Abstract
Topsoil samples were collected from plots in a dry cropland in the North China Plain 3 years after a single incorporation of biochar at 20 and 40 t ha−1 and analyzed for abundances and composition of microbial community and for respiration under controlled laboratory conditions at 15, 20, and 25 °C. The addition of biochar generally reduced soil respirations at the three temperatures and the temperature sensitivity (Q10) at 15–20 °C. Biochar amendment significantly increased bacterial 16S rRNA gene abundances and fungal ITS gene diversity and induced clear changes in their community compositions due to improvements in soil chemical properties such as soil organic C (SOC) and available N contents and pH. Illumina Miseq sequencing showed that the relative abundances of Actinobacteria, Gammaproteobacteria, Firmicutes, and Alternaria within Ascomycota, capable of decomposing SOC, were significantly decreased under biochar at 40 t ha−1. The Q10 values at 15–20 °C were significantly correlated with fungal diversity and dehydrogenase activity. Our results suggest that after 3 years a single biochar amendment could induce a shift in microbial community composition and functioning towards a slower organic C turnover and stability to warming, which may potentially reduce soil C loss in dryland under climate warming in the future.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soil organic C (SOC) plays an important role in increasing ecosystem productivity for agriculture and mitigating climate change (Lal 2004; Pan et al. 2009). Being one of the largest agricultural countries in the world, China possesses 12% of the world’s total crop harvest area but has generally lower topsoil SOC contents in comparison with the global average (Song et al. 2005). Practices that could enhance soil fertility, increase SOC stocks and stability to environmental changes, are urgently needed to sustain a high crop productivity and mitigate climate change (Smith et al. 2008; Schmidt et al. 2011). Biochar application into soil has a great potential to improve soil fertility while increasing soil C sequestration (Atkinson et al. 2010; Sohi et al. 2010), enhance crop productivity, and reduce greenhouse gas emissions in soils in North China Plain (Zhang et al. 2012; Lu et al. 2014). Rapid increments of soil C contents, water retention, and reduction of nutrient leaching were also demonstrated in both short- and long-term incubation studies (Kuzyakov et al. 2009; Laird et al. 2010; Farrell et al. 2013; Singh and Cowie 2014; Chen et al. 2017). However, whether these effects can last for some years after a single biochar addition and its effect on soil biological functions are not known.
Soil respiration, leading to SOC decomposition, is a major contributor to the increase in atmospheric CO2 and depends on temperature and moisture (Schlesinger and Andrews 2000). Biochar amendment can increase or decrease soil respirations (Castaldi et al. 2011; Jones et al. 2011; Maestrini et al. 2014; Mitchell et al. 2015; Zhou et al. 2017). Increased soil respiration, particularly that due to SOC decomposition under warming, could be responsible for the release of organic C sequestered in the soil and may negate the C sequestration potential of biochar (Schulze and Freibauer 2005; Kuzyakov et al. 2009; Zimmerman et al. 2011). In contrast, increased resistance of soil respiration to warming could be beneficial for C storage (Davidson and Janssens 2006). The dependence of soil respiration to temperature (also refers to as Q10) is influenced by soil organic C quality, substrate availability, as well as stability of soil organic matter (SOM) (Fang et al. 2005; Conant et al. 2008; von Lützow and Kögel-Knabner 2009). Probably, the decomposition of more recalcitrant SOM is sensitive to temperature due to the fact that it requires high activation energy to break down the molecules (Davidson and Janssens 2006; Hartley and Ineson 2008). However, other studies suggested that the decay of stable SOM is not temperature sensitive, possibly due to the limitation of substrate availability or thermal adaptation of microbial respiration (Liski et al. 1999; Luo et al. 2001). Being considered a chemically and biologically recalcitrant C material, biochar application may increase recalcitrant C pools and the physical protection of OC and of biochar particles in soil, and this may decrease SOC decomposition and its response to warming (Keith et al. 2011; Singh and Cowie 2014; Fang et al. 2014). So far, however, only very limited studies suggested that the temperature sensitivity of soil respiration could be affected by biochar application. For example, He et al. (2016) reported that soil respiration was not changed whereas its sensitivity to temperature decreased due to 5 years of consecutive biochar addition in temperate croplands. Fang et al. (2014) suggested that biochar resulted in a decrease or no change in temperature sensitivity of soil respiration, which depends on soils and biochar type in a 2-year incubation study. Therefore, the effect of biochar on temperature sensitivity of soil respiration after several years since a single addition to soil remains largely unknown.
Soil microorganisms are sensitive to environmental changes and play a key role in nutrient cycling and organic matter decomposition (Marschner et al. 2003). Changes in soil properties under biochar amendment have been demonstrated to improve microbial habitat, such as providing available organic C as substrate for microbial growth and protection from predators (Pietikäinen et al. 2000; Quilliam et al. 2013; Farrell et al. 2013). In addition, biochar application altered microbial abundance and composition (Ameloot et al. 2013; Farrell et al. 2013; Jenkins et al. 2017) and affected the direction and magnitude of soil microbial respiration in short-term studies (Castaldi et al. 2011; Jones et al. 2011; Ameloot et al. 2013; Maestrini et al. 2014; Lu et al. 2014). However, other studies performed in the fields a few years after biochar addition found that the effect of biochar on microbial abundance was generally short lived, probably due to the consumption of labile C from biochar and decreased impact of biochar on soil fertility (Jones et al. 2012; Rousk et al. 2013; Noyce et al. 2015). In addition, a few studies demonstrated that the presence of recalcitrant C from biochar after several years changed soil microbial community composition and decreased soil C availability and microbial activity in terms of organic matter decomposition due to enhanced organic-mineral interactions (Ameloot et al. 2014; Singh and Cowie 2014; Zheng et al. 2016). However, as already mentioned, although the findings resulted from short-term biochar experiments generally gave results differing from those under long-term field conditions (Jones et al. 2012; Rousk et al. 2013; Domene et al. 2014), it is still uncertain whether biochar addition can change microbial activity and diversity several years after a single incorporation. Moreover, it should be noted that any shifts in microbial activity can potentially affect the microbial SOC decomposition, which may influence the C sequestration potential of biochar.
The objective of this study was to investigate the effects of biochar on physicochemical properties, soil respiration and its temperature sensitivity, microbial community composition, and activity in an organic C-poor dry cropland soil 3 years after a single addition. We hypothesized that after the decomposition of labile C, the recalcitrant C of biochar could modify soil physicochemical properties and decrease soil C availability 3 years after its application into soil, and this may have decreased microbial activity with shifts in community composition. Such alterations may further reduce the sensitivity of microbial heterogenic respiration to soil warming. To test this, a field experiment was performed in a low fertility dry cropland soil in the North China Plain. Soil physical and chemical properties amended with biochar for 3 years were investigated. Soil respiration rates under varying temperatures were evaluated under laboratory conditions. The activities of β-glucosidase and dehydrogenase were analyzed to characterize the degradation of labile organic C and microbial respiratory process with active cells, respectively (Alef and Nannipieri 1995; Sinsabaugh et al. 2008). Microbial biomass C (MBC) was determined to evaluate microbial abundance and to calculate the metabolic quotient (qCO2), a bioindicator of efficiency of substrate utilization and disturbance (Bardgett and Saggar 1994). Both composition and abundance of bacterial and fungal community were determined by pyrosequencing and quantitative real-time PCR, respectively.
Materials and methods
Site description and biochar
A field experiment with biochar amendments was conducted in June 2011 in a dry cropland (36° 10′ N and 117° 09′ E) located in Tai’an city, Shandong province, China, lying in the east part of the North China Plain. The soil is classified as ustic Alfisols in US Soil Taxonomy (Soil Survey Staff 1999) with a pH (H2O) of 5.93, soil organic C (SOC) content of 8.5 g kg−1, total N (TN) concentration of 0.8 g kg−1, and with a sandy loam texture. The climate of the study site is semi-humid temperate monsoon, and the mean annual temperature and rainfall for the last 10 years were 13.0 °C and 697 mm, respectively. Soils are usually cropped with winter wheat-summer maize rotation. Biochar used in this study was pyrolyzed from wheat straw at 350–550 °C in a vertical kiln. Properties of this biochar have been already reported (Chen et al. 2016). The biochar material produced was ground to pass through a 2-mm sieve and homogenized before application in the field.
Treatment of biochar soil amendment and soil sampling
Field treatments were as follows: a single biochar amendment at rates of 0 t ha−1 (control, C0), 20 t ha−1 (C20), and 40 t ha−1 (C40). The application rate of 40 t ha−1, which is generally higher than that used in agricultural practices, was chosen in order to exaggerate biochar effects on soil chemical and biological properties. Biochar material was evenly broadcast on soil surface by hand and incorporated thoroughly into soil to a depth of 0–10 cm with a tilling tractor before maize plantation in June 2011. No more biochar were added throughout the 3 years in this study. Each treatment was carried out in a plot of 4 m × 6 m, plots were separated by a row to protect them from external effects. Each treatment was replicated three times, and the experiment was organized in a randomized block design. Maize production had been managed with local cultivars (Zea mays, Zhengdan 958) and inorganic fertilization practices (225 kg N ha−1, 90 kg P2O5 ha−1, and 180 kg K2O ha−1) were consistently performed across the treatments. With N fertilization, urea was applied, of which 60% was applied as basal fertilizer and 40% as supplementary fertilizer during crop growth.
Soil sampling was conducted in October 2014, 3 years after biochar application. Five randomized soil cores were taken from the 0–15-cm depth of each plot and were mixed thoroughly to form a composite sample. The composite sample was sieved (< 2 mm) and stored in an ice box before being transported to the laboratory. A portion of each sample was stored at −70 °C for molecular analysis, and the rest was used for laboratory incubation and physicochemical analyses.
Soil physicochemical analyses, microbial biomass C, and enzyme assay
Soil bulk density was measured using a 100-cm3 cylinder at soil sampling. Soil pH was determined with a soil-to-water ratio of 1:2.5 using a pH meter (Mettler Toledo Seveneasy, Switzerland). Soil moisture was determined by mass difference after drying at 105 °C until constant weight. SOC and TN were determined by the CNS Macro Elemental Analyzer (Elementar Analysen Systeme GmbH, Germany, 2003). Dissolved organic C (DOC) was determined by a multi-N/C analyzer (Jena TOC Analyzer, Jena, Germany) according to Chen et al. (2016). Available N (alkaline hydrolysis diffusion method) was measured according to Mulvaney (1996). Soil microbial biomass C (MBC) was determined by the chloroform fumigation extraction as described by Wu et al. (1990). Though biochar was reported to decrease extraction efficiency of MBC in the fumigation procedure (Lehmann et al. 2011), no correction for sorption was performed in this study. Microbial quotient (MQ) was calculated as the ratio of MBC to SOC. Dehydrogenase activity was determined as reported by Serra-Wittling et al. (1995). The activity of β-glucosidase was analyzed according to the standard fluorescence enzyme protocols described by Bell et al. (2013) using 4-methylumbelliferone-β-d-glucoside as a substrate. Fluorescence was quantified using a microplate fluorometer (Synergy™ H1, Biotek) at 365 nm excitation and 450 nm emission wavelengths.
Soil respiration
Soil respiration was determined by laboratory incubation according to Zheng et al. (2016). For its response to varying temperatures, soil was incubated at 15, 20, and 25 °C at 60% water-holding capacity. CO2 produced in the headspace was collected with a gas-tight syringe every day in the first 8 days, every other day from days 9 to 22, and every 3 days from days 23 to 31. After each gas sampling, the headspace was flushed with ambient air for 10 min to allow oxygen to diffuse into the soil to maintain aerobic conditions and then the jars were resealed for the next measurement. The CO2 concentration was analyzed by a gas chromatograph (Agilent 7890A, Santa Clara, CA, USA) equipped with a flame ionization detector.
Respiration quotient (RQ, percentage of SOC respired to CO2-C) and metabolic quotient (qCO2, the ratio between CO2-C and MBC) were calculated based on the CO2 emission at 25 °C. The temperature sensitivity coefficient (Q10 value) was calculated according to the equation of Kirschbaum (1995) that considers two temperatures: Q10 = (A2/A1)10/(T2-T1), where A2 and A1 are the C mineralization rate at temperatures T1 and T2, respectively.
Soil DNA extraction and real-time PCR
DNA of each soil was extracted with a PowerSoil® DNA isolation kit (Mo Bio-Laboratories Inc., Carlsbad, CA, USA) according to the manufacturer’s protocol. Bacterial 16S rRNA (primer set 338F/518R) and fungal internal transcribed spacer (ITS) (5.8 s/ITS1F; Fierer et al. 2005) fractions were amplified with an iCycler IQ5 Thermocycler (Bio-Rad Laboratories Inc., Hercules, CA, USA) by fluorometric monitoring with SYBR Green 1 dye. The 25-μL polymerase chain reaction (PCR) mixture contained 1–10 ng DNA, 0.2 mg mL−1 BSA, 0.2 μM of each primer, and 12.5 μL of SYBR premix EX Taq™ (Takara Shuzo, Shiga, Japan). The standard curves were constructed with plasmids from cloned rRNA genes separately for bacteria and fungi and generated with tenfold dilutions of plasmid DNA in triplicate. Melting curve analysis was done following each assay to confirm specific amplification. After amplification, a 1.5% agarose gel electrophoresis was carried out on the PCR amplification products and blanks to assess whether an appropriate size of gene fraction had been achieved.
Illumina Miseq sequencing and sequence processing
Both soil bacterial and fungal community compositions in each plot were investigated by Illumina Miseq sequencing. In brief, primer sets (515F and 907R) targeting the V4–V5 region and sets (1737F and 2043R) targeting the ITS fraction in combination with an eight-base barcodes sequence were used to amplify the bacterial 16S ribosomal RNA and fungal ITS gene, respectively (White et al. 1990; Zhou et al. 2011). PCR reactions were carried out in a thermal cycler (Bio-Rad Laboratories, Hercules, CA) in triplicate with 20 μL mixture consisting of 10 ng of DNA, 4 μL of 5 × FastPfu buffer, 0.8 μL of each primer (5 μM), 2 μL of 2.5 mM dNTPs, and 0.4 μL of FastPfu Polymerase. After a 2% (w/v) agarose gel, the band of PCR products were excised, solubilized with deionized water, and purified with the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, USA) and quantified via QuantiFluor™-ST (Promega, USA) kit. Purified amplicons were pooled in equimolar and paired-end sequenced (2 × 250) on an Illumina MiSeq platform according to the standard protocols. The obtained raw reads were deposited into the NCBI Sequence Read Archive (SRA) database (accession number: SRR2146924 and SRR2146952).
After split to each sample, raw sequences were trimmed using QIIME (version 1.17) (Caporaso et al. 2010) with threshold of quality score higher than 20 over a 10-bp sliding window and the minimum length of 200 bp. Forward and reverse reads with at least 10 bp overlap and lower than 5% mismatches were joined using FLASH and subjected to chimera removal by U-Chime (Edgar et al. 2011). Sequences were discarded if they contained any ambiguous characters and could not be assembled. Using the average neighbor algorithm with a cut-off of 97% similarity, the sequences were clustered into operational taxonomic units (OTUs). The representative sequences of each OTU for bacteria and fungi were selected and classified by Ribosomal Database Project (RDP) Classifier with a confidence threshold of 70%. To correct for sampling effect, we randomly resampled the sequences obtained from UPARSE treatment according to the minimum numbers of sequences across all samples for downstream analysis. A subset of 15,338 sequences for bacteria and 29,818 sequences for fungi per sample were randomly selected.
Statistical analysis
Statistical procedures were conducted with the SPSS 18.0 package for Windows. The statistical significance of the differences between samples was determined by One-way analysis of variance (ANOVA) followed by Duncan’s multiple range test with p < 0.05. An OTU-based analysis was performed to calculate the richness and diversity index of samples including Chao1, the abundance-based coverage estimator (ACE), and Shannon diversity index in these samples with a cutoff of 3% dissimilarity. Redundancy analysis (RDA) was performed based on the weighted pairwise UniFrac distances of OTU composition with Vegan 2.0 in R (V2.15.3) to determine the main environmental factors (soil moisture, bulk density, pH, C/N and SOC, TN, DOC, and available N contents) affecting the microbial community composition (Legendre and Anderson 1999). The significance of these environmental variables was tested with “envfit” based on 999 permutations in R.
Results
Changes in selected soil physical and chemical properties
Biochar changed topsoil physicochemical properties 3 years after a single amendment (Table 1). Soil moisture, pH, C/N and SOC, TN, DOC, and available N contents under 40 t ha−1 biochar treatment were significantly (p < 0.05) higher than under the control, though the increases were relatively small in most of the selected parameters. Bulk density of both 20 and 40 t ha−1 plots were both significantly (p < 0.05) lower than that of the control. There were no clear differences in pH and TN, DOC and available N contents between 20 and 40 t ha−1 plots.
Microbial biomass C, soil basal respiration, and dehydrogenase and β-glucosidase activities
Microbial biomass C (MBC) in biochar-amended soils at 20 and 40 t ha−1 were almost doubled compared with that of the control (Table 2), whereas soil basal respiration rates at 20 and 40 t ha−1 were significantly (p < 0.05) decreased by 12 and 20% compared with the control, respectively. RQ and qCO2 were significantly (p < 0.05) decreased with both two biochar addition rates. Dehydrogenase activity was decreased by 64 and 59% at 20 and 40 t ha−1 compared with the control. There were no significant changes in β-glucosidase activity under biochar amendment.
Temperature sensitivity of soil respiration and its correlation with microbiological properties
Cumulative CO2 emissions increased with increasing temperature across all treatments (Fig. 1). The cumulative CO2 emissions under 20 and 40 t ha−1 biochar amendments during the whole incubation were generally smaller than that of the control across all three temperatures. The temperature sensitivity of soil respiration (Q10) at 15–20 and 15–25 °C were significantly (p < 0.05) lower in biochar-amended soils than that in the control (Fig. 1b), while no significant changes were found in Q10 at 20–25 °C range. Moreover, the Q10 values at 15–20C and 15–25 °C were significantly (p < 0.05) and positively correlated with RQ and dehydrogenase activity but negatively correlated with fungal diversity (Table 3). The Q10 at 15–20 °C were also positively correlated with qCO2, while the Q10 at 15–25 °C were negatively correlated with MBC and bacterial abundance.
The cumulative CO2 respired over time from biochar-amended and non-amended samples at three temperatures (15, 20, and 25 °C) during a 31-day incubation (a) and temperature sensitivity (Q10) of soil respiration calculated based on respiration rates of the three temperatures (b). C0, 0 t ha−1, control; C20, 20 t ha−1; C40, 40 t ha−1. The different letters above the bars denoted significant differences among treatments (p < 0.05)
Abundance and alpha diversity of bacteria and fungi
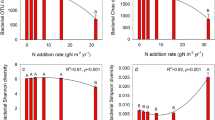
Biochar soil amendment significantly (p = 0.02) increased bacterial 16S rRNA gene copy numbers both at 20 and 40 t ha−1 3 years after a single incorporation (Table 4). The OTU richness and diversity index including Chao1, ACE, and Shannon indices were not altered under biochar amendment at both 20 and 40 t ha−1. In contrast, the abundance of fungal ITS rRNA gene was not changed but the OTU richness and Shannon index were increased with biochar amendment at 40 t ha−1.
Bacterial and fungal community composition
The relative abundances of Actinobacteria, Gammaproteobacteria, and Firmicutes were significantly (p < 0.05) decreased by 23, 10, and 23% under 40 t ha−1 biochar treatment compared with the control (Fig. 2). Whereas Gemmatimonadetes and Deltaproteobacteria were increased by 12 and 31% under 40 t ha−1 compared with the control. Ascomycota predominated in the dry cropland soil, and the abundance did not change under 20 t ha−1 but it increased by 19% under 40 t ha−1 compared with the control. The relative abundances of Basidiomycota were generally decreased with increasing biochar addition rate. Chytridiomycota was rarely detected in the control, but their abundance significantly (p < 0.01) increased by nearly 12 times under 40 t ha−1.
Biochar amendment changed the abundance of some bacterial and fungal genera particularly at the 40 t ha−1 (Table 5). The relative abundances of Lactococcus and Nocardioides were strongly decreased at the 40 t ha−1. Some low abundant genera, such as Arthrobacter and Marmoricola, in the control soil, were also significantly reduced under biochar. Alternaria and Guehomyces were the predominant fungal genera, accounting for 29 and 27% of the sequences and were decreased to 18 and 17% under biochar addition at 40 t ha−1. In contrast, the abundance of Chrysosporium, affiliated with Onygenaceae, were increased by almost seven times under biochar addition at 40 t ha−1.
Redundancy analysis of bacterial and fungal community composition
RDA indicated clear differences in microbial community composition between the biochar-amended plots at 40 t ha−1 and non-amended plots (Fig. 3). The first two components explained 60 and 86% of the total variability for bacterial and fungal community composition, respectively. The bacterial communities under biochar amendment at 40 t ha−1 were clearly separated from other two treatments in the ordination space. The bacterial communities positively and significantly varied with SOC (r 2 = 0.790, p = 0.01) and available N (r 2 = 0.706, p = 0.01) contents, C/N (r 2 = 0.668, p = 0.04), and pH (r 2 = 0.629, p = 0.04) (Table S1). Similarly, SOC (r 2 = 0.788, p = 0.01) and available N (r 2 = 0.760, p = 0.01) contents, C/N (r 2 = 0.667, p = 0.04), and pH (r 2 = 0.661, p = 0.03) significantly correlated with the fungal communities.
Redundancy analysis (RDA) of bacterial (a) and fungal community compositions (b) and environmental variables for individual samples from control and biochar-amended soils. Arrows represent environmental variables that yielded significant correlations with the microbial community composition. C0, 0 t ha−1, control; C20, 20 t ha−1; C40, 40 t ha−1. SOC, soil organic carbon. *p < 0.05 and **p < 0.01, significant correlations
Discussion
Effect of biochar amendment on soil physicochemical properties
Improvements in soil physicochemical properties, such as bulk density, pH, SOC, and TN, due to biochar amendment have been reported (Zhang et al. 2012; Farrell et al. 2013; Major et al. 2012; He et al. 2016; Chen et al. 2017). However, most of these findings are derived from either pot experiments or short-term field studies, whereas few studies have examined the long-term impacts of biochar, particularly in the fields (Jones et al. 2012; Zheng et al. 2016). Field conditions are characterized by frequent tillage practices and cycles of wetting and drying, which may decrease the impacts of biochar on soil physicochemical and biological properties. However, we found that most of the soil physicochemical properties were changed 3 years after a single biochar addition, indicating a persistent enhancement of soil quality under biochar. These findings confirm what reported by Zheng et al. (2016) in an acid rice paddy 4 years after application and by Yao et al. (2017a) in a fertile Mollisol 3 years of biochar amendment. In particular, we observed that the soil moisture was much higher in biochar-amended soils and generally increased with increasing biochar addition rates, suggesting that biochar may improve soil moisture storage and hydrological property, which is particularly important for soils poor in organic C in arid/semiarid zones (Omondi et al. 2016). The reported effects of biochar on DOC and available N are highly variable and dynamic across both short-term and long-term studies (Jones et al. 2012; Farrell et al. 2013; Zheng et al. 2016; Chen et al. 2017). The relatively higher DOC contents in this study may result from the decomposition of biochar since it had some biologically degradable organic molecules that could potentially contribute to the increase in DOC over 3 years as observed in our previous study (Chen et al. 2016). Others suggested that the increase of available N content could be ascribed to the retention effects of biochar or to the improved N fertilizer utilization efficiency (Lehmann et al. 2011; Taghizadeh-Toosi et al. 2012). Nevertheless, our study provides clear evidence that biochar could continue to modify some physicochemical properties, such as soil moisture, soil C and N contents, 3 years after a single amendment in an unfertile dry cropland soil.
Soil respiration under biochar amendment and its response to warming
In the present study, the soil basal respiration rates under biochar amendment were lower than the non-amended soils (Table 2), probably due to a negative priming effect (Maestrini et al. 2015). This finding confirms what reported under long-term field conditions (Jin 2010; Zimmerman et al. 2011; Domene et al. 2014; Chen et al. 2016). Increase in soil respiration was usually attributed to the increased labile organic C content after biochar addition (Farrell et al. 2013) or to the enhanced soil microbial activity (Castaldi et al. 2011; Jones et al. 2011), whereas the decrease in soil respiration was often explained by the toxic effect of biochar (Zimmerman et al. 2011) or reductions in substrate availability, microbial abundance and enzymatic activity in short-term studies (Lehmann et al. 2011; Maestrini et al. 2014). For example, the reduction in phenol-oxidase activity may contribute to the decrease of SOC mineralization induced by biochar addition (Maestrini et al. 2014). However, neither reductions in nutrient availability nor decrease in MBC was observed in our study. Lehmann et al. (2011) suggested that long-term experiments might improve efficiency in the C use because of co-location of microorganisms and C on biochar surfaces, which reduces the need for enzyme production. Our findings of lower qCO2 and dehydrogenase activity observed in biochar-amended soils could provide evidence for such hypothesis. qCO2 may also be an indicator of “microbial efficiency” even if it has been also used as a bioindicator of disturbance and environmental stress (Bardgett and Saggar 1994). A meta-analysis showed an overall decrease by 13% in qCO2 due to biochar addition (Zhou et al. 2017). The decreases of qCO2, particularly after some years since biochar application, may be ascribed to the improvement of microbial habitat as biochar may protect microorganisms from disturbance and provide nutrient and water for microbial growth (Lehmann et al. 2011; Quilliam et al. 2013; Chen et al. 2016). The reduced respiration quotient (RQ) under biochar treatment may indicate that SOC is protected against microbial decomposition or a shift in microbial community composition with prevalence of microbial species having a higher C use efficiency (Jiang et al. 2016). This explanation was evidenced by our previous findings that the composition of metabolically active soil microbial communities was changed, causing a decrease in SOC turnover after 18 months of biochar amendment (Chen et al. 2016). In summary, our findings suggest that a biochar amendment after 3 years decreased soil respiration, thus improving organic C accumulation in soil.
Temperature sensitivity of soil respiration under biochar decreased at 15–20 °C but not at 20–25 °C (Fig. 1), partially confirming previous studies (Fang et al. 2014; He et al. 2016) and supporting our initial hypothesis. Indeed, Fang et al. (2014) observed that temperature sensitivity of organic C mineralization due to biochar addition decreased in the Entisol, Vertisol, and Inceptisol, but not in the Oxisol, at 20–40 °C. He et al. (2016) found that the Q10 at higher biochar addition rate was not significantly decreased compared with the non-amended soil under a wheat-maize rotation. These contrasting results suggest that effects of biochar on sensitivity of soil respiration to temperature can vary and depend on various factors, such as soil type, temperature, and type and addition rate of biochar. Soil warming can affect temperature sensitivity of soil respiration by influencing soil water and nutrient availability and microbial and enzyme activity (Hartley and Ineson 2008; von Lützow and Kögel-Knabner 2009; Menichetti et al. 2015). He et al. (2016) suggested that biochar may decrease soil water availability due to its water absorption capacity, thus decreasing the temperature sensitivity of soil respiration under field conditions. However, water was not a limiting factor in our study since the soil moisture was maintained constant during incubation. Decomposition of recalcitrant soil organic matter was suggested to be not temperature-sensitive possibly due to the limitation of substrate availability (Liski et al. 1999; Luo et al. 2001; von Lützow and Kögel-Knabner 2009). Long-term biochar addition was shown to decrease organic C availability due to enhanced organic-mineral interactions (Ameloot et al. 2014; Singh and Cowie 2014). Therefore, it is possible that the incorporation of recalcitrant biochar C into soil after a few years may have decreased the temperature sensitivity of soil C decomposition, as suggested by Fang et al. (2014). In addition, the lower Q10 in the present study might result from stabilization of SOC and decreased soil microbial C turnover in response to biochar addition as suggested by Zimmermann et al. (2012). Spatial inaccessibility to microbes and enzymes due to the occlusion of SOC by aggregation, and hydrophobicity is crucial for SOC stabilization (von Lützow and Kögel-Knabner 2009; Zimmermann et al. 2012). Biochar amendment in the long-term may increase the stability of soil aggregates, leading the labile C compounds physically or chemically entrapped in aggregates or interior pores of biochar (Keith et al. 2011; Zimmermann et al. 2012; Singh and Cowie 2014), and thus being protected against microbial decomposition as supported by the decrease in the RQ and microbial activity of biochar amended soils even under elevated temperature (Pronk et al. 2012; Zimmermann et al. 2012). Dehydrogenases are intracellular enzymes participating in the oxidative phosphorylation in microbial cells, which is thus related to microbial respiratory processes (Insam 2001; Sinsabaugh et al. 2008). The decline in dehydrogenase activity and its positive correlation with Q10 in this study may confirm the decrease in microbial activity under biochar amendment. However, the Q10 values at 20–25 °C were not changed, which may be probably due to the fact that the two temperatures were suitable for microbial activities or microbial substrates became a limiting factor after their rapid consumption at warm condition (von Lützow and Kögel-Knabner 2009). Thus, our results suggest that the decreased temperature sensitivity of soil respiration under biochar amendment may potentially imply a reduction in soil C loss under climate warming.
Effects of biochar amendment on microbial abundance and diversity
Being consistent with earlier studies (Domene et al. 2014; Zheng et al. 2016; Jiang et al. 2016; Yao et al. 2017a), we found that soil MBC and bacterial abundances increased with a single biochar addition after 3 years, suggesting a persistent effect on microbial abundance. However, no changes were found in fungal abundance, which agrees with the findings by Jones et al. (2012) in an agricultural soil sampled 2 years after biochar addition. Observed increases in bacterial abundances are commonly attributed to greater C and nutrient availability provided by labile C of biochar, especially in short-term studies (Lehmann et al. 2011; Luo et al. 2013; Farrell et al. 2013; Maestrini et al. 2014; Chen et al. 2015). However, such direct effect of biochar may disappear within some months after biochar added to soils (Kuzyakov et al. 2009; Farrell et al. 2013). One possible explanation for the increase in bacterial abundance in this study may be ascribed to the indirect improvement in soil quality induced by biochar amendment, such as the increase in the SOC content and water retention. The presence of recalcitrant C of biochar may act as slow-releasing nutrient pools for microorganisms during the aging process (Kuzyakov et al. 2009; Lehmann et al. 2011; Singh and Cowie 2014). Improvements in water retention may also enhance microbial abundance through dissolving nutrients and causing a greater resistance of microbial cells to drying, particularly in dry lands (Maestrini et al. 2015). Domene et al. (2014) suggested that their observed increase in MBC was mainly due to increased soil moisture after a 3-year biochar addition. In our study, the higher soil moisture under biochar treatments and the significant correlations between soil moisture and bacterial or fungal abundances also provided evidence of the biochar’s role in promoting microbial biomass (Table S2). Other hypotheses, such as an improved protection of microorganisms from predators and drying, have been proposed to explain the increased microbial abundance in the long-term after biochar addition (Quilliam et al. 2013). Our earlier study also implied that the high porosity and large surface area of biochar could improve the habitat of soil microorganisms, in particular for bacteria rather than fungi, protecting them from grazing and drying after an 18-month field treatment (Chen et al. 2013).
After 3 years of biochar application, we found that the diversity of bacteria was not changed, which is in contrast with what was found by Zheng et al. (2016) in an acid rice paddy 4 years after addition. It is suggested that pH is a key factor determining bacterial diversity (Lauber et al. 2009). Rousk et al. (2010) have also showed the importance of soil pH in affecting bacterial diversity. In this study, we found that although soil pH was higher in biochar-amended than non-amended soils, bacterial diversity was not changed. In contrast, fungal diversity was increased under biochar addition in our study, which contrasts with the findings by Yao et al. (2017b). The different responses of bacterial and fungal diversities to biochar could be attributed to their preferences for different C sources (Rousk et al. 2009). Compared with bacteria, fungi are able to colonize poor C sources with a high C/N, such as biochar (Warnock et al. 2007). Therefore, we speculate that the incorporated recalcitrant C from biochar may play a role in stimulating fungal diversity. Microbial diversity is of great importance to ecosystem stability and resilience towards stress and disturbance. It could be inferred that the positive correlation between fungal diversity and Q10 in this study may have contributed to the resistance of soil respiration to warming through their functional redundancy in C mineralization (Rousk et al. 2009).
Changes of microbial community composition and its implication for soil C sequestration under biochar amendment
Shifts in microbial community composition are related to changes in soil environments caused by biochar amendment (Lehmann et al. 2011; Mitchell et al. 2015; Zhang et al. 2017; Chen et al. 2017). In our study, SOC and available N contents, pH, and C/N were significantly correlated to the shifts in bacterial community compositions (Fig. 3). Zheng et al. (2016) and Yao et al. (2017a) observed that microbial community compositions in biochar-amended soils were different from those of non-amended soils and were shaped by pH, SOC, and TN contents and C/N. However, we found available N rather than the TN significantly correlated with both bacterial and fungal community compositions, which suggests that microbial communities are more sensitive to the available than total N contents since the bioavailable N could be easily utilized by microorganisms particularly in N limited soils with a high C/N. Fungal community composition was affected by the SOC content and C/N, probably because fungi are main decomposers of SOC, particularly the recalcitrant C, while organic matter with a high C/N ratio is believed to have strong effect on fungal composition (Thiet et al. 2006).
A few studies suggested that biochar effects on microbial community composition could be hardly detected after 2 years since biochar application (Rousk et al. 2013; Noyce et al. 2015). However, our study showed some clear shifts in both bacterial and fungal groups even at phylum level 3 years after biochar incorporation. The abundances of three key bacterial phyla, Actinobacteria, Gammaproteobacteria, and Firmicutes, were significantly decreased by biochar amendment in this study, indicating their sensitivity to biochar. Zheng et al. (2016) observed that the relative abundance of Actinobacteria decreased by 21% in biochar-treated soils 4 years after application, whereas other studies reported that the relative abundance of Firmicutes was not affected by the biochar amendment in both short-term and long-term experiments (Chen et al. 2013; Yao et al. 2017a). Khodadad et al. (2011) suggested that the abundance of gram-positive bacteria, such as Actinobacteria and Firmicutes, could be increased by the presence of labile C in biochar but not by that of recalcitrant C. Thus, it is possible that when the labile C was exhausted, the recalcitrant C might have had an opposite effect on such copiotrophs (Fierer et al. 2007). Acidobacteria, an oligotrophic group widely distributed in agricultural soils, are enriched in soils with low resource availability and associated with lower SOC mineralization rate (Fierer et al. 2007). The generally higher abundance of Acidobacteria in our non-fertile soil and under biochar treatments may suggest that the biochar-amended soil involved a low bioavailability of C. The abundance of Ascomycota, dominating in the fungal community, was significantly enhanced by biochar. Alternaria, a genus of Ascomycota, was decreased by biochar. Alternaria species are known as major plant pathogens and can degrade SOC (Arfi et al. 2012); the decrease in this genus abundance under biochar may be beneficial to crop health. Besides, we found the relative abundance of Mortierella was significantly increased by biochar at 20 t ha−1. Species of Mortierella act as saprotrophs in soil and live on decaying leaves or other organic materials, and some are psychrophiles (Alexopoulos et al. 1996). Also Zheng et al. (2016) found a positive effect of biochar on Mortierella abundance, but Yao et al. (2017b) showed that the relative abundances of Mortierella was not changed by the biochar amendment. Actinobacteria, gram-positive bacteria, play an important role in the decomposition of organic matter and C cycling, and are sensitive to disturbance (Kramer and Gleixner 2008). Firmicutes are described as fast-growing copiotrophs and their growth is stimulated by fresh organic matter (Fierer et al. 2007). Together with the incorporation of recalcitrant C in biochar-amended soils, we speculate that the decreases in abundance of such gram-positive bacteria involved in C degradation under biochar may contribute to the decreases in soil respiration and soil organic matter turnover, even when the temperature is elevated. However, due to the inconsistent observations, additional research is needed to unravel the mechanisms of the effects of biochar on microbial community composition after few years.
Conclusions
The present study demonstrated that biochar amendment showed persistent effects on soil physicochemical properties 3 years after a single addition to a C-poor dry cropland soil. Biochar amendment decreased soil respiration, microbial metabolic quotient, and sensitivity of soil respiration to warming, which may increase the C use efficiency of microorganisms and stabilize SOC due to enhanced organic-mineral interactions. Our study also demonstrated that the abundances of some gram-positive bacteria, such as Actinobacteria and Firmicutes, were decreased and changes in soil physicochemical properties were important factors in shaping the microbial community composition. We suggest that biochar amendment caused a shift in microbial community functioning towards slower organic C turnover and stability to warming in dry cropland after 3 years, which may potentially reduce soil C loss.
References
Alef K, Nannipieri P (1995) Chapter 7-enzyme activities. In: Alef K, Nannipieri P (eds) Methods in applied soil microbiology and biochemistry. Academic Press, London, pp 311–373
Alexopoulos CJ, Mims CW, Blackwell M (1996) Introductory mycology, Fourth edn. John Wiley & Sons, New York
Ameloot N, De Neve S, Jegajeevagan K, Yildiz G, Buchan D, Funkuin YN, Prins W, Bouckaert L, Sleutel S (2013) Short-term CO2 and N2O emissions and microbial properties of biochar amended sandy loam soils. Soil Biol Biochem 57:401–410
Ameloot N, Sleutel S, Case SDC, Alberti G, McNamara NP, Zavalloni C, Vervisch B, Gd V, De Neve S (2014) C mineralization and microbial activity in four biochar field experiments several years after incorporation. Soil Biol Biochem 78:195–203
Arfi Y, Marchand C, Wartel M, Record E (2012) Fungal diversity in anoxic-sulfidic sediments in a mangrove soil. Fungal Ecol 5:282–285
Atkinson CJ, Fitzgerald JD, Hipps NA (2010) Potential mechanisms for achieving agricultural benefits from biochar application to temperate soils: a review. Plant Soil 337:1–18
Bardgett R, Saggar S (1994) Effects of heavy metal contamination on the short-term decomposition of labelled [14C] glucose in a pasture soil. Soil Biol Biochem 26:727–733
Bell CW, Fricks BE, Rocca JD, Steinweg JM, McMahon SK, Wallenstein MD (2013) High-throughput fluorometric measurement of potential soil extracellular enzyme activities. J Visual Experi: JoVE 50961
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pena AG, Goodrich JK, Gordon JI (2010) QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7:335–336
Castaldi S, Riondino M, Baronti S, Esposito FR, Marzaioli R, Rutigliano FA, Vaccari FP, Miglietta F (2011) Impact of biochar application to a Mediterranean wheat crop on soil microbial activity and greenhouse gas fluxes. Chemosphere 85:1464–1471
Chen J, Liu X, Zheng J, Zhang B, Lu H, Chi Z, Pan G, Li L, Zheng J, Zhang X, Wang J, Yu X (2013) Biochar soil amendment increased bacterial but decreased fungal gene abundance with shifts in community structure in a slightly acid rice paddy from Southwest China. Appl Soil Ecol 71:33–44
Chen J, Liu X, Li L, Zheng J, Qu J, Zheng J, Zhang X, Pan G (2015) Consistent increase in abundance and diversity but variable change in community composition of bacteria in topsoil of rice paddy under short term biochar treatment across three sites from South China. Appl Soil Ecol 91:68–79
Chen J, Sun X, Li L, Liu X, Zhang B, Zheng J, Pan G (2016) Change in active microbial community structure, abundance and carbon cycling in an acid rice paddy soil with the addition of biochar. Eur J Soil Sci 67:857–867
Chen J, Li S, Liang C, Xu Q, Li Y, Qin H, Fuhrmann JJ (2017) Response of microbial community structure and function to short-term biochar amendment in an intensively managed bamboo (Phyllostachys praecox) plantation soil: effect of particle size and addition rate. Sci Total Environ 574:24–33
Conant RT, Steinweg JM, Haddix ML, Paul EA, Plante AF, Six J (2008) Experimental warming shows that decomposition temperature sensitivity increases with soil organic matter recalcitrance. Ecology 89:2384–2391
Davidson EA, Janssens IA (2006) Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 440:165–173
Domene X, Mattana S, Hanley K, Enders A, Lehmann J (2014) Medium-term effects of corn biochar addition on soil biota activities and functions in a temperate soil cropped to corn. Soil Biol Biochem 72:152–162
Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R (2011) UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27:2194–2200
Fang C, Smith P, Moncrieff JB, Smith JU (2005) Similar response of labile and resistant soil organic matter pools to changes in temperature. Nature 433:57–59
Fang Y, Singh BP, Singh B (2014) Temperature sensitivity of biochar and native carbon mineralisation in biochar-amended soils. Agric Ecosyst Environ 191:158–167
Farrell M, Kuhn TK, Macdonald LM, Maddern TM, Murphy DV, Hall PA, Singh BP, Baumann K, Krull ES, Baldock JA (2013) Microbial utilisation of biochar-derived carbon. Sci Total Environ 465:288–297
Fierer N, Jackson JA, Vilgalys R, Jackson RB (2005) Assessment of soil microbial community structure by use of taxon-specific quantitative PCR assays. Appl Environ Microbiol 71:4117–4120
Fierer N, Bradford MA, Jackson RB (2007) Toward an ecological classification of soil bacteria. Ecology 88:1354–1364
Hartley IP, Ineson P (2008) Substrate quality and the temperature sensitivity of soil organic matter decomposition. Soil Biol Biochem 40:1567–1574
He X, Du Z, Wang Y, Lu N, Zhang Q (2016) Sensitivity of soil respiration to soil temperature decreased under deep biochar amended soils in temperate croplands. Appl Soil Ecol 108:204–210
Insam H (2001) Developments in soil microbiology since the mid 1960s. Geoderma 100:389–402
Jenkins JR, Viger M, Arnold EC, Harris ZM, Ventura M, Miglietta F, Girardin C, Edwards RJ, Rumpel C, Fornasier F, Zavalloni C, Tonon G, Alberti G, Taylor G (2017) Biochar alters the soil microbiome and soil function: results of next-generation amplicon sequencing across Europe. GCB Bioenergy 9:591–612
Jiang X, Denef K, Stewart C, Cotrufo MF (2016) Controls and dynamics of biochar decomposition and soil microbial abundance, composition, and carbon use efficiency during long-term biochar-amended soil incubations. Biol Fertil Soils 52:1–14
Jin H (2010) Characterization of microbial life colonizing biochar and biochar-amended soils. Dissertation, Cornell University
Jones DL, Murphy DV, Khalid M, Ahmad W, Edwards-Jones G, DeLuca TH (2011) Short-term biochar-induced increase in soil CO2 release is both biotically and abiotically mediated. Soil Biol Biochem 43:1723–1731
Jones DL, Rousk J, Edwards-Jones G, DeLuca TH, Murphy DV (2012) Biochar-mediated changes in soil quality and plant growth in a three year field trial. Soil Biol Biochem 45:113–124
Keith A, Singh B, Singh BP (2011) Interactive priming of biochar and labile organic matter mineralization in a smectite-rich soil. Environ Sci Technol 45:9611–9618
Khodadad CLM, Zimmerman AR, Green SJ, Uthandi S, Foster JS (2011) Taxa-specific changes in soil microbial community composition induced by pyrogenic carbon amendments. Soil Biol Biochem 43:385–392
Kirschbaum MUF (1995) The temperature dependence of soil organic matter decomposition, and the effect of global warming on soil organic carbon storage. Soil Biol Biochem 27:753–760
Kramer C, Gleixner G (2008) Soil organic matter in soil depth profiles: distinct carbon preferences of microbial groups during carbon trasformation. Soil Biol Biochem 40:425–433
Kuzyakov Y, Subbotina I, Chen H, Bogomolova I, Xu X (2009) Black carbon decomposition and incorporation into soil microbial biomass estimated by 14C labeling. Soil Biol Biochem 41:210–219
Laird D, Fleming P, Wang BQ, Horton R, Karlen D (2010) Biochar impact on nutrient leaching from a Midwestern agricultural soil. Geoderma 158:436–442
Lal R (2004) Soil carbon sequestration impacts on global climate change and food security. Science 11:1623–1627
Lauber CL, Hamady M, Knight R, Fierer N (2009) Pyrosequencing-based assessment of soil pH as a predictor of soil bacterial community structure at the continental scale. Appl Environ Microb 75:5111–5120
Lehmann J, Rillig MC, Thies J, Masiello CA, Hockaday WC, Crowley D (2011) Biochar effects on soil biota—a review. Soil Biol Biochem 43:1812–1836
Legendre P, Anderson MJ (1999) Distance-based redundancy analysis: testing multispecies responses in multifactorial ecological experiments. Ecol Monogr 69:1–24
Liski J, Ilvesniemi H, Mäkelä A, Westman CJ (1999) CO2 emissions from soil in response to climatic warming are overestimated: the decomposition of old soil organic matter is tolerant of temperature. Ambio 28:171–174
Lu W, Ding W, Zhang J, Li Y, Luo J, Bolan N, Xie Z (2014) Biochar suppressed the decomposition of organic carbon in a cultivated sandy loam soil: a negative priming effect. Soil Biol Biochem 76:12–21
Luo Y, Wan S, Hui D, Wallace L (2001) Acclimation of soil respiration to warming in a tall grass prairie. Nature 413:622–625
Luo Y, Durenkamp M, De Nobili M, Lin Q, Devonshire BJ, Brookes PC (2013) Microbial biomass growth, following incorporation of biochars produced at 350 °C or 700 °C, in a silty-clay loam soil of high and low pH. Soil Biol Biochem 57:513–523
Maestrini B, Herrmann AM, Nannipieri P, Schmidt MWI, Abiven S (2014) Ryegrass-derived pyrogenic organic matter changes organic carbon and nitrogen mineralization in a temperate forest soil. Soil Biol Biochem 69:291–301
Maestrini B, Nannipieri P, Abiven S (2015) A meta-analysis on pyrogenic organic matter induced priming effect. GCB Bioenergy 7:577–590
Major J, Rondon M, Molina D, Riha SJ, Lehmann J (2012) Nutrient leaching in a Colombian savanna Oxisol amended with biochar. J Environ Qual 41:1076–1086
Marschner P, Kandeler E, Marschner B (2003) Structure and function of the soil microbial community in a long-term fertilizer experiment. Soil Biol Biochem 35:453–461
Menichetti L, Reyes Ortigoza AL, García N, Giagnoni L, Nannipieri P, Renella G (2015) Thermal sensitivity of enzyme activity in tropical soils assessed by the Q10 and equilibrium model. Biol Fertil Soils 51:299–310
Mitchell PJ, Simpson AJ, Soong R, Simpson MJ (2015) Shifts in microbial community and water-extractable organic matter composition with biochar amendment in a temperate forest soil. Soil Biol Biochem 81:244–254
Mulvaney RL (1996) Nitrogen-inorganic forms. In: Bigham JM (ed) Methods of soil analysis, part 3 chemical methods, The Soil Science Society of American Book Series no 5. Soil Science Society of American, Inc., American Society of Agronomy, Inc., Madison, WI, pp 1123–1184
Noyce G, Basiliko N, Fulthorpe R, Sackett T, Thomas S (2015) Soil microbial responses over 2 years following biochar addition to a north temperate forest. Biol Fertil Soils 51:649–659
Omondi MO, Xia X, Nahayo A, Liu X, Korai PK, Pan G (2016) Quantification of biochar effects on soil hydrological properties using meta-analysis of literature data. Geoderma 274:28–34
Pan G, Smith P, Pan W (2009) The role of soil organic matter in maintaining the productivity and yield stability of cereals in China. Agric Ecosyst Environ 129:344–348
Pietikäinen J, Kiikkilä O, Fritze H (2000) Charcoal as a habitat for microbes and its effect on the microbial community of the underlying humus. Oikos 89:231–242
Pronk GJ, Heister K, Ding GC, Smalla K, Kögel-Knabner I (2012) Development of biogeochemical interfaces in an artificial soil incubation experiment; aggregation and formation of organo-mineral associations. Geoderma 189:585–594
Quilliam RS, Glanville HC, Wade SC, Jones DL (2013) Life in the ‘charosphere’—does biochar in agricultural soil provide a significant habitat for microorganisms? Soil Biol Biochem 65:287–293
Rousk J, Brookes PC, Bååth E (2009) Contrasting soil pH effects on fungal and bacterial growth suggest functional redundancy in carbon mineralization. Appl Environ Microb 75:1589–1596
Rousk J, Bååth E, Brookes PC, Lauber CL, Lozupone C, Caporaso JG, Knight R, Fierer N (2010) Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J 4:1340–1351
Rousk J, Dempster DN, Jones DL (2013) Transient biochar effects on decomposer microbial growth rates: evidence from two agricultural case-studies. Eur J Soil Sci 64:770–776
Schlesinger W, Andrews J (2000) Soil respiration and the global carbon cycle. Biogeochemistry 48:7–20
Schmidt MWI, Torn MS, Abiven S, Dittmar T, Guggenberger G, Janssens IA, Kleber M, Kogel-Knabner I, Lehmann J, Manning DAC, Nannipieri P, Rasse DP, Weiner S, Trumbore SE (2011) Persistence of soil organic matter as an ecosystem property. Nature 478:49–56
Schulze ED, Freibauer A (2005) Environmental science: carbon unlocked from soils. Nature 437:205–206
Serra-Wittling C, Houot S, Barriuso E (1995) Soil enzymatic response to addition of municipal solid-waste compost. Biol Fertil Soils 20:226–236
Singh BP, Cowie AL (2014) Long-term influence of biochar on native organic carbon mineralisation in a low-carbon clayey soil. Sci Rep-UK 4:e3687
Sinsabaugh RL, Lauber CL, Weintraub MN, Ahmed B, Allison SD, Crenshaw C, Contosta AR, Cusack D, Frey S, Gallo ME, Gartner TB, Hobbie SE, Holland K, Keeler BL, Powers JS, Stursova M, Takacs-Vesbach C, Waldrop MP, Wallenstein MD, Zak DR, Zeglin LH (2008) Stoichiometry of soil enzyme activity at global scale. Ecol Lett 11:1252–1264
Smith P, Marino D, Cai ZC, Gwary D, Janzen H, Kumar P (2008) Greenhouse gas mitigation in agriculture. Philos Trans R Soc B 363:789–813
Sohi S, Krull E, Lopez-Capel E, Bol R (2010) A review of biochar and its use and function in soil. Adv Agronomy 105:47–82
Soil Survey Staff (1999) Soil taxonomy, a basic classification for making and interpreting soil surveys. In: Agriculture Handbook 436, 2nd edn. Natural Resources Conservation Service, Washington, pp 869
Song GH, Li LQ, Pan GX, Zhang Q (2005) Topsoil organic carbon storage of China and its loss by cultivation. Biogeochemistry 74:47–62
Taghizadeh-Toosi A, Clough T, Sherlock R, Condron L (2012) Biochar adsorbed ammonia is bioavailable. Plant Soil 350:57–69
Thiet RK, Frey SD, Six J (2006) Do growth yield efficiencies differ between soil microbial communities differing in fungal:bacterial ratios? Reality check and methodological issues. Soil Biol Biochem 38:837–844
von Lützow M, Kögel-Knabner I (2009) Temperature sensitivity of soil organic matter decomposition—what do we know? Biol Fertil Soils 46:1–15
Warnock D, Lehmann J, Kuyper T, Rillig M (2007) Mycorrhizal responses to biochar in soil—concepts and mechanisms. Plant Soil 300:9–20
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic Press, New York, pp 315–322
Wu J, Joergensen RG, Pommerening B, Chaussod R, Brookes PC (1990) Measurement of soil microbial biomass C by fumigation-extraction—an automated procedure. Soil Biol Biochem 22:1167–1169
Yao Q, Liu J, Yu Z, Li Y, Jin J, Liu X, Wang G (2017a) Changes of bacterial community compositions after three years of biochar application in a black soil of northeast China. Appl Soil Ecol 113:11–21
Yao Q, Liu J, Yu Z, Li Y, Jin J, Liu X, Wang G (2017b) Three years of biochar amendment alters soil physiochemical properties and fungal community composition in a black soil of northeast China. Soil Biol Biochem 110:56–67
Zhang A, Liu Y, Pan G, Hussain Q, Li L, Zheng J, Zhang X (2012) Effect of biochar amendment on maize yield and greenhouse gas emissions from a soil organic carbon poor calcareous loamy soil from Central China Plain. Plant Soil 351:263–275
Zhang K, Chen L, Li Y, Brookes PC, Xu J, Luo Y (2017) The effects of combinations of biochar, lime, and organic fertilizer on nitrification and nitrifiers. Biol Fertil Soils 53:77–87
Zheng J, Chen J, Pan G, Liu X, Zhang X, Li L, Bian R, Cheng K, Jinwei Z (2016) Biochar decreased microbial metabolic quotient and shifted community composition four years after a single incorporation in a slightly acid rice paddy from southwest China. Sci Total Environ 571:206–217
Zhou H, Zhang D, Wang P, Liu X, Cheng K, Li L, Zheng J, Zhang X, Zheng J, Crowley D, van Zwieten L, Pan G (2017) Changes in microbial biomass and the metabolic quotient with biochar addition to agricultural soils: a meta-analysis. Agric Ecosyst Environ 239:80–89
Zhou J, Wu L, Deng Y, Zhi X, Jiang Y-H, Tu Q, Xie J, Van Nostrand JD, He Z, Yang Y (2011) Reproducibility and quantitation of amplicon sequencing-based detection. ISME J 5:1303–1313
Zimmerman AR, Gao B, Ahn M-Y (2011) Positive and negative carbon mineralization priming effects among a variety of biochar-amended soils. Soil Biol Biochem 43:1169–1179
Zimmermann M, Leifeld J, Conen F, Bird MI, Meir P (2012) Can composition and physical protection of soil organic matter explain soil respiration temperature sensitivity? Biogeochemistry 107:423–436
Acknowledgements
This work was funded by the National Science Foundation of China under grant numbers 41401318, 41371300, and 41371298, and the Scientific Research Foundation of Zhejiang A & F University under grant number 2014FR024. We thank Dr. Paolo Nannipieri, the Editor-in-Chief, and the anonymous reviewers for their very valuable comments in improving both the language and scientific quality of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 15 kb)
Rights and permissions
About this article
Cite this article
Chen, J., Sun, X., Zheng, J. et al. Biochar amendment changes temperature sensitivity of soil respiration and composition of microbial communities 3 years after incorporation in an organic carbon-poor dry cropland soil. Biol Fertil Soils 54, 175–188 (2018). https://doi.org/10.1007/s00374-017-1253-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-017-1253-6