Abstract
Elevated atmospheric CO2 concentration ([CO2]) may change litter chemistry which affects litter decomposability. This study investigated respiration and microbial biomass of soils amended with litter of Pinus densiflora (a coniferous species; pine) and Quercus variabilis (a deciduous species; oak) that were grown under different atmospheric [CO2] and thus had different chemistry. Elevated [CO2] increased lignin/N through increased lignin concentration and decreased N concentration. The CO2 emission from the soils amended with litter produced under the same [CO2] regime was greater for oak than pine litter, confirming that broadleaf litter with lower lignin decomposes faster than needle leaf litter. Within each species, however, soils amended with high lignin/N litter grown under elevated [CO2] emitted more CO2 than those with low lignin/N litter grown under ambient [CO2]. Such contrasting effects of lignin/N on inter- and intra-species variations in litter decomposition should be ascribed to the effects of other litter chemistry variables including nonstructural carbohydrate, calcium and manganese as well as inhibitory effect of N on lignin decomposition. The microbial biomass was also higher in the soils amended with high lignin/N litter than those with low lignin/N litter probably due to low substrate use efficiency of lignin by microbes. Our study suggests that elevated [CO2] increases lignin/N for both species, but increased lignin/N does not always reduce soil respiration and microbial biomass. Further study investigating a variety of tree species is required for more comprehensive understanding of inter- and intra-species variations of litter decomposition under elevated [CO2].
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In forests, decomposition of leaf litter (referred to as litter hereafter) is the pathway through which carbon (C) and nutrients such as nitrogen (N) and phosphorus (P) are recycled, and thus the changes in the decomposition pattern of litter affect soil C storage as well as forest productivity, eventually affecting global C cycling (Berg and McClaugherty 2008; Prescott 2010). Though many factors including litter quality, soil properties, and climatic variables affect litter decomposition (Zhang et al. 2008; Zhong et al. 2017), litter quality is the principal factor that determines litter decomposability at a local scale where the variations in the climate and site conditions are confined (Prescott 2010; Zhang et al. 2008).
A large number of publications reported that litter quality is determined by litter chemistry such as elemental compositions including C, N, P, calcium (Ca), manganese (Mn), and aluminum (Al) as well as the constitution of organic compounds ranging from nonstructural carbohydrate (NSC) to recalcitrant lignin (Prescott 2010; Rahman et al. 2013; Zhang et al. 2008). Among those, the ratio of lignin to N (lignin/N) is commonly used as a robust predictor of litter quality and thus decomposability (e.g., Prescott 2010; Taylor et al. 1989; Zhang et al. 2008). It is widely reported that high-quality litter with low lignin and high N concentrations decomposes faster than low-quality litter with high lignin and low N, resulting in a greater CO2 emission due to easily accessibility of high-quality litter by microbes (Cotrufo et al. 2013; Manzoni et al. 2010; Rahman et al. 2013; Zhang et al. 2008). Readily decomposability of high-quality litter was also believed to result in a greater microbial biomass compared to low-quality litter (Cheng et al. 2013; Iovieno et al. 2010; Ushio et al. 2008).
However, such lignin/N effects on soil respiration, litter decomposition, and microbial biomass are based on inter-species variations that compared decomposability of litters from a variety of functionally different plant species under current atmospheric CO2 concentration ([CO2]) (e.g., Prescott 2010). For example, several studies ascribed faster decomposition of broadleaf litter than that of needle leaf litter to a lower lignin/N of the former than the latter (Chodak et al. 2016; Lorenz et al. 2004; Wang and Yang 2007). As elevating CO2 concentration ([CO2]) is likely to cause carbohydrates accumulation, lignin/N may increase due to N dilution effect (Norby et al. 2001). Therefore, understanding the effects of elevated [CO2] (E[CO2]) on lignin/N of litter and subsequent changes in microbial respiration of soils amended with the litter is critical in estimating potential changes in forest C dynamics under the projected climate change. In addition, as not only lignin/N but also other litter chemistry (e.g., NSC, P, Ca, and Mn) differs with plant species (Prescott 2010), investigation of both inter- and intra-species variations in the litter decomposition may provide further insight into the effects of the lignin/N changed by E[CO2] on litter decomposition. However, our understanding of the effect of lignin/N on soil respiration and microbial biomass is limited to litter from different plant species grown under the ambient [CO2] (A[CO2]).
To fill the research gap, we investigated respiration and microbial biomass of soils amended with litter of Pinus densiflora (a needle-leaved coniferous species; pine) and Quercus variabilis (a broad-leaved deciduous species; oak) that was grown under different [CO2] and thus had different chemistry. We hypothesized that (1) E[CO2] would increase lignin/N due to carbohydrates accumulation and associated N dilution effect as stated above, (2) increased lignin/N would decrease soil respiration and microbial biomass due to retardation of litter decomposition, and (3) such effects of increased lignin/N by E[CO2] on soil respiration and microbial biomass would differ with tree species as not only lignin/N but also other chemical properties differ with species.
Materials and methods
Preparation of litter samples
Litter samples of pine and oak were collected from trees exposed to different [CO2] regimes for two growing seasons (2012 and 2013). The details of the conditions of the tree growth are described in Park (2016) who conducted a series of experiments to investigate the effects of [CO2] on the growth of the two functionally different tree species. Briefly, 2-year-old seedlings were planted in pots (28 cm in diameter × 35 cm in height) packed with a forest soil (40 kg on dry basis). The forest soil had a pH of 5.10, 7.2 g C kg−1 of total C, 0.9 g N kg−1 of total N, 9.4 mg N kg−1 of exchangeable NH4+, 0.2 mg N kg−1 of NO3−, and 8.5 mg P2O5 kg−1of available P (Bray #1 P). The pots were put into field chambers with or without CO2 fumigation located at the experiment field of Chonnam National University (126° 53′ E, 35° 10′ N, alt. 33 m), Gwangju, South Korea. The chambers (2.4 m in width × 24 m in length × 2.0 m in height for each chamber) were composed of six independent chambers; three of which were allocated to the A[CO2] condition, and the remaining were allocated to the E[CO2] condition. The [CO2] in the chambers allocated to E[CO2] was set at 660 ppmv and controlled by fumigating CO2 from a pure CO2 cylinder. Average data for 5 min of [CO2] in the chambers were monitored every 5 s with a CO2 analyzer (GMT 222, Vaisala, Switzerland) and were stored in a data logger (CR100, Campbell Scientific, USA) (Kim et al. 2011). In each chamber, three pots for each tree species were placed. The pots received 6.7 g N m−2 (as NH4NO3), 7.6 g P2O5 m−2 and 5.2 g K2O5 m−2 (as KH2PO4) in May of each year. Water was supplied to the pots using a semi-automatic drip irrigation system equipped with soil moisture sensors (EC5, Decagon Devices, Inc., Pullman, WA, USA) to maintain soil moisture content between 0.1 and 0.2 m3 m−3 during the growing seasons. The [CO2] across the chambers during the study period were 331.3 ± 1.3 (mean ± SD) ppmv for A[CO2] and 647.7 ± 4.8 ppmv for E[CO2] in 2012 and 320.0 ± 1.3 ppmv for A[CO2] and 604.6 ± 3.3 ppmv for E[CO2] in 2013. At the end of the second growing season, the seedlings were covered with nylon nets to collect all the litterfall.
Analysis of litter chemistry
The collected litter samples were washed with distilled water to remove dust and soil particles and dried at 60 °C in an oven for 5 days. The litter samples were hand-mixed thoroughly and a portion of the litter samples (10 g) was ground to fine powder with a ball mill (MM-200, Retsch GmbH 88 & Co., KG, Haan, Germany) and used for chemical analysis and the incubation experiment. Total C and N concentrations were determined by a combustion method (Nelson and Sommers 1996) using an elemental analyzer (FLASHEA-1112, Thermo, USA). The lignin contents were analyzed by a gravimetric method using hot sulfuric acid digestion which allows lignin content analysis with a small-sized sample (King and Heath 1967). Although the determined lignin fraction may contain not only true lignin but also other lignin-like materials, this fraction was defined as lignin in this study (Osono and Takeda 2004). The NSC contents were determined by a gravimetric method after gelatinization and enzyme (α-amylase and amyloglucosidase) reaction that solubilize the NSC (Ohnish and Horie 1999). The concentrations of minerals (P, Ca, Mn, and Al) in the litter were determined by using an inductively coupled plasma (ICP) emission spectrophotometer (IRIS-AP, Thermo Jarrell Ash Corp., Fanklin, MA, USA) after digestion of 0.5 g of sample with a HNO3-HClO4-H2SO4 mixture (1:8:1) (Kwak et al. 2009).
Incubation experiment and CO2 measurement
Litter decomposition was investigated by measuring CO2 emission from soils amended with litter in the laboratory incubation experiment. Litter decomposition is affected by not only litter chemistry but also physical properties such as leaf specific area (Cortez et al. 2007) and toughness (Pérez-Harguindeguy et al. 2000). In this experiment, therefore, finely ground litter samples were used to rule out the effects of physical properties on litter decomposition (Cleveland et al. 2014). However, it is also important to underline that the effects of grinding litter on CO2 emission may differ with litter types (Bremer et al. 1991; Handayanto et al. 1997; Rinkes et al. 2014).
Soils (2 kg) were collected from randomly selected 10 pots in which seedlings were grown, and mixed thoroughly, air-dried, sieved (< 2-mm) and used for chemical analysis (see Table 1 for the soil properties) and the incubation experiment. Thirty grams of the soil was placed into a 100-mL beaker; a total of 15 beakers were prepared because there were two species with two litter and each treatment was replicated three times; in addition, the control was also replicated three times. The moisture content of the soils in the beakers was brought to 60% of water holding capacity by adding distilled water, and the soils were pre-incubated at 25 ± 1 °C in darkness for 5 days to restore and stabilize the microbial activity. The ground litter sample (1 g) was placed into the beakers and mixed thoroughly with a spatula. The amount of litter applied to soils was determined by considering the quantity of litterfall in the pots (range 400–700 g m−2). The 100-mL beaker containing the soils and a 20-mL vial with 10 mL 0.5 N NaOH (CO2 trap) were placed into 1-L air-tight Mason jar. Additional three jars with an empty beaker (without soil) were also prepared as blanks for the CO2 measurement.
The jars were incubated at 25 ± 1 °C in the darkness for 45 days. During the incubation, the jars were opened for 10 min every other day to maintain an adequate O2 level. In a previous study, the CO2 loss during the aeration was estimated to be negligible because the CO2 loss was below 0.7% of total CO2 emission (Lee et al. 2011; Lim et al. 2012). At 1, 2, 3, 5, 7, 10, 15, 20, 25, 30, 35, 40, and 45 days of the incubation, the CO2 trap was collected from the jars and analyzed for CO2, and a new vial containing fresh NaOH solution was placed back in the jar except for the final CO2 measurement. Soil moisture content was adjusted by adding distilled water to the initial weight of the soil container at each CO2 measurement. The incubation experiment was stopped at 45 days as no significant flush of CO2 was observed and thus the soil respiration could be assumed to be stabilized (see Fig. 1). The amount of CO2 trapped within the NaOH solution was determined via titration of the solution with 0.5 N HCl solution after the addition of 2 mL 1 N BaCl2 (Choi et al. 2005). The rate of CO2 respiration was expressed as mg C kg soil−1 day−1. The cumulative CO2-C evolved over the 45-day incubation was calculated as percentage of total litter-C after subtracting the amounts of CO2-C emitted from the control soil without litter from those of litter-amended soils as in a preliminary study with 13C-depleted (−35.5 ± 0.2‰) pine and oak litters, we found that priming effect is negligible for the soil.
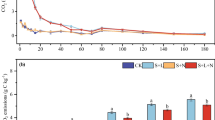
CO2 emission from soils amended with litters of (a) pine and (b) oak with different litter chemistry during the 45-days incubation. Values are the means of triplicate, and vertical bars indicate standard errors of the means (n = 3). Error bars are often too small to be depicted. Detailed litter chemistry is provided in Table 2, and ANOVA is provided in Table 3. Data for control without litter addition are depicted in all the figure for better comparison
Analysis of soil pH and microbial biomass and community composition
At the end of incubation experiment, the soil samples were freeze-dried and analyzed for soil pH and microbial community composition. Soil pH was measured with a pH meter at 1-to-5 of soil-to-water ratio. Microbial biomass and community composition was determined by using the fatty acid methyl ester (FAME) analysis method, as described by Lee and Yun (2011). Individual FAME was used as biomarkers for various groups of microbes and reported as nmol FAMEs g soil−1, and the total viable microbial biomass was calculated as the sum of FAMEs concentrations (Diedhiou et al. 2009; Frostegård and Bååth 1996). The FAMEs iso (i) 15 carbon chain with zero double bonds (15:0), anti-iso (α) 15:0, 15:0, i16:0, 16:1ω9, 16:1ω7, i17:0, α17:0, 17:0, cyclopropane (cy) 17:0, 18:1 with the first double bond from the 7th carbon (ω7c), and cy19:0 were chosen to represent bacterial biomass (Schutter and Dick 2000). The FAMEs 18:1 ω9c and 18:2ω6c were used as biomarkers of fungi (Bradleya et al. 2006) and 16:1ω5c was used as an indicator of Arbuscular mycorrhizal fungi (Frostegård et al. 1993). The major bacterial groups were calculated by summing the FAME biomarkers as follows: 16:1ω7c, 18:1ω7c, cy17:0, and cy19:0 were used as indicators of Gram-negative bacteria and the branched FAMEs i15:0, α 15:0, i16:0, i17:0, and α17:0 were chosen to represent Gram-positive bacteria (Zelles 1997). The FAME 10-methyl (Me) 18:0 was used to indicate Actinomycetes (Schutter and Dick 2000).
Kinetic model fitting and statistical analysis
Data were tested for normality of distribution and homogeneity of variance with Shapiro-Wilk and Levene’s tests, respectively. Data transformation was not needed since no heterogeneity was detected and the distribution was normal. To estimate litter decomposition kinetics parameters (the size of mineralizable litter C and decomposition rate constant), the CO2 emission data were fitted to the single exponential first-order kinetic model with the Fit Curve procedures of SigmaPlot 10.0 (Systat Software Inc., IL), which uses the Marquardt-Levenberg algorithm and an iterative process to determine the parameter values that minimize the residual sum of squares (Lim et al. 2012):
where %Ccum is the cumulative CO2 expressed as a percentage of total C mineralized from the litter at time t, Cmin is the size of mineralizable litter-C pool as a percentage of total C of litter, and k is litter decomposition rate constant (day−1). Although the double exponential model is believed to be better in estimating C mineralization than the single exponential model as it separates soil C pool into rapidly and slowly decomposable C pools (Ajwa and Tabatabai 1994), our data did not fit to the double exponential model. The significance of the first-order kinetic model of CO2 emission was analyzed with the F-test.
Changes in litter chemistry with tree species and [CO2] and the effects of tree species (pine and oak) and litter chemistry (e.g., low and high lignin/N) on cumulative CO2 emission and microbial biomass and community composition were assessed by analysis of variance (ANOVA) using SPSS 21.0 package (SPSS Inc., Chicago). The effect of litter addition on the microbial biomass and community composition was also assessed by ANOVA. When their effects were significant, the means were separated by Tukey’s test. The level of significance for all statistical tests was set α = 0.05.
Results
Litter chemistry
Litter chemistry differed with species (e.g., P = 0.020 for lignin and P = 0.003 for NSC) and [CO2] regimes (e.g., P = 0.035 for lignin and P = 0.050 for NSC); under A[CO2], pine litter had a greater (P < 0.05) lignin concentration and a lower NSC than oak litter (Table 2). For both species, E[CO2] increased lignin concentration (P = 0.035) and decreased (P < 0.001) N concentration, resulting in increased (P < 0.001) lignin/N ratio. The NSC concentration of oak litter was greater (P = 0.003) than that of pine litter, and E[CO2] increased (P = 0.050) the NSC concentration (Table 2). The litter also had different P, Ca, Mn, and Al concentrations (Table 2); specifically, oak litter had higher (P < 0.001) Ca and Mn concentrations than pine litter.
CO2 emission and litter decomposition kinetics
Litter amendments stimulated microbial respiration as indicated by the significantly (P < 0.001) high CO2 emission in the soils amended with litter compared to those without litter (Fig. 1). In the soils amended with litter, the daily rate of CO2 emission from the soils reached peaks within 2 days of incubation followed by a gradual decrease up to 25 days; thereafter it remained at a stable level until the end of the incubation regardless of species and litter chemistry (Fig. 1).
During the 45-day of incubation, when compared between pine and oak litters, the cumulative CO2 emission from litter and/or soil was higher (P = 0.008) for the soils amended with oak litter which had lower lignin concentrations and significantly higher NSC, Ca, and Mn concentrations than those for the soils with pine litter (Table 3). The inter-species difference in CO2 emission was more apparent for litters grown under E[CO2] than those under A[CO2]. Within the same species that were grown under different [CO2] regime, however, the soils amended with litter of significantly higher lignin/N, grown under E[CO2], showed greater (P < 0.05) cumulative CO2 than those with litter of significantly lower lignin/N, grown under A[CO2] (Table 3). The effects of tree species (P < 0.001) and litter chemistry on the size of mineralizable litter-C pool were similar to those on the cumulative CO2 emission (Table 3). The decomposition rate constant was not affected by species and the effect of litter chemistry was only significant (P = 0.048) for pine (Table 3).
Soil pH and microbial biomass and community composition
The effects of species and litter chemistry on soil pH were not statistically significant (P > 0.05) though pine litter application resulted in a slightly lower pH (5.36–5.54) than that (5.70–5.83) of oak litter application (Table 4).
Litter addition increased the contents of FAMEs from Gram-positive, Gram-negative bacteria, and fungi compared to the control soils by 5–17 folds with the greatest increases in fungal FAMEs (Table 4). The total FAMEs were higher (P < 0.001) in the soils amended with pine litter than those with oak litter by 32.6%, and the effect of litter chemistry on the total FAMEs was only significant for pine; litter with high lignin/N litter increased (P > 0.05) the total FAMEs by 13.4% compared to those with low lignin/N litter (Table 4).
The variations in the bacterial FAMEs as affected by litter species and chemistry were the same as those of the total FAMEs; among the major bacterial group, Gram-positive bacterial FAMEs increased (P > 0.05) by amending litter with high lignin/N for pine but not for oak, whereas Gram-negative bacterial FAMEs were not affected by litter chemistry but were higher (P < 0.001) in the soils with pine litter than those with oak litter (Table 4). Fungal FAMEs were not affected by litter chemistry but were higher (P = 0.001) in the soils amended with pine litter than those with oak litter.
Discussion
Litter chemistry with [CO2] and species
The increased NSC and lignin and decreased N concentration by E[CO2] should be attributed to accumulation of carbohydrates caused by “CO2 fertilization” effect (e.g., Norby et al. 2001), leading to increased lignin/N (Table 2). Comparing between species, the lower lignin, Ca and Mn concentrations of pine litter than those of oak litter are consistent with other studies that compared litter chemistry between needle-leaved coniferous and broad-leaved deciduous (Berg and McClaugherty 2008; Gholz et al. 2000).
Inter-species variations in litter decomposition
When litters of pine and oak grown under the same [CO2] regime were incorporated into the soils, the CO2 emission and the size of mineralizable litter-C pool for soils amended with oak litter were greater than those with pine litter (Table 3 and Fig. 2), which coincided with the lower lignin concentration of oak than pine litters (Table 2) (Chodak et al. 2016; Lorenz et al. 2004; Wang and Yang 2007). Several studies consistently reported that deciduous broadleaf litter (high-quality litter) were more readily decomposed than coniferous needle leaf litter (low-quality litter) (Chodak et al. 2016; Lorenz et al. 2004; Wang and Yang 2007). Specifically, for pine and oak species, many studies also repeatedly reported that CO2 emission from soil respiration was greater for oak than pine in in situ field experiments (e.g., Chodak et al. 2016; Wang and Yang 2007; Wu et al. 2006). However, the results from field conditions may not represent the pure effect of litter chemistry on CO2 emission because CO2 emission via soil respiration is affected not only by litter chemistry but also many other factors such as soil organic matter contents and microbial abundance that differ with site conditions (Chodak et al. 2016). For example, it is often reported that soils under broad-leaved trees receive more litter and have more microbial biomass compared to the soils under needle-leaved trees, leading to greater soil respiration in the soils under broad-leaved trees (Chodak et al. 2016; Wang and Yang 2007).
Cumulative litter-C mineralized expressed as a percentage of total litter-C of (a) pine and (b) oak with different litter chemistry during the 45-day incubation. Values are the means of triplicate, and vertical bars indicate standard errors of the means. Error bars are often too small to be depicted. Detailed litter chemistry is provided in Table 2
In the controlled laboratory incubation experiments in which litter was amended to the same soil at the same quantity, however, Wang et al. (2014) reported that CO2 emission from soils amended with needle leaf litter of Pinus massoniana was greater than that with broadleaf litter of Michelia macclurei. In our study, when litter of pine grown under E[CO2] and litter of oak grown under A[CO2] was compared, the soils amended with pine litter (high lignin/N) resulted in a greater CO2 emission than the soils amended with oak litter (low lignin/N) (Table 3), which suggests that broadleaf litter does not always decompose faster than needle leaf litter. In addition, when litters of the different species grown under the same [CO2] regime are compared, though the lignin/N ratio was not different between species (Table 2), CO2 emission from soils amended with oak litter was greater than that with pine litter (Table 3). Therefore, the frequently reported pattern of greater CO2 emission from soils of deciduous broadleaf tree stands compared to needle leaf tree stands (Chodak et al. 2016; Wang and Yang 2007; Wu et al. 2006) may not be ascribed solely to lower lignin/N of deciduous broadleaf trees. Among many litter chemical variables, in our study, NSC, Ca, and Mn seemed to play important roles in litter decomposition as these were consistently higher for oak than pine litter (Table 2) as also supported by other studies (Berg and McClaugherty 2008; Gholz et al. 2000). The NSC consists of starch which is readily decomposable by microbes, Ca is a nutrient for microbes, and Mn is an essential element for manganese peroxidases which play important roles in lignin decomposition by saprotrophic white-rot fungi; therefore, high NSC, Ca, and Mn concentrations of oak litter might favor for litter decomposition (Prescott 2010). Therefore, it is suggested that faster decomposition rate of broadleaf than needle leaf litter may be in part due to the difference in other litter chemical variables such as NSC (Skorupski et al. 2012), Ca (Aponte et al. 2012), and Mn (Davey et al. 2007) as well as lignin.
Intra-species variation in litter decomposition
The current understanding of the effects of litter chemistry such as lignin, N, and NSC concentrations on litter decomposition are largely based on the comparison between litter with high N, low lignin, and high NSC concentrations vs. litter with low N, high lignin, and low NSC concentrations (Prescott 2010; Skorupski et al. 2012). In our study, we compared litters with high N, low lignin, and low NSC concentrations (for A[CO2]) vs. low N, high lignin, and high NSC concentrations (for E[CO2]) (Table 2). Therefore, our study provides a novel picture of the effect of litter chemistry on litter decomposition. When litters of the same species grown under different [CO2] regime are compared, the greater CO2 emissions for litter grown under E[CO2] than those under A[CO2] in spite of the higher lignin/N of the litter grown under E[CO2] (Table 3) suggests that lignin/N is not a robust predictor of intra-species variations in the litter decomposability. The effects of changed atmospheric conditions on litter chemistry and subsequent litter decomposition are still controversial; e.g., Norby et al. (2001) reported that litter produced under E[CO2] showed high lignin/N, but did not affect soil CO2 emission when compared to the litter produced under A[CO2]; whereas, Wang et al. (2018) reported that decreased lignin/N caused by elevated N and S deposition increased decomposition of trembling aspen litter. As litter decomposition and subsequent release of CO2 are complicated processes, it is difficult to define a clear mechanism for such CO2 emission pattern seen in this study, which is in contradiction to the current understanding. Nevertheless, as litter with a high lignin/N that was collected from trees grown under E[CO2] had a greater NSC concentration than litter with a low lignin/N collected from trees grown under A[CO2] (Table 2) as also reported by Coley et al. (2002), the higher NSC concentration might contribute to the greater CO2 emission for the soils amended with high lignin/N litter than those with low lignin/N litter. In addition, particularly for oak, increased Ca and Mn concentrations under E[CO2] (Table 2) might be also responsible for the greater CO2 emission from the soils amended with litter with high lignin/N (in E[CO2]) than that with low lignin/N (in A[CO2]) since Ca and Mn stimulate the production of Mn peroxidase, laccase, and lignin peroxidase, thereby increasing the decomposition of lignin (Berg 2014; Eriksson et al. 1990; Perez and Jeffries 1992).
The greater CO2 emission from soils with high lignin/N litter than those with low lignin/N litter could be also explained in the context of lignin decomposability. Studies have repeatedly shown that lignin is not as stable as generally considered (Heim and Schmidt 2006; Prescott 2010; Rasse et al. 2005) and thus lignin is likely to be decomposed through co-metabolism with less recalcitrant compounds such as starch in the early stage of litter decomposition (Bahri et al. 2008; Klotzbücher et al. 2011). It is also well established that microbial assimilation of lignin C is low (i.e., loss of lignin C via respiration is high) (Bahri et al. 2008) due to low substrate use efficiency (SUE), the proportion of assimilated substrate which is utilized for microbial biomass growth relative to the substrate being mineralized and respired (Cotrufo et al. 2013). The SUEs of simple compounds are high (e.g., 73% for glucose, Dijkstra et al. 2012) compared to complex structural compounds (e.g., <31% for lignin, Bahri et al. 2008). Therefore, decomposition of litter with a high lignin concentration in the early stage of microbial decomposition process may emit more CO2 compared to litter with a low lignin concentration due to low SUE of lignin. In addition, it is also reported that lignin decomposition is retarded under high N availability due to inhibition of lignolytic enzyme activity (Ge et al. 2016; Hobbie et al. 2012; Knorr et al. 2005). Such inhibitory effects of N on lignin decomposition are evident when inorganic N is abundant (DeForest et al. 2004; Treseder 2008; Tu et al. 2014). In this context, the lower CO2 emission in the soils amended with low lignin/N (and thus low C/N) litter than those with high lignin/N (and thus high C/N) could be attributed in part to the retardation of lignin decomposition for litter with low lignin/N (and thus high N concentration) due to increased N availability through mineralization of N (Berg and McClaugherty 2008).
However, it should also be mentioned that in the present study, using finely ground litter may alter natural litter decomposition processes as grinding may enhance CO2 emission from high C/N substrate but may not affect CO2 emission from low C/N substrate (Bremer et al. 1991). In this context, the greater CO2 emission from soils amended with litter produced under E[CO2] (thus has a greater lignin/N and C/N), compared to soils amended with litter produced under A[CO2] (thus has a lower lignin/N and C/N) should be ascribed in part to the grinding effect.
Microbial biomass and community composition
The greater FAMEs of the soils amended with pine litter than those with oak litter (Table 4) are not consistent with many studies that repeatedly reported that microbial biomass is higher in the soils under broad-leaved trees than those under needle-leaved trees (e.g., Cheng et al. 2013; Iovieno et al. 2010; Ushio et al. 2008). Such difference reported in the literature can be basically attributed to both a greater amount of litterfall and high litter quality of the broad-leaved trees compared to the needle-leaved species (Chodak et al. 2016). However, some studies which were conducted in laboratory incubation experiments with the addition of the same quantity of litter to soils reported that microbial biomass was greater in the soils amended with needle leaf litter than those with broadleaf litter (Wang et al. 2014, 2016), supporting our findings. Therefore, our results in combination with the studies of Wang et al. (2014, 2016) suggest that the greater microbial biomass under broad-leaved trees that are frequently observed in the field conditions should be ascribed to the greater quantity of litterfall rather than high litter quality. As very few studies directly compared the changes in microbial biomass by addition of litter of different tree species (Wang et al. 2014, 2016), the exact mechanisms of the greater microbial biomass in the soils amended with pine litter than that with oak litter are not clear. Despite the uncertainty, however, our study suggests that inherent C use efficiency of the microbial community in the soils amended with pine litter are not lower than that with oak litter.
Within the species, the greater FAMEs for the soils with high lignin/N litter than with low lignin/N litter (Table 4) suggest that microbes assimilate litter C more efficiently when lignin/N is high in spite of the low SUE of lignin. This might be associated again with the inhibitory effect of N on lignolytic enzymes activity (DeForest et al. 2004; Treseder 2008). As lignin hinders the accessibility of microbes to other compounds such as cellulose in plant tissues, retardation of lignin decomposition under high N availability in the soils amended with low lignin/N litter could result in lower FAMEs by reducing C substrate available for microbial growth compared to those with high lignin/N litter (DeForest et al. 2004; Treseder 2008). In addition, we also postulate that such pattern of FAMEs between litters with contrasting lignin/N is linked in part to the potentially different composition of organic components of litter as litter with a high lignin/N collected from trees grown under E[CO2] had higher NSC concentration (Table 2). As previously mentioned, increased NSC concentration for litter exposed to E[CO2] might stimulate microbial growth when the litter was added to the soils. It is also possible that litter with different chemical composition changed the soil microbial community, which in turn affected soil organic C turnover (Don et al. 2017).
Compared to other microbial community, fungi responded more greatly to the addition of litter (Table 4). This is consistent with other findings that leaf litter addition stimulates the growth of fungi rather than bacteria (Neely et al. 1991; Wang et al. 2014) as fungal growth is more favored by the addition of cellulose and lignin whereas bacterial growth is stimulated by addition of readily decomposable substrate (Meidute et al. 2008). In this context, the greater fungal FAMEs in the soils amended with pine litter than those with oak litter (Table 4) could be attributed to the higher lignin concentration in the pine litter (Table 2) (Weand et al. 2010). The greater response of Gram-negative bacteria than Gram-positive bacteria (Table 4) also corroborates other studies which reported that leaf litter added to soils is utilized to a greater extent by Gram-negative bacteria (Garcia-Pausas and Paterson 2011) because Gram-negative bacteria preferentially utilize plant tissues whereas Gram-positive bacteria have preference to indigenous soil organic matter (Kramer and Gleixner 2008; Tavi et al. 2013).
Addition of litter with different chemical composition including lignin and nutrients are reported to affect microbial community composition through their influences on the soil environment (Chodak et al. 2016; Iovieno et al. 2010; Ushio et al. 2008). Among the soil environmental parameters, soil pH has been shown to be the principal factor being correlated with microbial community composition though the exact mechanisms are not discovered (Iovieno et al. 2010; Jiang et al. 2014; Ushio et al. 2008). For example, increase in fungal biomass and decrease in bacterial biomass with decreasing soil pH has been consistently reported (e.g., Bååth and Anderson 2003). In those studies, the soils had a wide pH gradient from 3 to 8; however, in our study, the narrow soil pH range (5.5 to 5.8) may not allow for the exhibition of such a pattern of fungal and bacterial biomass with respect to soil pH. More comprehensive study is required to understand the changes in the microbial community composition caused by addition of litter with different chemistry by sequencing marker genes that allow direct assessment of microbial community composition (Schöler et al. 2017; Vestergaard et al. 2017).
Conclusions
To eliminate the effects of physical attributes of litter on decomposition, our experiments were conducted with finely ground litter samples under laboratory incubation conditions that are different from natural conditions in which litter is broken down by soil fauna and soluble materials such as dissolved organics and ions are leached down. For those reasons, our results may not reflect the litter decomposition in the natural forest soils. Nevertheless, this study provides a novel finding on how the changed litter chemistry under rising [CO2] affects respiration and microbial biomass of soils amended with the litter. Our results suggest that lignin/N is a useful parameter in understanding the differences in the litter decomposition between tree species grown under the same [CO2] regime; however, it does not explain successfully the variations in the litter decomposition within a species or across species grown under different [CO2] regimes. Not only lignin/N, but also the concentrations of NSC and elements particularly Ca and Mn need to be taken into account to explain the inter-species variations in the microbial decomposability of litter. As the projected E[CO2] may change not only lignin/N but also other litter chemical variables, such as NSC and elements concentration, a mechanistic understanding of the effects of litter chemistry on litter decomposability may help more accurate predictions of C dynamics in terrestrial ecosystems under rising [CO2].
References
Ajwa HA, Tabatabai MA (1994) Decomposition of different organic materials in soils. Biol Fertil Soils 18:175–182. https://doi.org/10.1007/BF00647664
Aponte C, Carcía LV, Marañón T (2012) Trees species effect on litter decomposition and nutrient release in Mediterranean oak forests changes over time. Ecosystems 15:1204–1218. https://doi.org/10.1007/s10021-012-9577-4
Bahri H, Rasseb DP, Rumpela C, Dignaca MF, Bardouxa G, Mariotti A (2008) Lignin degradation during a laboratory incubation followed by 13C isotope analysis. Soil Biol Biochem 40:1916–1922. https://doi.org/10.1016/j.soilbio.2008.04.002
Bååth E, Anderson TH (2003) Comparison of soil fungal/bacterial ratios in a pH gradient using physiological and PLFA-based technique. Soil Biol Biochem 35:955–963. https://doi.org/10.1016/S0038-0717(03)00154-8
Berg B (2014) Decomposition patterns for foliar litter—a theory for influencing factors. Soil Biol Biochem 78:222–232. https://doi.org/10.1016/j.soilbio.2014.08.005
Berg B, McClaugherty C (2008) Plant litter—decomposition, humus formation, carbon sequestration. Springer, Berlin
Bradleya K, Rhae A, Drijberb RA, Knopsc J (2006) Increased N availability in grassland soils modifies their microbial communities and decreases the abundance of arbuscular mycorrhizal fungi. Soil Biol Biochem 38:1583–1595. https://doi.org/10.1016/j.soilbio.2005.11.011
Bremer E, van Houtum W, van Kessel C (1991) Carbon dioxide evolution from wheat and lentil residues as affected by grinding, added nitrogen, and the absence of soil. Biol Fertil Soils 11:221–227
Cheng F, Peng X, Zhao P, Yuan J, Zhong C, Cheng Y, Cui C, Zhang S (2013) Soil microbial biomass, basal respiration and enzyme activity of main forest types in the Qinling mountains. PLoS One 8:e67353. https://doi.org/10.1371/journal.pone.0067353
Chodak M, Klimek B, Niklińska M (2016) Composition and activity of soil microbial communities in different types of temperate forests. Biol Fertil Soils 52:1093–1104. https://doi.org/10.1007/s00374-016-1144-2
Choi WJ, Chang SX, Hao X (2005) White spruce response to co-composted hydrocarbon-contaminated drilling waste: effects of compost age and nitrogen fertilization. J Envrion Qual 34:1319–1327. https://doi.org/10.2134/jeq2005.0436
Cleveland CC, Reed SC, Keller AB, Nemergut DR, O’Neill SP, Ostertag R, Vitousek PM (2014) Litter quality versus soil microbial community controls over decomposition: a quantitative analysis. Oecologia 174:283–294. https://doi.org/10.1007/s00442-013-2758-9
Coley PD, Massa M, Lovelock CE, Winter K (2002) Effects of elevated CO2 on foliar chemistry of saplings of nine species of tropical tree. Oecologia 133:62–69. https://doi.org/10.1007/s00442-002-1005-6
Cortez J, Garnier E, Pérez-Harguindeguy N, Debussche M, Gillon D (2007) Plant traits, litter quality and decomposition in a Mediterranean old-field succession. Plant Soil 296:19–34. https://doi.org/10.1007/s11104-007-9285-6
Cotrufo MF, Wallenstein MD, Boot CM, Denef K, Paul E (2013) The Microbial Efficiency-Matrix Stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: do labile plant inputs form stable soil organic matter? Glob Change Boil 19:988–995. https://doi.org/10.1111/gcb.12113
Davey MP, Berg B, Emmett BA, Rowland P (2007) Decomposition of oak leaf litter is related to initial litter Mn concentrations. Can J Bot 85:16–24. https://doi.org/10.1139/B06-150
DeForest JL, Zak DR, Pregitzer KS, Burton AJ (2004) Atmospheric nitrate deposition and the microbial degradation of cellobiose and vanillin in a northern hardwood forest. Soil Biol Biochem 36:965–971. https://doi.org/10.1016/j.soilbio.2004.02.011
Diedhiou S, Dossa EL, Badiane AN, Diedhiou I, Sene M, Dick RP (2009) Decomposition and spatial microbial heterogeneity associated with native shrubs in soils of agroecosystems in semi-arid Senegal. Pedobiologia 52:273–286. https://doi.org/10.1139/B06-150
Dijkstra FA, Pendall E, Morgan JA, Blumenthal DM, Carrillo Y, LeCain DR, Follett RF, Williams DG (2012) Climate change alters stoichiometry of phosphorus and nitrogen in a semiarid grassland. New Phytol 196:807–815. https://doi.org/10.1111/j.1469-8137.2012.04349.x
Don A, Böhme IH, Dohrmann AB, Poeplau C, Tebbe CC (2017) Microbial community composition affects soil organic carbon turnover in mineral soils. Biol Fertil Soils 53:445–456. https://doi.org/10.1007/s00374-017-1198-9
Eriksson KE, Blanchette RA, Ander P (1990) Microbial and enzymatic degradation of wood and wood components. Springer, Berlin
Frostegård Å, Bååth E (1996) Thgre use of phospholipid fatty acid analysis to estimate bacterial and fungal biomass in soil. Biol Fertil Soils 22:59–65. https://doi.org/10.1007/BF00384433
Frostegård Å, Tunlid A, Bååth E (1993) Phospholipid fatty acid composition, biomass and activity of microbial communities from two soil types experimentally exposed to different heavy metals. Appl Environ Microbiol 59:3605–3617
Garcia-Pausas J, Paterson E (2011) Microbial community abundance and structure are determinants of soil organic matter mineralization in the presence of labile carbon. Soil Biol Biochem 43:1705–1713. https://doi.org/10.1016/j.soilbio.2011.04.016
Ge X, Xiao W, Zeng L, Huang Z, Zhou B, Schaub M, Li MH (2016) Relationships between soil-litter interface enzyme activities and decomposition in Pinus massoniana Plantations in China. J Soils Sediments 4:996–1008. https://doi.org/10.1007/s11368-016-1591-2
Gholz HL, Wedin DA, Smitherman SM, Harmon ME, Parton WJ (2000) Long-term dynamics of pine and hardwood litter in contrasting environments: toward a global model of decomposition. Glob Change Boil 6:750–765. https://doi.org/10.1046/j.1365-2486.2000.00349.x
Handayanto E, Cadisch G, Giller KE (1997) Regulating N mineralization from plant residues by manipulation of quality. In: Cadisch G, Giller KE (eds) Driven by nature: plant litter quality and decomposition. CABI, Wallingford, pp 175–185
Heim A, Schmidt MWI (2006) Lignin turnover in arable soil and grassland analysed with two different labeling approaches. Eur J Soil Sci 58:599–608. https://doi.org/10.1111/j.1365-2389.2006.00848.x
Hobbie SE, Eddy WC, Buyarski CR, Adair EC, Ogdahl ML, Weisenhorn P (2012) Response of decomposing litter and its microbial community to multiple forms of nitrogen enrichment. Ecol Monogr 82:389–405. https://doi.org/10.1111/j.1365-2389.2006.00848.x
Iovieno P, Alfani A, Bååth E (2010) Soil microbial community structure and biomass as affected by Pinus pinea plantation in two Mediterranean areas. Appl Soil Ecol 45:56–63. https://doi.org/10.1016/j.apsoil.2010.02.001
Jiang X, Cao L, Zhang R (2014) Changes of labile and recalcitrant carbon pools under nitrogen addition in a city lawn soil. J Soils Sediments 14:515–524. https://doi.org/10.1007/s11368-013-0822-z
Keeney DR, Nelson DW (1982) Nitrogen-inorganic forms. In: Page AL (ed) Methods of soil analysis, part 2: chemical and microbiological properties. ASA and SSSA, Madison, WI, pp 643–698
Kim HY, Lim SS, Kwak JH, Lee DS, Lee SM, Ro HM, Choi WJ (2011) Dry matter and nitrogen accumulation and partitioning in rice (Oryza sativa L.) exposed to experimental warming with elevated CO2. Plant Soil 342:59–71. https://doi.org/10.1007/s11104-010-0665-y
King HGC, Heath GW (1967) The chemical analysis of small samples of leaf material and the relationship between the disappearance and composition of leaves. Pedobiologia 7:192–197
Klotzbücher T, Kaiser K, Guggenberger G, Gatzek C, Kalbitz K (2011) A new conceptual model for the fate of lignin in decomposing plant litter. Ecology 92:1052–1062. https://doi.org/10.1890/10-1307.1
Knorr M, Frey SD, Curtis PS (2005) Nitrogen additions and litter decomposition: a meta-analysis. Ecology 86:3252–3257. https://doi.org/10.1890/05-0150
Kramer C, Gleixner G (2008) Soil organic matter in soil depth profiles-distinct carbon preferences of microbial groups during carbon transformation. Soil Biol Biochem 42:92–100. https://doi.org/10.1016/j.soilbio.2007.09.016
Kuo S (1996) Phosphorus. In: Sparks DL, Page AL, Helmke PA, Loeppert RH, Soltanpour PN, Tabatabai MA, Johnston CT, Sumner ME (eds) Methods of soil analysis, part 3: chemical methods. ASA and SSSA, Madison, WI, pp 869–919
Kwak JH, Choi WJ, Lim SS, Arshad MA (2009) δ13C, δ15N, N concentration, and ca-to-Al ratios of forest samples from Pinus densiflora stands in rural and industrial areas. Chem Geol 264:385–−393. https://doi.org/10.1016/j.chemgeo.2009.04.002
Lee SI, Lim SS, Lee KS, Kwak JH, Jung JW, Ro HM, Choi WJ (2011) Kinetics responses of soil carbon dioxide emission to increasing urea application rate. Korean J Environ Agric 30:99–104. https://doi.org/10.5338/KJEA.2011.30.2.99
Lee YH, Yun HD (2011) Changes in microbial community of agricultural soils subjected to organic farming system in Korean paddy fields with no-till management. J Korean Soc Appl Biol Chem 54:434–441. https://doi.org/10.3839/jksabc.2011.067
Lim SS, Lee KS, Lee SI, Lee DS, Kwak JH, Hao X, Ro HM, Choi WJ (2012) Carbon mineralization and retention of livestock manure composts with different substrate qualities in three soils. J Soils Sediments 12:312–322. https://doi.org/10.1007/s11368-011-0458-9
Lorenz K, Preston CM, Krumrei S, Feger KH (2004) Decomposition of needle/leaf litter from scots pine, black cherry, common oak and European beech at a conurbation forest site. Eur. J For Res 123:177–188. https://doi.org/10.1007/s10342-004-0025-7
Manzoni S, Trofymow JA, Jackson RB, Porporato A (2010) Stoichiometric controls on carbon, nitrogen, and phosphorus dynamics in decomposing litter. Ecol Monogr 80:89–106. https://doi.org/10.1890/09-0179.1
Meidute S, Demoling F, Bååth E (2008) Antagonistic and synergistic effects of fungal and bacterial growth in soil after adding different carbon and nitrogen sources. Soil Biol Biochem 40:2334–2344. https://doi.org/10.1016/j.soilbio.2008.05.011
Neely CL, Beare MH, Hargrove WL, Coleman DC (1991) Relationships between fungal and bacterial substrate-induced respiration, biomass and plant residue decomposition. Soil Biol Biochem 23:947–954. https://doi.org/10.1016/0038-0717(91)90175-J
Nelson DW, Sommers LE (1996) Total carbon, organic carbon, and organic matter. In: Sparks DL, Page AL, Helmke PA, Loeppert RH, Soltanpour PN, Tabatabai MA, Johnston CT, Sumner ME (eds) Methods of soil analysis, part 3: chemical methods. ASA and SSSA, Madison, Wisconsin, WI, pp 961–1010
Norby RJ, Cotrufo MF, Ineson P, O’Neill EG, Canadell JG (2001) Elevated CO2, litter chemistry, and decomposition: a synthesis. Oecologia 127:153–165. https://doi.org/10.1007/s004420000615
Ohnish M, Horie T (1999) A proxy analysis of nonstructural carbohydrate in rice plant by using the gravimetric method. Jpn J Crop Sci 68:126–136. https://doi.org/10.1626/jcs.68.126
Osono T, Takeda H (2004) Accumulation and release of nitrogen and phosphorus in relation to lignin decomposition in leaf litter of 14 tree species. Ecol Res 19:593–602. https://doi.org/10.1111/j.1440-1703.2004.00675.x
Park HJ (2016) Climatic and soil environmental changes effects on litter quality and decomposition of coniferous and deciduous trees. MSc thesis, Chonnam National University, Gwangju, South Korea
Perez J, Jeffries TW (1992) Roles of manganese and organic acid chelators in regulating lignin degradation and biosynthesis of peroxidases by Phanerochaete chrysosporium. Appl Environ Microbiol 58:2402–2409
Pérez-Harguindeguy N, Díaz S, Cornelissen JHC, Vendramini F, Cabido M, Castellanos A (2000) Chemistry and toughness predict leaf litter decomposition rates over a wide spectrum of functional types and taxa in central Argentina. Plant Soil 218:21–30. https://doi.org/10.1023/A:1014981715532
Prescott C (2010) Litter decomposition: what controls it and how can we alter it to sequester more carbon in forest soils? Biogeochemistry 101:133–149. https://doi.org/10.1007/s10533-010-9439-0
Rahman MM, Tsukamoto J, Rahman MM, Yoneyama A, Mostafa KM (2013) Lignin and its effects on litter decomposition in forest ecosystems. Chem Ecol 29:540–553. https://doi.org/10.1080/02757540.2013.790380
Rasse DP, Rumpel C, Dignac MF (2005) Is soil carbon mostly root carbon? Mechanisms for a specific stabilization. Plant Soil 269:341–3563. https://doi.org/10.1007/s11104-004-0907-y
Rinkes ZL, DeForest JL, Grandy AS, Moorhead DL, Weintraub MN (2014) Interactions between leaf litter quality, particle size, and microbial community during the earliest stage of decay. Biogeochemistry 117:153–168. https://doi.org/10.1007/s10533-013-9872-y
Schutter ME, Dick RP (2000) Comparison of fatty acid methyl ester (FAME) methods for characterizing microbial communities. Soil Sci Soc Am J 64:1659–1668. https://doi.org/10.2136/sssaj2000.6451659x
Schöler A, Jacquiod S, Vestergaard G, Schulz S, Schloter M (2017) Analysis of soil microbial communities based on amplicon sequencing of marker genes. Biol Fertil Soils 53:485–489. https://doi.org/10.1007/s00374-017-1205-1
Skorupski M, Jagodziński AM, Żytkowiak R, Karolewski P (2012) Differences in chemical composition of needle and leaf litter from exotic and native tree species stands. Dendrobiology 68:101–112
Tavi NM, Martikainen PJ, Lokko K, Kontro M, Wild B, Richter A, Bisai C (2013) Linking microbial community structure and allocation of plant-derived carbon in an organic agricultural soil using 13CO2 pulse-chase labeling combined with 13C-PLFA profiling. Soil Biol Biochem 58:207–215. https://doi.org/10.1016/j.soilbio.2012.11.013
Taylor BR, Parkinson D, Parsons WFJ (1989) Nitrogen and lignin content as predictors of litter decay rates: a microcosm test. Ecology 70:97–104. https://doi.org/10.2307/1938416
Treseder KK (2008) Nitrogen additions and microbial biomass: a meta-analysis of ecosystem studies. Ecol Lett 11:1111–1120. https://doi.org/10.1111/j.1461-0248.2008.01230.x
Tu LH, Hu HL, Chen G, Peng Y, Xiao YL, Hu TX, Zhang J, Li XW, Liu L, Tang Y (2014) Nitrogen addition significantly affects forest litter decomposition under high levels of ambient nitrogen deposition. PLoS One 9:e88752. https://doi.org/10.1371/journal.pone.0088752
Ushio M, Wagai R, Balser T, Kitayama K (2008) Variations in the soil microbial community composition of a tropical montane forest ecosystem: dose tree species matter? Soil Biol Biochem 40:2699–2702. https://doi.org/10.1016/j.soilbio.2008.06.023
Vestergaard G, Schulz S, Schöler A, Schloter M (2017) Making big data smart − how to use metagenomics to understand soil quality. Biol Fertil Soils 56:479–484. https://doi.org/10.1007/ss00374-017-1191-3
Wang C, Yang J (2007) Rhizospheric and heterotrophic components of soil respiration in six Chinese temperate forests. Glob Change Boil 13:123–131. https://doi.org/10.1111/j.1365-2486.2006.01291.x
Wang H, Liu SR, Wang JX, Shin ZM, Xu J, Hong PZ, Ming AG, Yu HL, Chen L, Lu LH, Cai DX (2016) Differential effects of conifer and broadleaf litter inputs on soil organic carbon chemical composition through altered soil microbial community composition. Sci Rep 6:27097. https://doi.org/10.1038/srep27097
Wang Q, Kwak JH, Choi WJ, Chang SX (2018) Decomposition of trembling aspen leaf litter under long-term nitrogen and sulfur deposition: effects of litter chemistry and forest floor microbial properties. For Ecol Manag 412:53–61. https://doi.org/10.1016/j.foreco.2018.01.042
Wang Q, Wang S, He T, Liu L, Wu J (2014) Response of organic carbon mineralization and microbial community to leaf litter and nutrient additions in subtropical forest soils. Soil Biol Biochem 71:13–20. https://doi.org/10.1016/j.soilbio.2014.01.004
Weand M, Arthur MA, Lovett GM, McCulley RL, Weathers K (2010) Effect of tree species and N additions on forest floor microbial communities and extracellular enzyme activities. Soil Biol Biochem 42:2161–2173. https://doi.org/10.1016/j.soilbio.2010.08.012
Wu Y, Liu G, Fu B, Liu Z, Hu H (2006) Comparing soil CO2 emission in pine plantation and oak shrub: dynamics and correlations. Ecol Res 21:840–848. https://doi.org/10.1007/s11284-006-0040-x
Zelles L (1997) Phospholipid fatty acid profiles in selected members of soil microbial communities. Chemosphere 35:275–294. https://doi.org/10.1016/S0045-6535(97)00155-0
Zhang DQ, Hui DF, Luo YQ, Zhou GY (2008) Rates of litter decomposition in terrestrial ecosystems: global patterns and controlling factors. J Plant Ecol 1:85–93. https://doi.org/10.1093/jpe/rtn002
Zhong Y, Yan W, Wang R, Shangguan Z (2017) Differential responses of litter decomposition to nutrient addition and soil water availability with long-term vegetation recovery. Biol Fertil Soils 53:939–949. https://doi.org/10.1007/s00374-017-1242-9
Acknowledgements
This work was supported by the National Research Foundation of Korea funded by the Ministry of Education (NRF-2015R1D1A3A01018961) and by scholarship awarded to HJP by the Korean Government (NRF-2017H1A2A1044523) and Chonnam National University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Park, HJ., Lim, SS., Kwak, JH. et al. Elevated CO2 concentration affected pine and oak litter chemistry and the respiration and microbial biomass of soils amended with these litters. Biol Fertil Soils 54, 583–594 (2018). https://doi.org/10.1007/s00374-018-1282-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-018-1282-9