. 2018; Lehmann et al. 2011; O’Neill et al. 2009; Warnock et al. 2007). Recent studies have also given inconsistent results of biochar application with respect to soil invertebrates, some showing negative while others positive responses (Domene et al. 2015; Verheijen et al. 2010). Given the important role of soil fauna in moderating soil processes and functions, understanding the effects of biochar on spatial distribution of soil fauna can be a starting point towards achieving sustainable agroecosystems. This is especially important in Africa where majority of people obtain their livelihoods from subsistence farming (Kamau et al. 2017).
A major concern of biochar use is availability of adequate amounts of biomass due to the competing uses such as for feed and fuel (Mbau 2012). One potential source of biomass in the tropics that remains underutilized is Prosopis juliflora (Sw.) DC., a shrub that is considered one of the worst invasive plants in the region. Several studies have shown that the shrub remains a major threat to rangelands and croplands especially in Africa, Australia, Middle East and the Indian subcontinent due to its rapid rate of invasion (Kaur et al. 2012; Mbaabu et al. 2019; Mwangi and Swallow 2008; Shiferaw et al. 2019). For instance, in Eastern Africa, the shrub has spread from a few plots established in 1970s and 1980s to millions of hectares in recent years (Wakie et al. 2012). To contain the invasion, it has been suggested that P. juliflora biomass can be harvested for fuel due to its high calorific value and high biomass production, estimated to be 25 to 30 Mg ha−1 year−1 within a cycle of 4 to 5 years (Mwangi and Swallow 2008). In Kenya, one such option that is being tested is generation of electricity through gasification of the wood derived from the shrub. Such a process is expected to generate large amounts of biochar as a by-product which can then be utilized as a soil amendment.
The aim of this study was therefore, to evaluate the potential of biochar derived from P. juliflora and blends of the biochar with inorganic fertilizer in restoring the fertility of nutrient deficient soil and their effects on fauna abundance and diversity. It was hypothesized that: (i) soil chemical properties will change as a result of biochar application and, (ii) soil macrofauna abundance would increase with increased amounts of biochar, but that the magnitude of these effects would be modulated by the type of inorganic fertilizer.
Materials and methods
Description of the study site
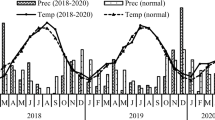
The study was conducted at the University of Nairobi’s Upper Kabete Field Station, located about 10 km Northwest of Nairobi City at latitude 1° 15′ S and longitude 36° 44′ E, with an elevation of approximately 1900 m above sea level. The area is classified as an upper sub-humid midland (UM2) agroecological zone (Jaetzold et al. 2006), receiving an average annual precipitation of about 1000 mm in a bimodal rainfall pattern. Approximately 600 mm of the rainfall is received between March and May often locally referred to as “long-rains” and 400 mm between October and December, called “short-rains”. Temperatures are fairly constant throughout the year with a minimum and maximum mean temperature of 14 °C and 24 °C, respectively. The average monthly rainfall and temperature over the study period are given in Fig. 1. Soils where the study was conducted are classified as Nitisols (Jaetzold et al. 2006). Soils before the experiment were slightly acidic (pH of 5.5), with low available P (10.0 mg kg−1), exchangeable K (0.6 g kg−1), total C (15.0 g kg−1) and N (1.0 g kg−1) (Table 1).
Monthly average rainfall and temperature during the study seasons — 2014/15, 2015/16 and a 20-year average (1994–2013). Source: Gitari et al. 2019
Chemical characterization of the biochar
Biochar used in this study was obtained from Cummins Cogeneration (Kenya), a private electricity-producing company, which uses Prosopis juliflora (Sw.) DC trees as raw materials in its operations. Biochar is one of the by-products from the power generation process and was made at 800 °C with an approximate residence time of 5 min. Fresh biochar was obtained from the company in sealed bags. Before its application at the experimental site or making the fertilizer blends, the biochar was homogenized and about 50 g of sample was retained for chemical analyses. The sample was air dried until a constant weight, fine-ground and stored in bags awaiting analysis. The samples were analysed for pH and macro-elements (C, N, P, K, Ca and Mg). The pH was determined in water using a 1:5 biochar/water ratio. Total C and N were determined by FLASH 2000 NC Analyser (ThermoFisher Scientific, Cambridge, UK) while P, K, Ca and Mg were extracted through a closed-vessel microwave-assisted digestion system (Miller 1998) and determined using inductively coupled plasma atomic emission spectroscopy (Isaac and Johnson 1998). The biochar was found to have relatively low total N, and available P and Mg contents with values of 14.0 g, 4.2 g and 6.5 g kg−1, respectively compared to available K and Ca, with values of 135.6 g and 153.7 g kg−1, respectively (Table 1). The biochar had a high pH (8.6).
Experimental design
The farm used in the study had been under maize for three consecutive years with no organic or inorganic inputs added during this period. The farm was divided into 5 m by 4 m plots and the amendments randomly allocated in these plots. The amendments comprised: (i) biochar applied alone at a rate of 5 and 10 Mg ha−1; (ii) three fertilizer types applied separately (di-ammonium phosphate (18:46:0), urea (46:0:0) and composite NPK (23:23:0)). The DAP was applied at the rates commonly used by smallholder farmers of 75 kg ha−1 (13.5 kg N; 15 kg P), urea at a rate of 100 kg ha−1 (46 kg N ha−1) and NPK at 150 kg ha−1 (34.5 kg N; 15 kg P); (iii) six blends of fertilizer + biochar made from each fertilizer type and two biochar rates. The amount of biochar in each of the blend was equivalent to 0.05 Mg and 0.1 Mg ha−1. These blends were made before field application was done; and (iv) a control with no biochar or fertilizer applied. Several ratios of fertilizer + biochar were tested for physical consistency after blending to assess their ease of application. It was observed that more than 0.1 Mg ha−1 of biochar in the blend made the mixture to cake, thus making it difficult to apply. Therefore, we chose 0.1 Mg ha−1 and half this amount (0.05 Mg ha−1) as our final ratios.
The treatments were replicated four times in a randomized complete block design. In each plot, 0.1-m deep furrows, spaced at 0.75 m, were made in preparation for planting. Treatments were then spread evenly in the furrows and incorporated with the topsoil. Finally, two maize seeds were planted per hill at a spacing of 0.25 m within the furrows and covered to a depth of 0.05 m. Only one seedling was retained after thinning, which was done immediately after crop emergence. The study was done for four consecutive seasons, from the onset of short rains in 2014 (October) to the end of long rains in 2016 (July). The amendments were applied in the first two consecutive seasons; no inputs were applied in the last two seasons. After harvesting at the end of each season, the stalks were removed from the plots in order to retain the integrity of applied treatments for subsequent seasons. However, stubbles left after harvesting were incorporated into the soil during land preparation using hand hoes.
Soil sampling, preparation and analysis
In each of the four seasons, soil samples were randomly taken from four points on each plot using soil augers to a depth of 0.2 m at the sixth week after crop emergence. The soil was thoroughly mixed to make one composite sample for the analysis. The soil properties analysed included: soil pH, total C, total and available N, available P and exchangeable K. Soil pH was determined in water using a 1:2.5 soil/solution ratio. Inorganic N (NO3− and NH4+) was extracted using 2 M potassium chloride (KCl) and determined using steam distillation method (Bremner and Keeney 1965). Total C and N were determined by FLASH 2000 NC Analyser (ThermoFisher Scientific, Cambridge, UK). Available P and exchangeable K, Ca and Mg were extracted by Mehlich-3 procedure (Mehlich 1984) and measured using an Inductively Coupled Plasma Atomic Emission Spectrophotometre (Isaac and Johnson 1998).
Soil fauna sampling
Sampling for soil macrofauna was conducted using soil monolith (0.25 by 0.25 by 0.30 m), where two monoliths were randomly excavated from each plot following the standard Tropical Soil Biology and Fertility Programme (TSBF) sampling protocol (Anderson and Ingram 1993). Sampling was done once each season at 8 weeks after the crop emergence. The soil samples were placed in trays and large clods broken slowly to facilitate hand-sorting of soil macrofauna. All soil macrofauna were first placed in 75% ethanol and at the end of the sampling exercise, the macrofauna (except earthworms) were transferred into fresh ethanol. Earthworms were transferred into 4% formaldehyde for preservation. Soil macrofauna were identified to genera or species and the abundance calculated as number of individuals per square metre (individuals m−2). Sampling for nematodes was done using a soil auger at six points within each plot to a depth of 0.2 m. The six cores were mixed thoroughly and a sub-sample derived from them. Extraction of nematodes was done using Baerman pan technique (Forge and Kimpinski 2007) followed by identification and enumeration. Nematodes were identified to genera and the abundance reported as numbers per 100 g of soil (numbers 100 g−1 soil).
Statistical analysis and data management
Generalized linear mixed models (GLMM) were used to test the effects of biochar, fertilizer and fertilizer + biochar amendments on soil fauna, given that the data showed deviation from normality and lack of homogeneity of variance. The analysis was conducted using the package lme4 in R (Bates et al. 2015) in R statistical software, version 3.3.2 (R-Core Team 2016). Negative binomial regression analysis was chosen as an extension of the Poisson distribution to allow for the count data with a significant proportion of zero values. Maximum likelihood (ML) was used to estimate the model parameters and the model selection was based on Akaike Information Criterion (AIC). Where analysis of variance (ANOVA) showed significant effects, Tukey’s HSD test was used to separate the means at p < 0.05.
Results
Changes in soil chemical properties as a result of biochar and fertilizer + biochar application
All soil chemical properties measured (except pH) were significantly affected by the amendments in the first two seasons (Table 2). Total C and N contents were significantly lower in the unamended control (9.4 g and 0.9 g kg−1, respectively) compared to soils amended with either biochar, fertilizers and fertilizer + biochar which recorded more than 15.0 g kg−1 C and 1.9 g kg−1 N. This was about 50% higher C content and more than double the amount of N than in control plots. Exchangeable K, P, NH4+ and NO3− contents showed similar differences as C and N contents. In seasons 3 and 4, only available NO3− showed significant differences. Soil amended with fertilizer +0.05 Mg biochar ha−1, regardless of the fertilized type, had significantly higher NO3− content than soils with the other amendments. However, C content at the end of the fourth season was notably higher in all the plots that received biochar, fertilizer or fertilizer + biochar blends than in the first two seasons.
Response of soil macrofauna to biochar and fertilizer + biochar application
Of the 11 soil macrofauna groups recovered, only earthworms, were influenced by biochar and fertilizer additions (Table 3). The higher rate of biochar application appeared to attract earthworms to soils which received 10 Mg biochar ha−1 recording the highest number of earthworms (207 individuals m−2), compared to plots which received 5 Mg biochar ha−1 (105 individuals m−2) and control (97 individuals m−2). Application of inorganic fertilizer had negative effects on earthworm abundance. No earthworms were recovered from soils where either urea was applied alone or with 0.05 Mg biochar ha−1. The response of earthworms to NPK fertilizer showed a similar trend to that of urea whereas additions of DAP (with and without biochar) did not show any specific trends.
The diversity and taxonomic richness varied across soils with different additions (Table 3). Soils amended with biochar and fertilizer + biochar blends had more than 50% higher taxonomic richness compared with soils where DAP and NPK was applied alone (p < 0.001). For instance, soils which received biochar (either at 5 Mg or 10 Mg biochar ha−1) and fertilizer + biochar blends (either at 0.05 Mg or 0.1 Mg biochar ha−1) had on average a taxonomic richness of about 7.0 species compared to about 3.3 species in soils which received either DAP or NPK. On the contrary, soils which received urea alone had higher taxonomic richness (6.8 species) compared to those that received urea + 0.05 Mg biochar ha−1 (4.2 species) or urea + 0.1 Mg biochar ha−1 (5.0 species). Differences in soil macrofauna diversity were similar to taxonomic richness.
Response of nematodes to application of biochar and fertilizer + biochar blends
The most dominant functional group of nematodes were the bacterivores, which were five times more abundant than the other trophic groups studied here. Response to fertilizer and biochar application was also more conspicuous in this group (Table 4). Genera Eucephalobus, Rhabditis and Primastolaimus were the most numerous among the bacterivores. Population of the three bacterivorous nematodes in the control plots was more than eight times the number obtained from soils amended with biochar and more than three times the number obtained in soils amended with fertilizer or fertilizer + biochar. Majority of nematodes of the genus Wilsonema (13 nematodes 100 g−1 soil) was also recovered from unamended soil, as was the highest population (56 nematodes 100 g−1 soil) of the genus Aphelenchus belonging to the fungivorous group. The omnivorous nematodes, especially the genus Discolaimoides, were significantly lower in all the soils amended with fertilizer +0.1 Mg biochar ha−1, regardless of fertilizer type. Abundance of Trichodorus nematodes was highest in unamended soil (22 nematodes 100 g−1 soil) while Tylenchus did not show any specific trends.
Agronomic effectiveness of biochar and fertilizer + biochar for maize yield
In the first season, soils amended with 5 and 10 Mg biochar ha−1 resulted in about 5.0 Mg of maize grain yield ha−1, which was six times higher than the unamended soil (Table 5). Where fertilizer and fertilizer + biochar blends were applied (except urea and its blends), the yield ranged between 7.0 and 8.0 Mg ha−1 which was about 10 times higher than that obtained in unamended soil. These differences were sustained in the second season. In the third and the fourth seasons when no new inputs were added, biochar continued to show a residual effect on crop yield, though in a lower magnitude. For instance, in the third season, plots that had received fertilizer or their blends gave a yield of 3.0 Mg ha−1 which was about six times above unamended plots. Interestingly, plots that had received 10 Mg biochar ha−1 gave yields that were equal to those obtained from soils that had received fertilizers or with fertilizer + biochar. Maize yields declined in the fourth season where 1.0 to 2.3 Mg ha−1 were obtained, irrespective of the type of amendment applied in the first two seasons, compared to unamended soil where 0.4 Mg grain yield ha−1 was obtained.
Discussion
Chemical properties of soil amended with biochar and fertilizer + biochar blends
Biochar application as a soil amendment has been shown to change soil chemical properties through the release of elements or changes in soil pH (Lehmann et al. 2003; Van Zwieten et al. 2010). For instance, addition of pyrolysed materials has been shown to add substantial amounts of elements such as available P and bases (Ippolito et al. 2015; Warnock et al. 2007). Ash in biochar, on the other hand, has been shown to have significant effects on soil pH. Changes in soil pH could further affect availability of some elements through sorption-desorption processes. Results obtained in this study support these earlier findings since there was an increased concentration of all the elements measured compared to the unamended soil. This concurs with Novak et al. (2009) who reported that application of pecan-shell biochar increased soil organic C, P, K and Ca while Lehmann et al. (2003) reported an increase in P, K, Ca and Mg after biochar addition. Gaskin et al. (2010) also reported higher K, Ca and Mg after application of peanut-hull biochar. In the current study, the fact that there were no significant differences in nutrient status of the soil amended with biochar, fertilizer and fertilizer + biochar may imply that the biochar was supplying an equivalent amount of these nutrients as the fertilizer. Of importance, nitrate (NO3−) seem to have been retained in significant amounts in plots amended with fertilizer + 0.05 Mg biochar ha−1, two seasons after stopping biochar application. Other studies have reported nitrate retention in either biochar or biochar-rich soil. For instance, Hagemann et al. (2017) reported higher nitrate in biochar co-composted with manure than that obtained in pristine biochar, and Guerena et al. (2013) found lower nitrate leaching after biochar application. Hagemann et al. (2017) attributed this to the formation of organic coatings on the biochar, which they proposed as evidence that co-composted biochar could act as a slow release fertilizer. Guerena et al. (2013) found microbial biomass N and organic N to be enriched with added fertilizer N in combination with high adsorption of dissolved organic N pointing at organic N retention. In addition, 47% lower ammonia gas evolution from ultisols amended with 2.5 Mg ha−1 of eucalyptus biochar over 3 years in Western Kenya (Fungo et al. 2019) may explain part of the accumulation of nitrate found in our study. Changes in all other nutrients only seem to have lasted for one season. Nonetheless, C content at the end of the four seasons (where no amendments were applied during the third and fourth) was nearly double that recorded in the first two seasons. These differences may be attributed to the mode of application of amendments and the time of sampling. Application of amendments was done in furrows and soil sampling for chemical analysis was done at the end of the season. Thus, the contribution of these amendments to the changes in bulk soil properties could only have been detected in the subsequent season after incorporation of the stubble and residual amendments into the whole plot during land preparation. Therefore, the greatest portion of C content recorded at the end of the second season could have been drawn mainly from residual effects of the first season. Similarly, a greater portion of C recorded in the fourth season may mainly result from residual effects of first three seasons. Apart from changes in available nutrient contents, other benefits associated with application of biochar such as improved water holding capacity or soil structure were not measured in this study and can therefore not be estimated.
Response of soil fauna to application of biochar and fertilizer + biochar blends
Earthworms seemed to prefer biochar amended soils which concurs with other studies such as Van Zwieten et al. (2010) who reported that earthworms preferred soil (Ferrosol) amended with biochar over unamended soil. It has been suggested that the response of earthworms and other soil macrofauna to biochar application can result from short-term release of organic molecules from freshly added biochar (Lehmann et al. 2011). Several studies have indicated that a portion of C in biochar is readily available, which may encourage proliferation of soil microbes. Soil macrofauna such as earthworms (Kamau et al. 2017) or collembolans (Domene et al. 2015) could then benefit from such microbes directly or indirectly through a cascade of effects within the food chain. Other studies have suggested that earthworms may ingest biochar particles to benefit from their liming and detoxifying properties (Lehmann et al. 2011; Topoliantz and Ponge 2003). Such mechanisms may explain the observed increase in earthworms’ abundance in biochar-amended soil. Apart from the direct effects of biochar on food substrate availability, earthworms could have been attracted by amelioration of the physical properties. For instance, it has been suggested that biochar can improve soil porosity and aeration, and thus temperature and moisture regimes in the soil (McCormack et al. 2013). Though these were not measured, we cannot rule out the possibility of their contribution to the observed differences in earthworm abundance. Lack of significant differences in all the other soil macrofauna groups could be due to the fact that many of these are highly mobile and may not rely directly on biochar as a food substrate. Nonetheless, higher diversity and taxonomic richness in plots amended with biochar compared to fertilizer alone may indicate that biochar was providing better environmental conditions for earthworms. This may explain why blending fertilizers with biochar moderated negative effects of the chemical fertilizers.
Soil nematodes were negatively impacted by biochar application. The general decline in nematode numbers associated with biochar application could indicate that the amendment either increased predators of the nematodes or it was directly exerting negative effects on them. Studies have shown that incorporation of organic inputs attracts numerous organisms, some of which could be natural enemies to nematodes such as nematophagous fungi (e.g. Arthrobotrys brochopaga and A. oligospora), collembolans and tardigrades (Akhtar and Malik 2000; Wang and McSorley 2005). Though these were not measured in our study, we suggest that they could have played a role in the observed differences in nematode population. Among the nematode functional groups, bacterivorous and fungivorous nematodes were affected the most, with their population declining by more than eight times after biochar application. Some studies that have looked at the effects of biochar on nematode functional groups, have given mixed results. For instance, Zhang et al. (2013) observed significantly higher fungivorous nematodes on plots amended with large amounts of wheat-straw biochar (12 and 48 Mg ha−1) compared to unamended soil. However, in the same study, the authors reported that plant-parasitic nematodes significantly decreased in plots with biochar, whereas bacterivorous and omnivores-predators were not significantly affected compared to the control plots. Rahman et al. (2014), on the other hand, reported that application of poultry-litter biochar reduced plant parasitic nematodes eightfold, but showed no significant effects on free-living nematodes. These studies show that the response of nematodes is greatly dependent on the type of biochar.
Agronomic effectiveness of biochar and fertilizer + biochar blends on maize yield
In our study, application of biochar increased yield of maize fivefold in the first season. Generally, the response of crops to biochar application has been shown to vary depending on the type of biochar and application rates, soil type, climatic conditions and type of crop among other factors (Jeffery et al. 2011). For instance, the yield increase in our study was comparable to results by Kimetu et al. (2008) with application of 7 Mg ha−1 eucalyptus biochar per season over two years. Uzoma et al. (2011) reported that application of cow-manure biochar in a nutrient-deficient sandy soil at a rate of 15 and 20 Mg ha−1 led to an increase in maize yield by about 150% and 100%, respectively. The authors attributed the increase in maize yield to the two major changes after biochar application: (i) cow manure derived biochar increased nutrients (N, P and exchangeable cations) and (ii) changes in physical properties of the sandy soil, which could then have improved the water and nutrient use efficiency. There are several other studies which have reported marginal maize yield increases or even a decrease after biochar application. In their meta-analysis, Jeffery et al. (2011) reported that the combined average increase in maize productivity after biochar application, compared to the control with no biochar, did not exceed 10%. The authors noted that the marginal difference in crop productivity could have been caused by the high variation among the treatments of each study included in the meta-analysis. Zhang et al. (2012) also reported only a marginal increase in maize grain yield of about 16% and 7% after addition of wheat-straw biochar at a rate of 20 and 40 Mg ha−1, respectively to a SOC-poor calcareous soil with a pH of 8.4 in contrast to 5.5 in our soil. Similarly, Glaser et al. (2015) reported a significant increase in maize yield by about 20% after addition of 1 Mg ha−1 of biochar to mineral fertilizer. On the other hand, Gaskin et al. (2010) reported a significant decrease in maize grain yield after application of both pine-chip and peanut-hull biochar to a SOC and nutrient-poor ultisol with pH 5.5. In our study, we attribute the yield increase to addition of nutrients though application of biochar, retention of N and favourable climatic conditions. Application of 5 Mg biochar ha−1, for example, added an equivalent of 21 kg P to the soil. Since addition of small amounts of P have been shown to have significant positive effects on crop yields (Bationo 2008; Mbau 2012), such increases in P due to biochar addition may have led to the observed maize yield increases in soils amended with biochar. High amounts of K, Ca and Mg added through biochar application could have also led to an increase in maize yield which concurs with the study by Major et al. (2010). The lack of a pH increase in soils as a result of biochar additions does not allow confirmation of a liming effect. However, localized pH effects around biochars that are not detectable in bulk soil, cannot be excluded (Lehmann et al. 2015) but were not assessed here.
Apart from the direct nutrient addition with the biochar, relatively high rainfall amounts received within the study period could also have contributed to the greater response of the crop to biochar application. The rainfall amounts at the peak of growth period in the four seasons was close to or higher than the 20-year average. However, yield differences were sustained for only one season after the application of the amendments was stopped, which shows that the biochar has to be applied regularly to be able to sustain such yield increases. This was different with inorganic fertilizers applied years after biochar application to a similar soil in Western Kenya, where biochar effects were detectable for several years (Guerena et al. 2016).
With estimates of maize production in Kenya being 2.5 Mg ha−1 (De Groote 2002), the grain yield obtained as a result of biochar application was more than double the country’s average production. This shows that there is potential in P. juliflora biochar as a soil amendment in low fertility soils. Estimates of retail prices of $75 Mg−1 of charcoal fines calculate to a cost of $375 to $750 ha−1, generating a revenue increase by between $1000 and $1400 per season (estimated from an increase of about 4.5 Mg ha−1 due to use of biochar as calculated from Table 5 and the market price of maize grain in financial year 2016/2017, which was estimated at $400 Mg−1). However, there is a need for a full cost-benefit analysis of using biochar and their blends with fertilizers to ensure that farmers make an informed choice.
Conclusions
The results of this study have shown a significant change in soil nutrients as a result of biochar application. Notable trends were observed with NO3 which persisted two seasons after stopping application of inputs. Earthworms increased while nematodes decreased in biochar-amended plots. One reason for the higher earthworm abundance could be increased microbial population due to biochar application which may have benefited earthworms directly or indirectly through a cascade of effects within the food chain. On the other hand, a decrease in nematode numbers could have been caused by attraction of their natural enemies, such as nematophagous fungi, collembolans and tardigrades, which may suppress their population, or the biochar may have had deterring effects on nematodes, which could not be identified here. Maize grain yield increases from 0.9 to 5.6 Mg ha−1 after biochar application demonstrate the relevant potential of P. juliflora biochar in improving crop production, especially on smallholder farms where availability of fertilizers is limited. This can be an important step towards converting the shrub (referred to as a noxious weed) into a valuable soil amendment for improving productivity of soils that are deficient in P and low in organic matter. This strategy may build on the high biomass production capacity of the shrub which is estimated to be 25 to 30 Mg ha−1 year−1 within a cycle of 4 to 5 years. Further research with long-term application of biochar and its long-term residual effects is needed to investigate seasonal variations that could obscure the observed results at short-time scales.
References
Akhtar M, Malik A (2000) Role of organic soil amendments and soil organisms in the biological control of plant parasitic nematodes: a review. Bioresour Technol 74:35–47
Anderson JM, Ingram JSI (1993) Tropical soil biology and fertility: a handbook of methods. CAB International, Wallingford
Ayuke FO, Brussaard L, Vanlauwe B, Six J, Lelei DK, Kibunja CN, Pulleman MM (2011) Soil fertility management: impacts on soil macrofauna, soil aggregation and soil organic matter allocation. Appl Soil Ecol 48:53–62
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48
Bationo A (2008) Integrated soil fertility management options for agricultural intensification in the Sudano-Sahelian zone of West Africa. Academy of Science Publishers, Nairobi
Bremner JM, Keeney DR (1965) Steam distillation methods for determination of ammonium, nitrate and nitrite. Anal Chim Acta 32:485–495
Chan KY, Van Zwieten L, Meszaros I, Downie A, Joseph S (2008) Using poultry litter biochars as soil amendments. Aust J Soil Residues 46:437–444
Chen JH, Sun X, Zheng JF, Zhang XH, Liu XY, Bian RJ, Li LQ, Cheng K, Zheng JW, Pan GX (2018) Biochar amendment changes temperature sensitivity of soil respiration and composition of microbial communities 3 years after in-corporation in an organic carbon-poor dry cropland soil. Biol Fertil Soils 54:175–188
Cheng J, Li Y, Gao W, Chen Y, Pan W, Lee X, Tang Y (2018) Effects of biochar on Cd and Pb mobility and microbial community composition in a calcareous soil planted with tobacco. Biol Fertil Soils 54:373–383
De Groote H (2002) Maize yield losses from stem borers in Kenya. Insect Sci Appl 22:89–96
Domene X, Hanley K, Enders A, Lehmann J (2015) Short-term mesofauna responses to soil additions of corn stover biochar and the role of microbial biomass. Appl Soil Ecol 89:10–17
Forge TA, Kimpinski J (2007) Nematodes. In: Gregorich EG, Carter MR (eds) Soil sampling and methods of analysis, 2nd edn. CRC Press, Boca Raton, pp 415–425
Fungo B, Lehmann J, Kalbitz K, Thiong’o M, Tenywa M, Okeyo I, Neufeldt H (2019) Ammonia and nitrous oxide emissions from a field ultisol amended with tithonia green manure, urea, and biochar. Biol Fertil Soils 55:135–148
Gaskin JW, Speir RA, Harris K, Das KC, Lee RD, Morris LA, Fisher DS (2010) Effect of peanut hull and pine chip biochar on soil nutrients, corn nutrient status, and yield. Agron J 102:623–633
Gitari H, Gachene CKK, Karanja NN, Kamau S, Nyawade S, Schulte-Geldermann E (2019) Potato-legume intercropping on a sloping terrain and its effects on soil physico-chemical properties. Plant Soil 438:447–460
Glaser B, Lehmann J, Zech W (2002) Ameliorating physical and chemical properties of highly weathered soils in the tropics with charcoal - a review. Biol Fertil Soils 35:219–230
Glaser B, Wiedner K, Seelig S, Schmidt H-P, Gerber H (2015) Biochar organic fertilizers from natural resources as substitute for mineral fertilizers. Agron Sustain Dev 35:667–678
Guerena D, Lehmann J, Hanley K, Enders A, Hyland C, Riha S (2013) Nitrogen dynamics following field application of biochar in a temperate north American maize-based production system. Plant Soil 365:239–254
Guerena D, Kimetu J, Riha S, Neufeldt H, Lehmann J (2016) Maize productivity dynamics in response to mineral nutrient additions and legacy organic soil inputs of contrasting quality. Field Crop Res 188:13–120
Hagemann N, Joseph S, Schmidt HP, Kammann CI, Harter J, Borch T, Young RB, Varga K, Taherymoosavi S, Elliott KW, McKenna A, Albu M, Mayrhofer C, Obst M, Conte P, Dieguez-Alonso A, Orsetti S, Subdiaga E, Behrens S, Kappler A (2017) Organic coating on biochar explains its nutrient retention and stimulation of soil fertility. Nat Commun 8:1089. https://doi.org/10.1038/s41467-017-01123-0
Ippolito JA, Spokas KA, Novak JM, Lentz RD, Cantrell KB (2015) Biochar elemental composition and factors influencing nutrient retention. In: Lehmann J, Joseph S (eds) Biochar for environmental management: science, technology and implementation, 2nd edn. Routledge, New York, pp 139–163
Isaac RA, Johnson WC Jr (1998) Elemental determination by inductively coupled plasma atomic emission spectrometry. In: Kalra YP (ed) Handbook of reference methods for plant analysis. CRC Press, Boca Raton, pp 165–170
Jaetzold R, Schemidt H, Hornetz B, Shisanya C (2006) Farm management handbook of Kenya, vol II/B2. Ministry of Agriculture. Kenya and German Agency for Technical Cooperation (GTZ), Nairobi
Jeffery S, Verheijen FG, van der Velde M, Bastos AC (2011) A quantitative review of the effects of biochar application to soils on crop productivity using meta-analysis. Agric Ecosyst Environ 144:175–187
Kamau S, Barrios E, Karanja NK, Ayuke FO, Lehmann J (2017) Spatial variation of soil macrofauna and nutrients in agricultural landscapes dominated by historical charcoal production. Appl Soil Ecol 119:286–293
Kaur R, Gonzales WL, Llambi LD, Soriano PJ, Callaway RM, Rout ME, Gallaher JT, Inderjit (2012) Community impacts of Prosopis juliflora invasion: biogeographic and congeneric comparisons. PLoS One 7:e44966
Kimetu J, Lehmann J, Ngoze S, Mugendi D, Kinyangi J, Riha S, Verchot L, Recha J, Pell A (2008) Reversibility of soil productivity decline with organic matter of differing quality along a degradation gradient. Ecosystems 11:726–739
Lehmann J, da Silva JP Jr, Steiner C, Nehls T, Zech W, Glaser B (2003) Nutrient availability and leaching in an archaeological Anthrosol and a Ferrasol of the Central Amazon basin: fertilizer, manure, and charcoal amendments. Plant Soil 249:343–357
Lehmann J, Gaunt J, Rondon M (2006) Bio-char sequestration in terrestrial ecosystems – a review. Mitig Adapt Strateg Glob Chang 11:403–427
Lehmann J, Rillig MC, Thies J, Masiello CA, Hockaday WC, Crowley D (2011) Biochar effects on soil biota – a review. Soil Biol Biochem 43:1812–1336
Lehmann J, Kuzyakov Y, Pan G, Ok YS (2015) Biochars and the plant-soil interface. Plant Soil 395:1–5
Liang B, Lehmann J, Solomon D, Kinyangi J, Grossman J, O’Neill B, Skjemstad JO, Thies J, Luizão FJ, Petersen J, Neves EG (2006) Black carbon increases cation exchange capacity in soils. Soil Sci Soc Am J 70:1719–1730
Major J, Rondon M, Molina D, Riha S, Lehmann J (2010) Maize yield and nutrition during 4 years after biochar application to a Colombian savanna oxisol. Plant Soil 333:117–128
Mao J-D, Johnson RL, Lehmann J, Olk DC, Neves EG, Thompson ML, Schmidt-Rohr K (2012) Abundant and stable char residues in soils: implications for soil fertility and carbon sequestration. Environ Sci Technol 46:9571–9576
Mbaabu PR, Ng W-T, Schaffner U, Gichaba M, Olago D, Choge S, Oriaso S, Eckert S (2019) Spatial evolution of Prosopis invasion and its effects on LULC and livelihoods in Baringo, Kenya. Remote Sens 11:1217
Mbau SK (2012) Evaluating quality of composts made from organic agro-wastes and their influence on maize yield and soil fauna in Western Kenya. MSc. Thesis. University of Nairobi
Mbau SK, Karanja N, Ayuke F (2015) Short-term influence of compost application on maize yield, soil macrofauna diversity and abundance in nutrient deficient soils of Kakamega County, Kenya. Plant Soil 387:379–394
McCormack S, Ostle N, Bardgett RD, Hopkin DW, VanBergen AJ (2013) Biochar in bioenergy cropping systems: impacts on soil faunal communities and linked ecosystem processes. Glob Change Biol Bioenergy 5:81–95
Mehlich M (1984) Mehlichs-3 soil test extractant: a modification of the Mehlich 2 extractant. Commun Soil Sci Plant Anal 15:1409–1416
Miller RO (1998) Microwave digestion of plant tissue in a closed vessel. In: Kalra YP (ed) Handbook of reference methods for plant analysis. CRC Press, Boca Raton, pp 69–73
Mwangi E, Swallow B (2008) Prosopis juliflora invasion and rural livelihoods in the Lake Baringo area of Kenya. Conserv Soc 6:130–140
Novak JM, Busscher WJ, Laird DL, Ahmedna M, Watts DW, Niandou MAS (2009) Impact of biochar amendment on fertility of a southeastern coastal plain soil. Soil Sci 174:105–112
O’Neill B, Grossman J, Tsai M, Gomes J, Lehmann J, Peterson J, Neves E, Thies J (2009) Bacterial community composition in Brazilian Anthrosols and adjacent soils characterized using culturing and molecular identification. Microb Ecol 58:23–35
Rahman L, Whitelaw-Weckert MA, Orchard B (2014) Impact of organic soil amendments, including poultry-litter biochar, on nematodes in a Riverina, New South Wales, vineyard. Soil Res 52:604–619
R-Core Team (2016) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Schimmelpfennig S, Glaser B (2012) One step forward toward characterization: some important material properties to distinguish biochars. J Environ Qual 41:1001–1013
Shiferaw H, Bewket W, Alamirew T, Zeleke G, Teketay D, Bekele K, Schaffner U, Eckert S (2019) Implications of land use/land cover dynamics and Prosopis invasion on ecosystem service values in Afar Region, Ethiopia. Sci Total Environ 675:354–366
Topoliantz S, Ponge JF (2003) Burrowing activity of geophagus earthworm Pontoscolex corethrurus (Oligochaeta: Glossoscolecidae) in the presence of charcoal. Appl Soil Ecol 23:267–271
Uzoma KC, Inoue M, Andry H, Fujimaki H, Zahoor A, Nishihara E (2011) Effect of cow manure biochar on maize productivity under sandy soil condition. Soil Use Manag 27:205–212
Van Zwieten L, Kimber S, Morris S, Chan KY, Downie A, Rust J, Joseph S, Cowie A (2010) Effects of biochar from slow pyrolysis of papermill waste on agronomic performance and soil fertility. Plant Soil 327:235–246
Vanlauwe B, Kihara J, Chivenge P, Pypers P, Coe R, Six J (2011) Agronomic use efficiency of N fertilizer in maize-based systems in sub-Saharan Africa within the context of integrated soil fertility management. Plant Soil 339:35–50
Ventura M, Alberti G, Panzacchi P, Vedove GD, Miglietta F, Tonon G (2019) Biochar mineralization and priming effect in a poplar short rotation coppice from a 3-year field experiment. Biol Fertil Soils 55:67–78
Verheijen F, Jeffery SL, Bastos AC, van der Velde M, Diafas I (2010) Biochar application to soils: a critical scientific review of effects on soil properties, processes and functions. European Commission, Luxembourg
Wakie T, Evangelista P, Laituri M (2012) Utilization assessment of Prosopis juliflora in Afar region, Ethiopia. Pastoral Livelihoods Initiative II Project (PLI II), US Forest Service, USDA Office of International Programs, USAID. https://www.researchgate.net/publication/307560598. Accessed 28 June 2019
Wang K-H, McSorley R (2005) Effects of soil ecosystem management on nematode pests, nutrient cycling, and plant health. APSnet Features. https://doi.org/10.1094/APSnetFeatures/2005-0105; https://www.researchgate.net/publication/255671573. Accessed 05 Sept 2018
Warnock DD, Lehmann J, Kuyper TW, Rillig MC (2007) Mycorrhizal responses to biochar in soil – concepts and mechanisms. Plant Soil 300:9–20
Waters D, Van Zwieten L, Singh BP, Downie A, Cowie AL, Lehmann J (2011) Biochars in soil for climate change mitigation and adaptation. In: Singh BP, Cowie AL, Chan KY (eds) Soil health and climate change. Springer-Verlag, Berlin, pp 345–368
Zhang A, Liu Y, Pan G, Hussain Q, Li L, Zheng J, Zhang X (2012) Effect of biochar amendment on maize yield and greenhouse gas emissions from a soil organic carbon poor calcareous loamy soil from Central China plain. Plant Soil 351:263–275
Zhang XK, Li Q, Liang WJ, Zhang M, Bao XL, Xie ZB (2013) Soil nematode response to biochar addition in a Chinese wheat field. Pedosphere 23:98–103
Acknowledgements
This work was supported financially by MEA Ltd. Staff time for the first author was supported by the Fondation des Fondateurs, Biochar for Sustainable Soils (B4SS) (ST2F-1166), a Global Environment Facility (GEF) funded project. We wish to thank Andrew Thuo for his assistance in nematode identification, John Kimotho for soil analyses and Dr. Harun Gitari for proofreading this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kamau, S., Karanja, N.K., Ayuke, F.O. et al. Short-term influence of biochar and fertilizer-biochar blends on soil nutrients, fauna and maize growth. Biol Fertil Soils 55, 661–673 (2019). https://doi.org/10.1007/s00374-019-01381-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-019-01381-8