Abstract
Formulating biochar-based nitrogen fertilisers from charred livestock manure and urea, the two largest emitters of ammonia (NH3) may help to abate particulate matter emitted from agricultural operations. However, animal manure biochar inadequately retains carbon, thus impairing its primary role of carbon sequestration. Co-pyrolysis of animal manure with phosphorus (P) may improve quality of the biochar, but with the phosphate rock reserves expected to vanish soon, a shift to renewable P sources is desirable. Bone waste is laden with P and can be a viable replacement of the phosphate rock. In the current study, we assessed the efficiency of bone waste as a P source in the co-pyrolysis of cow dung and quantified the NH3 emitting potentials of the biochar-based urea and UHP fertilisers formulated with the co-pyrolysed biochar. Co-pyrolysis of cow dung with bone waste increased yield and carbon retentions of biochar and boosted biochar’s capacity to attenuate NH3 emissions. UHP fertilisers formulated from the co-pyrolysed biochar lessened NH3 evolutions by as high as 85.93% and were more effective in reducing NH3 volatilisations than co-pyrolysed biochar-based urea fertilisers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Animal excrement and nitrogen (N) fertilisers are by far the largest emitters of NH3 which through intricate atmospheric reactions forms constituent chemical species of particulate matter (PM) [1, 2] and is thus a vital air pollutant [3, 4]. The general importance of NH3 emissions to air quality has been underscored by several studies including but not limited to Tsimpidi et al. [5], Pinder et al. [6], Wang et al. [7], Megaritis et al. [8] and Bessagnet et al. [9] with each of them calling for effective curtailment of NH3 emissions for the betterment of human health and environment. An overwhelming interest in using biochar to curb NH3 volatilisations has ensued in recent past, although different studies have come up with contrasting observations.
Mandal et al. [10] found that combined applications of biochar and N fertilisers effectively attenuated NH3 volatilisations by as high as 77.2% in comparison with N fertiliser applications without any biochar amendments. The observation was later backed by Sun et al. [11] who reported diminished emissions of NH3 from N fertilisers added to biochar amended soils even though the rates of reduction recorded were way smaller than those reported by the former i.e. the slowdown in NH3 volatilisations prompted by biochar amendments ranged between a paltry 4.2% and 11.2%. On the contrary, however, studies by Subedi et al. [12], Fan et al. [13], Feng et al. [14] and He et al. [15] registered elevated levels of NH3 discharge from N fertilisers applied in biochar amended soils.
To ensure optimal slowdown of N release and possibly NH3 emissions from N fertilisers, studies by Chen et al. [16], Punga et al. [17] and Liu et al. [8] for example formulated slow release fertilisers. The former pelletised biochar and urea hydrogen peroxide (UHP) using kaolin as a binding material while both Punga et al. [17] and Liu et al. [8] prepended bentonite in the biochar-N fertiliser mixtures. The inclusion of clay in the biochar-based N fertilisers might have been intended to boost the adsorption of N by biochar-based fertilisers due to the high surface areas of clay. Developing a slow release fertiliser from urea and charred livestock manure (the largest emitters of NH3) may offer a sustainable solution to the pressing issue of PM emissions ensuing from agriculture. However, the very low carbon retention capabilities of high mineral animal manure derived biochar weakens its key role of sequestering carbon.
Studies by Zhao et al. [19] and Zhao et al. [20] espoused that co-pyrolysis of biomass with P improves carbon retentions in biochar which is particularly important for animal manure biochars with exceedingly low carbon retention capabilities. Amidst prognosticated scarcity of the finite phosphate rock (PR) reserves [21, 22], it is appropriate to surmise that renewable P sources such as BM will have a central role in the sustainability of agricultural production systems in the future. Besides supplying P needed for the improvement of biochar quality especially carbon retention, bone char is an efficient adsorbent and thus, co-pyrolysing it with animal manure may enhance the resultant biochar’s ability to adsorb added N which may concomitantly reduce NH3 discharges to the environment. Objectives of this study were to assess the ammonia emitting potentials of biochar-based urea and UHP fertilisers (CCDBFs) formulated out of co-pyrolysed BM and animal manure using cow dung (CD) as a representative for animal manure and to delineate some of the mechanisms which may underlie the gaseous volatilisations.
Materials and methods
Biochar and CCDBFs (CCDB-Urea and CCDB-UHP) production
Dried cow dung and rendered bone waste were each ground and sieved through a 1.8 mm sieve. The sieved materials were blended in varying proportions to obtain final mixtures containing 0%, 5%, 10%, 25% and 50% of BM by weight. The mixtures were rewetted to ensure cohesion amongst the admixed components, dried again and then pyrolysed at 500 °C. Pyrolysis followed packing feedstock into a ceramic container covered with a lid and combusting in a muffle furnace (Lindberg/Blue M) for 2 h. Additionally, bone char (BC) was produced from unmixed bone meal pyrolysed in the same way as adumbrated earlier but at two different temperatures of 500 °C (BC500) and 750 °C (BC750). Another treatment with triple superphosphate (TSP) as a P source added at a rate of 25% of the mixture was included in the experiment to aid in the comparison of the efficacy of BM with conventional P sources. 3.5 mm extruded activated carbon (AC) was purchased from Duksan chemicals®, South Korea. Formulation of co-pyrolysed bone meal and cow dung-based UHP fertilisers (CCDB-UHP) followed soaking biochar in a UHP solution for 24 h at room temperature adopting mixing ratios of 5:1, respectively, while the same mixing ratios were adopted for making co-pyrolysed bone meal and cow dung-based urea fertilisers (CCDB-Urea). The mixtures were oven dried at 105 °C for 24 h, cooled and packed for further analysis and usage. 0%, 5%, 10%, 25% and 50% BM containing biochar-based urea fertilisers were denoted as CDB-Urea, CD + BM 5-Urea, CD + BM 10-Urea, CD + BM 25-Urea and CD + BM 50-Urea, respectively, while CDB-UHP, CD + BM 5-UHP, CD + BM 10-UHP, CD + BM 25-UHP and CD + BM 50-UHP symbolised their corresponding CCDB-UHP fertilisers.
Experimental set up
NH3 Emissions were measured through two incubation experiments. Firstly, the effects of CD biochar (CDB), AC and BC (BC 500 and BC 750) on NH3 volatilisations from urea and UHP were investigated by using both paddy and upland soils. That incubation experiment was executed to identify the best experimental conditions for the second and main incubation experiment. Paddy soils were kept under saturated conditions while upland soils were maintained at 60% of the water holding capacity following recommendations by Punga et al. [17] and biochar was added at a rate of 3% of the soil. N in forms of Urea and UHP was applied to the soil at rates of 500 mg per kg of soil paying strict adherence to the mixing procedure adopted by Mandal et al. [10]. Paddy and upland soils were respectively picked from paddy and upland fields, air dried, ground and strained through a 2.0 mm sieve. Upland soil which emitted the highest volumes of NH3 in the first incubation experiment was selected for usage in the main incubation experiment where NH3 volatilisation potentials of the different co-pyrolysed BM and CD biochars (CCDB), CCDB-Urea and CCDB-UHP were measured. Both CCDB-Urea and CCDB-UHP fertilisers were added to the soil at rates that maintained the N application rate of 500 mg/kg.
The relationships between each of the surface area (SA), micro-pore volume (MpV) and total pore volume (Vt) of CDB, AC, BC 500, BC 750 and CCDB on NH3 emissions were also investigated. This followed recommendations by Mandal et al. [10] that the nature and properties of biochar’s pores may have direct impacts on NH3 volatilisations from the soil. Alongside NH3 volatilisation measurements, mineralisation rates of the CCDBFs were determined and the relationship between them and cumulative NH3 volatilisations explicated. This is because the rate at which N fertilisers mineralise may have direct implications on gaseous NH3 discharges from the soil. Both incubation experiments proceeded at room temperature and each lasted for 28 days.
Measurement of NH3 volatilisations and N mineralisation rates
Measurement of NH3 volatilisations followed a procedure espoused by Mandal et al. [10] where NH3 was captured by 0.2 M sulphuric acid and the unreacted acid titrated with 0.4 M sodium hydroxide. In brief, 100 g of the soil containing different amendments was placed in a 500 ml jar (respiration jar) and scintillation vials (20 ml) containing 10 ml of 0.2 M sulphuric acid were hung inside the jar to capture NH3 emitted from the soil. Schematic diagram of the experimental set up is shown in Figure S1 in the supplementary file. N mineralisation rates of the CCDBFs were derived from the equation adopted by Thangarajan et al. [23] and given below:
where Nmr is the net N mineralisation rate (mg/kg of soil per day), Nft and Nit are the total mineral N concentration of the amended soil on the final and initial incubation days, respectively, Nfc and Nic are the total mineral N concentration of the control on the final and initial incubation days, respectively, while fd and id are the respective final and initial incubation days. Mineral N is the sum of ammonium nitrogen (NH4+-N) and nitrate nitrogen (NO3−-N) concentrations. NH4+-N and NO3−-N concentrations were measured colourimetrically following the analytical methods adopted by Kalra and Crumbaugh et al. [24] after extraction with 2 M KCl.
Biochar and soil analysis
The pH was determined by adding 25 ml of 0.01 M CaCl2 solution to 5 g of either air dried biochar or soil samples followed by overhead rotation of the mixture for 1 h after which pH of the suspension was measured directly using a pH metre. The EC measurement followed addition of 200 ml of desalinated water to 20 g of either soil or biochar and shaking for 1 h. The solution was filtered and EC was measured in the filtrate using pH and EC metre. Surface area (SA) of biochar was computed as an equivalence of iodine numbers following proposals by Mianowski et al. [25] and Phuong [26]. The iodine numbers of different biochar samples were determined by paying strict adherence to the ASTM D4607-94 method. Micro-pore volume (MpV) and total volumes (Vt) of biochar were determined from the modelled equations propounded by Nunes and Guerreiro, [27] using both methylene blue and iodine numbers. Biochar carbon (C) and nitrogen (N) were assessed with C/N analyser while mass yields were obtained through direct weighing of the biomass feed stocks and obtained biochar. Soil organic carbon (OC) was determined following the Walkley and Black method with organic matter (OM) computed by multiplying 1.72 with the OC value obtained. Available phosphorus was extracted with Olsen solution and determined colourimetrically for orthophosphate by paying strict adherence to the ascorbic acid method using a UV–Vis spectrophotometer (Evolution 300; Thermo Scientific, Inc.). Soil exchangeable cations including K+, Ca2+, Mg2+ and Na+ were measured with ICP–OES (GBC Scientific, Australia) after leaching the samples with 1 N ammonium acetate solution at a neutral pH (7.0). Carbon retentions were calculated from the equation set forth by Zhao et al. [20] and is given below:
where Cpm and Wpm are carbon content and weight of the pyrolysed materials, respectively, Cfb is carbon content of the feed stock biomass, Wfb is the weight of the feed stock biomass, Cam is the carbon content of the added material while Wam is the weight of the added material. The chemical properties of the soil are presented in Table 1 while those of the biochars are shown in Table 2. Co-pyrolysed biochars were also characterised with scanning electron microscope (results shown in Figure S2) in the supplementary file.
Statistical analysis
Data analysis was conducted through Microsoft Excel version 2016 and included (1) a correlation analysis to discern the extent of the relationships between NH3 volatilisations and SA, MpV and Vt of biochar. (2) A linear regression analysis to establish the relationship between NH3 volatilisations from the soil and rate of N mineralisation. (3) Analysis of variance (ANOVA) on the results for the effect of CCDBFs on NH3 evolutions and N mineralisation at 5% level of significance followed by post hoc t-tests with studentised q tables to quantify the significant differences between the different treatments.
Results and discussion
Effects of co-pyrolysis on biochar properties
As indicated in Table 2, co-pyrolysed biochars had slightly lower pH values than the pure CDB biochars, i.e. pH of CCDB ranged between 10.19 and 9.71 while that of CD + TSP was 6.33 against a pH value of 10.57 registered by CDB. This observation accorded with that of Zhao et al. [19] who noted that co-pyrolysing switch grass and saw dust with BM and TSP decreased pH to 8.45−8.67 and 4.96−5.12, respectively, from 8.56−9.56 in pure biochars. They ascribed the acidic pH of biochar produced from co-pyrolysis of biomass and TSP to the strong acidity of TSP. The slightly lower pH of CCDB biochars than pristine CDB might have been due to the marginally low intrinsic pH of BC, although more investigations may be needed to properly expound the reasons underlying this observation. Additionally, co-pyrolysis of CD with BM augmented resultant biochar’s surface area from 77.57 mg/g of CDB to a maximum value of 104.11 mg/g in the CD + BM 50 biochar, with SA values rising along increasing BM contents in the biomass feed stocks. This effect is likely to have stemmed from the high SA of BC itself.
In agreement with a previous study by Zhao et al. [19], co-pyrolysis increased yield of the charred materials and the increments magnified with increasing BM content in the biomass feed stocks pyrolysed. This outcome might have ensued from the recalcitrance of the additives to disintegration upon heating as evidenced from the high yields obtained when BM was pyrolysed. Carbon content reduced to 40.40–26.81% in co-pyrolysed biochars from 41.30% in CDB, an observation that concurred with the results obtained by Zhao et al. [19], Zhao et al. [20] and Lustosa-Filho et al. [28]. The low C content of the additives diluted the carbon concentration of the biomass feed stock, an effect that was carried over to biochars. On the contrary, however, C retentions increased with increasing concentrations of BM in the samples from 47.92% in CDB to a maximum concentration of 63.81% in CD + BM 50 while CD + TSP biochar retained 79.26% of its carbon. The high C retentions may be due to the passivation of carbon brought about by P additives added to biomass which form a durable coating on the surface of biochar preventing C decomposition as delineated by Jiang et al. [29] and Zhao et al. [20] and shown in SEM images (Figure S2) in the supplementary file. The higher efficiency of TSP than BM in retaining biochar C stems from the differences in their P species and availability [20]. For instance, Zhao et al. [20] demonstrated that readily available P supplied by TSP bonded to C and oxygen atoms of the biomass forming hard to break C–O–PO3 chemical groups which could not be formed with BM.
NH3 emissions from upland and paddy soils in the first incubation experiment
Although the first incubation experiment was a preliminary one intended to aid in the selection of the right experimental conditions for the main incubation experiment, it helped to quantify NH3 volatilisations from a range of pyrolysed materials including BC, CDB and AC. From Fig. 2, the cumulative NH3 volatilisations were higher in the upland soil than in the paddy one. This could have been due to the higher pH of the upland soil since Mandal et al. [10] demonstrated that gaseous NH3 loss from the soil increased along increasing soil pH. Another reason for this difference might be the differences in the OM contents of the soils, because Verdi et al. [46] found that applying urea to OM-rich soil led to more NH3 volatilisations than when urea was applied to low OM containing soil. At cumulative volatilisations of 141.6 mg/kg and 94.6 mg/kg from upland and paddy soils, respectively (Fig. 1a, b), urea-only amendment constituted the greatest loss of applied N of all the treatments. N lost as NH3 from upland soil accounted for 28.32% of the total quantity of N applied while the loss from paddy soil amounted to 18.92%. The obtained values tallied well with a majority of former studies for instance by Subedi et al. [12], Mandal et al. [10], Feng et al. [14], Sun et al. [11] and others which reported gaseous NH3 emissions amounting to between 10 and 40% of the applied urea N.
Applying UHP instead of urea faintly subsided NH3 volatilisations by 15.20% and 18.02% in upland and paddy soils, respectively. Hydrogen peroxide (H2O2) protonates in water according to Eq. 1, increasing the concentration of hydrogen ions in soil solution which neutralise \({\rm O}{\rm H}^{-}\) produced when carbamide (\(\complement {\rm O}({\rm N}{\rm H}_{2}{)}_{2})\) hydrolyses as was explained by Bolan et al. [30] and Mandal et al. [10] and shown in Eq. 2;
In the absence of \({\rm O}{\rm H}^{-}\) to reduce \({\rm N}{\rm H}_{4 }^{+}\) to NH3 as indicated by Bolan et al. [30], gaseous NH3 emissions subsidise while ammonification and possibly nitrification proliferate. Another fate of H2O2 applied along with urea in the biochar-UHP complex in the soil may be dissociation in soil water producing free oxygen radicals and water as shown in the following equation:
Oxygen released enhances nitrification by oxidising \({\rm N}{\rm H}_{4 }^{+}\) to \({{\rm N}{\rm O}}_{2}^{-}\) and then \({{\rm N}{\rm O}}_{3}^{-}\) as demonstrated by McElroy [31] and elaborated in Eqs. 4 and 5. These chemical reactions prevent breakdown of \({\rm N}{\rm H}_{4 }^{+}\) to NH3 by \({\rm O}{\rm H}^{-}\) which may offer another explanation for the observed reductions in the gaseous emissions where UHP instead of urea was applied.
From Fig. 1a, and b, CDB, BC 500 and BC750 reduced NH3 emissions by 46.7%, 55.8% and 50.6%, respectively, when added to upland soil while the reductions in the paddy soil were smaller than in the upland soil and stood at 30.3%, 42.0% and 32.5%, respectively. These observations were in agreement with those made by Mandal et al. [10] and Sun et al. [11] who reported lessened emissions of NH3 upon biochar additions to the soil. The rates of reductions in the current study were however, lower than those reported by Mandal et al. [10] possibly due to low biochar application rates used but higher than the ones recorded by Sun et al. [11]. Another clear observation was that BC was more efficient in combating volatilisations of NH3 than biochar (CDB) possibly due to differences in surface area and pore properties as was propounded by Mandal et al. [10]. Indeed, strong negative correlation coefficients were found between NH3 volatilisations and MpV (− 0.83243), SA (− 0.83177) and Vt (− 0.77119) of BC, CDB, AC and CCDB. These results of correlation analysis imply that biochar’s micro-porosity and surface area take precedence over total volume in influencing the quantity of NH3 discharge from the soil. The results also augur well with observations made by Subedi et al. [12] who found biochar with a large SA more effective than hydrochar (with a small SA) in abating NH3 volatilisations. Later on, Feng et al. [14] showed that paddy soils amended with wheat straw biochar produced at 700 °C emitted lower quantities of NH3 than soil that received biochar produced at 500 °C, because the latter had lower SA than the former. From Figure S2 (in the supplementary file), SEM images reveal that increasing concentration of BM in the biomass mixture led to creation of porous biochar which may explain the high efficiency of the co-pyrolysed biochars in attenuating NH3 emissions. Micro-pore volume is particularly important, because Sarkar and Naidu [32] disclosed that a material with a large pore diameter is likely to retain large quantities of water/moisture with accompanying reduction in the amount of \({\rm N}{\rm H}_{4 }^{+}\) adsorbed. Of all the amendments, AC invoked the highest rates of NH3 emission reductions standing at 62.40% and 46.72% in upland and paddy soils, respectively. This result was in harmony with that of Wang et al. [33] who found AC more efficient than biochar in adsorbing \({\rm N}{\rm H}_{4 }^{+}\) from a biogas slurry and attributed its stellar performance to AC’s superior SA. This could offer a good explanation to the observations made in this study since AC’s SA was at least more than nine-fold that of BC and or CDB.
Besides direct adsorption of \({\rm N}{\rm H}_{4 }^{+}\), there are several mechanisms underlying reduced NH3 emissions observed with biochar amendments as were demonstrated by Mandal et al. [10]. For example NH3 produced may act as a Brownsted and or Lewis acid accepting a \({\rm H}^{+}\) from the carboxyl groups on the surface of biochar-producing ammonium salts, hence abating gaseous emissions. Additionally, alkaline NH3 gas can be protonated to \({\rm N}{\rm H}_{4 }^{+}\) by acidic groups on the surface of biochar and then adsorbed.
Effects of CCDB, CCDB-Urea and CCDB-UHP on NH3 volatilisations from upland soil
With reference to Fig. 2a, b and c, applying CCDB biochars instead of BC and CDB decreased NH3 discharges from urea applied to upland soils. Gaseous NH3 volatilisations in CD + BM 5, CD + TSP, CD + BM 10, CD + BM 25 and CD + BM 50 amended soils reduced by 48.02%, 49.10%, 51.07%, 52.29% and 53.53%, respectively. Similar to the observations made earlier with BC, AC and CDB, these rates of gaseous reduction were commensurate with the SA, MpV and Vt of the CCDB biochars. This observation is a demonstration that co-pyrolysis of CD (animal manure) with large quantities of BM produces biochar with increased capacities to adsorb \({\rm N}{\rm H}_{4 }^{+}\) thus reducing NH3 discharges from soil applied N fertilisers. Relatedly, Zhao et al. [20] demonstrated that biochar produced by co-pyrolysing BM and biomass was more efficient than one produced from a mixture of TSP and biomass in stabilising heavy metals in the soil. Although they attributed their observation to P in hydroxyapatite (Ca10(PO4)6(OH)2) that did not react with C during pyrolysis but ended up solubilising to PO43− and precipitating heavy metals, elevated SA following the co-pyrolysis may have contributed to that efficiency.
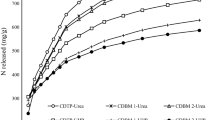
It was also observed that CCDB-Urea and CCDB-UHP were more efficient in reducing gaseous NH3 emissions than separate applications of fertilisers and CCDB biochars. In comparison to the urea-only amendment, CDB-Urea, CD + TSP-Urea, CD + BM 5-Urea, CD + BM 10-Urea, CD + BM 25-Urea and CD + BM 50-Urea reduced NH3 volatilisations by 58.47%, 65.50%, 69.15%, 69.77%, 70.84%, and 74.80%, respectively. On the other hand, CDB-UHP, CD + TSP-UHP, CD + BM 5-UHP, CD + BM 10-UHP, CD + BM 25-UHP and CD + BM 50-UHP lessened NH3 emissions by 71.77%, 73.04%, 76.38%, 79.69%, 83.45%, and 85.93%, respectively. Until now, there has been only a handful of studies into N release potentials of biochar-based N fertilisers, because research into this field is still in a nascent stage. However, the smatterings of data available show that biochar-based N fertilisers are efficient at slowing down N release into the environment.
For example, one of the early studies by Chen et al. [16] discovered that pelletised biochar-based UHP fertilisers reduced leaching of N by up to 74.32% in comparison to UHP only amendment. Later on, Punga et al. [17] found that N containing biochar prills with high biochar and low N contents volatilised lesser gaseous NH3 than urea. Dong et al. [34] in a bid to develop an efficient slow release biochar-based fertiliser, used different blending ratios of biochar, bentonite, humic acid and a range of adhesive materials and found that the slow release ability of biochar was optimised with 25% biochar, 4% bentonite, and 10% humic acid using modified corn starch adhesive material. Additionally, Liu et al. [18] found that prepending urea to hydrothermally decomposed biomass was more effective than blending biochar and urea in lessening N release into the soil. Shi et al. [35] discovered that urea bound to a blend of biochar and clay minerals reduced N leaching by 70% in comparison to conventional urea and boosted plant growth. Although none of the above-cited studies directly measured gaseous NH3 emitting potentials of the biochar-based N fertilisers, their inherent slow release abilities may concomitantly slowdown NH3 volatilisations which may explain the low emitting capacities of the CCDB-Urea and CCDB-UHP fertilisers observed in the current study. That line of argument is supported by the strong coefficients of linear regression (Fig. 2d) obtained between the cumulative gaseous NH3 emissions and rates of mineralisation of CCDB-Urea (r2 = 0.8137) and CCDB-UHP (r2 = 0.9036). This is because rates of N mineralisation are quantified from inorganic forms of N available in the soil which are potent sources of NH3 and thus, a reduction in the rate of their release the soil portends reductions in the quantities of NH3 emitted to the atmosphere. Quantities of volatilised N decreased along increasing BM content in the CCDB, CCDB-Urea and CCDB-UHP which indicates that biochar’s SA plays a crucial role in adsorbing \({\rm N}{\rm H}_{4 }^{+}\) as indicated by Subedi et al. [12] and Feng et al. [14].
Mineralisation of different CCDB-Urea and CCDB-UHP fertilisers
CCDB-Urea and CCDB-UHP amended soils registered lower concentrations of NH4+-N and \({{\rm N}{\rm O}}_{3}^{-}\)-N than both urea and UHP amended soils throughout the incubation period as shown in Fig. 3. The observation accorded with previous studies by Mandal et al. [10] and Dong et al. [34] which reported scaled down mineralisations of N applied to soil with biochar amendments or in form of biochar-based fertilisers. In the current study, levels of mineralised N (NH4+-N and \({{\rm N}{\rm O}}_{3}^{-}\)-N) were lower in UHP and CCDB-UHP amended soils than in urea and CCDB-Urea, respectively, which might have stemmed from the lower rates of N release by UHP in comparison to urea as was demonstrated earlier. Another observation was that increasing concentrations of \({{\rm N}{\rm O}}_{3}^{-}\)-N followed decrements in the concentrations of NH4+-N in all the treatments possibly because of the direct formation of \({{\rm N}{\rm O}}_{3}^{-}\)-N from NH4+-N which was in agreement with the deductions made by Mandal et al. [10].
There are several reasons that may account for the reduced concentration levels of inorganic N observed in the CCDB-Urea and CCDB-UHP fertilisers. Adsorption of \({{\rm N}{\rm O}}_{3}^{-}\) and \({\rm N}{\rm H}_{4 }^{+}\) may be the leading mechanism accounting for the decrease because several studies have confirmed biochar’s high affinity for the inorganic N forms. For example, a study by Taghizadeh-Toosi et al. [36] found biochar very efficient in adsorbing NH4+-N, although the adsorbed N was highly bioavailable. Another study by Sarkhot et al. [37] discovered that biochar was an efficient adsorbent for NH4+-N from dairy manure. Tian et al. [38] found both poultry litter and hardwood biochars effective adsorbents of NH4+ from storm water. Similarly, Kammann et al. [39] reported improved plant growth using co-composted biochar and suggested that the improvement was due to \({{\rm N}{\rm O}}_{3}^{-}\)-N capture by biochar during composting. The captured \({{\rm N}{\rm O}}_{3}^{-}\)-N is strongly held up by biochar and not sufficiently extractable with the standard methods as indicated by Haider et al. [40] who had to repeat the extractions several times to be able to effectively remove the captured \({{\rm N}{\rm O}}_{3}^{-}\). Haider et al. [41] also found that biochar applied to a sandy soil greatly retained \({{\rm N}{\rm O}}_{3}^{-}\)-N in the top soil preventing it from leaching to lower soil layers.
Another weighty reason for the reduced concentrations of inorganic N pools observed in the current study may be N immobilisation. N immobilisation tends to increase with increasing C: N ratios and thus, adding biochar C is likely to proliferate immobilisation of N by soil micro-organisms [42, 43]. That is because fresh biochars may contain appreciable quantities of labile C that can be readily consumed by soil microbes leading to temporary immobilisation of N in the soil. Indeed, Bruun et al. [44] demonstrated that biochar which contained a large fraction of labile C immobilised more N than the one with low concentration of labile C. N immobilisation following biochar addition to soil has been shown to slowdown leaching of \({{\rm N}{\rm O}}_{3}^{-}\) by Ippolito et al. [45]. The decreased release of inorganic N was the precursor for the reduced net N mineralisation of CCDB-Urea and CCDB-UHP fertilisers added to soil. For example, net N mineralisation rates slowed down from 8.233 mg kg−1 day−1 in urea amended soil to the lowest value of 3.74 mg kg−1 day−1 in CD + BM 50-Urea amendment. A similar trend was observed in the CCDB-UHP amendments where net mineralisation rates reduced from 6.12 mg kg−1 day−1 in UHP amended soils to the lowest value of 2.86 mg kg−1 day−1 recorded in the CD + BM 50-UHP amendment as shown in Fig. 4 below.
The observed decreases in net N mineralisation rates concurred with previous studies by Dempster et al. [42] and Castaldi et al. [43] who reported reduced net mineralisation rates of N in biochar amended soils. N mineralisation rates are derived from the concentrations of NH4+-N and \({{\rm N}{\rm O}}_{3}^{-}\)-N in the soil and hence slowed down mineralisation rates of the biochar fertilisers ensued from reduced release of the inorganic N forms. Therefore, the same reasons given for reduced releases of mineral N hold for the mineralisation rates. Strong linear relationships obtained between cumulative NH3 volatilisations and mineralisation rates (Fig. 2d) indicate that reduced N mineralisation is a crucial pathway through which biochar-based fertilisers and or biochar attenuate NH3 volatilisations to the atmosphere.
Conclusion
The results demonstrate that BM (bone waste) is capable of replacing PR as a source of P in the co-pyrolysis of biomass for improved carbon retention and biochar yield although a higher dosage of BM may be required to produce a similar effect achievable with lower dosages of conventional P sources. That was evidenced when biomass mixed with 25% TSP produced a 14.2 percentage increase in biochar yield while the same mixing ratio with BM only elicited an 8.4 percentage rise in biochar yield. The results also confirm dependence of gaseous NH3 emissions on biochar’s surface area, micro-porosity and even total volume indicating the importance of adsorption in the abatement of NH3 volatilisations. The decreasing NH3 volatilisations along increasing bone meal concentration in CCDB is because bone char generally has a higher adsorption capacity than animal manure biochar. Therefore, BM included in the biochar feed stock helps to boost resultant biochar’s adsorption potential of N concomitantly checking NH3 emissions.
The study also confirms that applying biochar-based N fertilisers is more effective in controlling NH3 volatilisations than separate applications of biochar and N fertilisers. CCDB-UHP attenuated gaseous NH3 evolutions by as high as 85.93%, while the highest rate of reduction achieved from CCDB-Urea stood at 74.80% in comparison to urea. Therefore, CCDB-UHP was more efficient than CCDB-Urea in controlling NH3 volatilisations from the soil and is recommended as a replacement for conventional urea. Attenuation of N mineralisation is a prominent pathway through which both CCDB-Urea and CCDB-UHP reduced NH3 emissions from the soil. However, slowed down net mineralisation rates of the applied N observed in both CCDB-Urea and CCDB-UHP may also hinder proper plant growth. Therefore, experiments are needed to elucidate the agronomic efficiencies of the different CCDB-Urea and CCDB-UHP.
References
Goebes MD, Strader R, Davidson C (2003) An ammonia emission inventory for fertilizer application in the United States. Atmos Environ 37(18):2539–2550. https://doi.org/10.1016/s1352-2310(03)00129-8
Pozzer A, Tsimpidi AP, Karydis VA, de Meij A, Lelieveld J (2017) Impact of agricultural emission reductions on fine-particulate matter and public health. Atmos Chem Phys 17(20):12813–12826. https://doi.org/10.5194/acp-17-12813-2017
Roumeliotis TS, Van Heyst BJ (2008) Summary of ammonia and particulate matter emission factors for poultry operations. J Appl Poult Res 17(2):305–314. https://doi.org/10.3382/japr.2007-00073
Wang S, Nan J, Shi C, Fu Q, Gao S, Wang D, Cui H, Saiz-Lopez A, Zhou B (2015) Atmospheric ammonia and its impacts on regional air quality over the megacity of Shanghai, China. Sci Rep. https://doi.org/10.1038/srep15842
Tsimpidi AP, Karydis VA, Pandis SN (2007) Response of inorganic fine particulate matter to emission changes of sulfur dioxide and ammonia: the Eastern United States as a case study. J Air Waste Manag Assoc 57(12):1489–1498. https://doi.org/10.3155/1047-3289.57.12.1489
Pinder RW, Gilliland AB, Dennis RL (2008) Environmental impact of atmospheric NH3 emissions under present and future conditions in the eastern United States. Geophys Res Lett. https://doi.org/10.1029/2008gl033732
Wang S, Xing J, Jang C, Zhu Y, Fu JS, Hao J (2011) Impact Assessment of ammonia emissions on inorganic aerosols in East China using response surface modeling technique. Environ Sci Technol 45(21):9293–9300
Megaritis AG, Fountoukis C, Charalampidis PE, Pilinis C, Pandis SN (2013) Response of fine particulate matter concentrations to changes of emissions and temperature in Europe. Atmos Chem Phys 13(6):3423–3443. https://doi.org/10.5194/acp-13-3423-2013
Bessagnet B, Beauchamp M, Guerreiro C, de Leeuw F, Tsyro S, Colette A, Meleux F, Rouıl L, Ruyssenaars P, Sauter F, Velders GJM, Foltescu VL, Aardenne JV (2014) Can further mitigation of ammonia emissions reduce exceedances of particulate matter air quality standards? Environ Sci Policy 44:149–163. https://doi.org/10.1016/j.envsci.2014.07.011
Mandal S, Thangarajan R, Bolan NS, Sarkar B, Khan N, Ok YS, Naidu R (2016) Biochar-induced concomitant decrease in ammonia volatilization and increase in nitrogen use efficiency by wheat. Chemosphere 142:120–127
Sun X, Zhong T, Zhang L, Zhang K, Wu W (2019) Reducing ammonia volatilization from paddy field with rice straw derived biochar. Sci Total Environ 660:512–518. https://doi.org/10.1016/j.scitotenv.2018.12.450
Subedi R, Kammann C, Pelissetti S, Taupe N, Bertora C, Monaco S, Grignani C (2015) Does soil amended with biochar and hydrochar reduce ammonia emissions following the application of pig slurry? Eur J Soil Sci 66(6):1044–1053. https://doi.org/10.1111/ejss.12302
Fan C, Chen H, Li B, Xiong Z (2017) Biochar reduces yield-scaled emissions of reactive nitrogen gases from vegetable soils across China. Biogeosciences 14(11):2851–2863. https://doi.org/10.5194/bg-14-2851-2017
Feng Y, Sun H, Xue L, Liu Y, Gao Q, Lu K, Yang L (2017) Biochar applied at an appropriate rate can avoid increasing NH 3 volatilization dramatically in rice paddy soil. Chemosphere 168:1277–1284. https://doi.org/10.1016/j.chemosphere.2016.11.151
He T, Liu D, Yuan J, Luo J, Lindsey S, Bolan N, Ding W (2018) Effects of application of inhibitors and biochar to fertilizer on gaseous nitrogen emissions from an intensively managed wheat field. Sci Total Environ 628–629:121–130. https://doi.org/10.1016/j.scitotenv.2018.02.048
Chen L, Chen Q, Rao P, Yan L, Shakib A, Shen G (2018) Formulating and optimizing a novel biochar-based fertilizer for simultaneous slow-release of nitrogen and immobilization of cadmium. Sustainability 10(8):2740
Puga AP, de Queiroz MCA, Ligo MAV, Carvalho CS, Pires AMM, de Marcatto JOS, Andrade CA (2019) Nitrogen availability and ammonia volatilization in biochar-based fertilizers. Arch Agron Soil Sci. https://doi.org/10.1080/03650340.2019.1650916
Liu X, Liao J, Song H, Yang Y, Guan C, Zhang Z (2019) A biochar-based route for environmentally friendly controlled release of nitrogen: urea-loaded biochar and bentonite composite. Sci Rep. https://doi.org/10.1038/s41598-019-46065-3
Zhao L, Cao X, Zheng W, Kan Y (2014) Phosphorus-assisted biomass thermal conversion: reducing carbon loss and improving biochar stability. PLoS ONE 9(12):e115373. https://doi.org/10.1371/journal.pone.0115373
Zhao L, Cao X, Zheng W, Scott JW, Sharma BK, Chen X (2016) Co-pyrolysis of biomass with phosphate fertilizers to improve biochar carbon retention, slow nutrient release, and stabilize heavy metals in soil. ACS Sustain Chem Eng 4(3):1630–1636. https://doi.org/10.1021/acssuschemeng.5b01570
Cordell D, Drangert J-O, White S (2009) The story of phosphorus: global food security and food for thought. Glob Environ Change 19(2):292–305. https://doi.org/10.1016/j.gloenvcha.2008.10.009
Dawson CJ, Hilton J (2011) Fertiliser availability in a resource-limited world: production and recycling of nitrogen and phosphorus. Food Policy 36:S14–S22. https://doi.org/10.1016/j.foodpol.2010.11.012
Thangarajan R, Bolan NS, Tian G, Naidu R, Kunhikrishnan A (2013) Role of organic amendment application on greenhouse gas emission from soil. Sci Total Environ 465:72–96. https://doi.org/10.1016/j.scitotenv.2013.01.031
Maynard DG, Kalra YP, Crumbaugh JA (2007) Nitrate and exchangeable ammonium nitrogen (chapter 6) Pages 71–80 in M.R. Carter and E.G. Gregorich (eds). Soil sampling and methods of analysis (2nd edn). CRC Press, Taylor and Francis Group, Boca Raton, FL. 1264 p 26
Mianowski A, Owczarek M, Marecka A (2007) Surface area of activated carbon determined by the iodine adsorption number. Energy Sour Part A Recovery Util Environ Eff 29(9):839–850. https://doi.org/10.1080/00908310500430901
Phuong, D.T.M (2018) Physicochemical Properties and Adsorption Capacity of Biochars Produced from Residues of Two Rice Varieties (Oryza sativa). (Ph.D. thesis) Japanese Koshihikari and Vietnamese IR50404. https://hdl.handle.net/10069/38684
Nunes CA, Guerreiro MC (2011) Estimation of surface area and pore volume of activated carbons by methylene blue and iodine numbers. Química Nova 34(3):472–476. https://doi.org/10.1590/s0100-40422011000300020
Lustosa Filho JF, Penido ES, Castro PP, Silva CA, Melo LCA (2017) Co-pyrolysis of poultry litter and phosphate and magnesium generates alternative slow-release fertilizer suitable for tropical soils. ACS Sustain Chem Eng 5(10):9043–9052. https://doi.org/10.1021/acssuschemeng.7b01935
Jiang T, Feng X, Wang Q, Xiao Z, Wang F, Xie Y (2014) Fire performance of oak wood modified with N-methylol resin and methylolated guanylurea phosphate/boric acid-based fire retardant. Constr Build Mater 72:1–6
Bolan NS, Saggar S, Luo J, Bhandral R, Singh J (2004) Gaseous emissions of nitrogen from grazed pastures: processes, measurements and modelling, environmental implications, and mitigation. Adv Agron 84:37–120
McElroy MB (2002) The atmospheric environment: effects of human activity. Princeton University Press, Princeton
Sarkar B, Naidu R (2014) Nutrient and water use efficiency in soil: the influence of geological mineral amendments. Nutr Use Effic Basics Adv. https://doi.org/10.1007/978-81-322-2169-2_3
Wang B, Lee X, Theng BKG, Zhang L, Cheng H, Cheng J, Lyu W (2019) Biochar addition can reduce NOx gas emissions from a calcareous soil. Environ Pollut Bioavailab 31(1):38–48
Dong D, Wang C, Van Zwieten L, Wang H, Jiang P, Zhou M, Wu W (2019) An effective biochar-based slow-release fertilizer for reducing nitrogen loss in paddy fields. J Soils Sediments. https://doi.org/10.1007/s11368-019-02401-8
Shi W, Ju Y, Bian R, Li L, Joseph S, Mitchell DRG, Munroe P, Taherymoosavi S, Pan G (2019) Biochar bound urea boosts plant growth and reduces nitrogen leaching. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2019.134424
Taghizadeh-Toosi A, Clough TJ, Sherlock RR, Condron LM (2012) Biochar adsorbed ammonia is bioavailable. Plant Soil 350:57–69
Sarkhot DV, Berhe AA, Ghezzehei TA (2012) Impact of biochar enriched with dairy manure effluent on carbon and nitrogen dynamics. J Environ Qual 41(4):1107. https://doi.org/10.2134/jeq2011.0123
Tian J, Miller V, Chiu PC, Maresca JA, Guo M, Imhoff PT (2016) Nutrient release and ammonium sorption by poultry litter and wood biochars in storm water treatment. Sci Total Environ 553:596–606. https://doi.org/10.1016/j.scitotenv.2016.02.129
Kammann CI, Schmidt H-P, Messerschmidt N, Linsel S, Steffens D, Müller C, Koyrol H-W, Conte P, Joseph S (2015) Plant growth improvement mediated by nitrate capture in co-composted biochar. Sci Rep. https://doi.org/10.1038/srep11080
Haider G, Steffens D, Müller C, Kammann CI (2016) Standard extraction methods may underestimate nitrate stocks captured by field aged biochar. J Environ Qual 45:1196–1204
Haider G, Steffens D, Moser G, Müller C, Kammann CI (2017) Biochar reduced nitrate leaching and improved soil moisture content without yield improvements in a four-year field study. Agr Ecosyst Environ 237:80–94
Dempster DN, Gleeson DB, Solaiman ZM, Jones DL, Murphy DV (2011) Decreased soil microbial biomass and nitrogen mineralisation with Eucalyptus biochar addition to a coarse textured soil. Plant Soil 354(1–2):311–324. https://doi.org/10.1007/s11104-011-1067-5
Castaldi S, Riondino M, Baronti S, Esposito FR, Marzaioli R, Rutigliano FA, Vaccari FP, Miglietta F (2011) Impact of biochar application to a Mediterranean wheat crop on soil microbial activity and greenhouse gas fluxes. Chemosphere 85(9):1464–1471. https://doi.org/10.1016/j.chemosphere.2011.08.031
Bruun E, Ambus P, Egsgaard H, Hauggaard-Nielsen H (2012) Effects of slow and fast pyrolysis biochar on soil C and N turnover dynamics. Soil Biol Biochem 46(4):73–79. https://doi.org/10.1016/j.soilbio.2011.11.019
Ippolito JA, Novak JM, Busscher WJ, Ahmedna M, Rehrah D, Watts DW (2012) Switchgrass Biochar Affects Two Aridisols. J Environ Qual 41(4):1123. https://doi.org/10.2134/jeq2011.0100
Verdi L, Mancini M, Ljubojevic M, Orlandini S, Dalla Marta A (2018) Greenhouse gas and ammonia emissions from soil: the effect of organic matter and fertilisation method. Ital J Agron. https://doi.org/10.4081/ija.2018.1124
Acknowledgements
This research study was conducted with support from a research grant from the Cooperative Research Program for Agricultural Science & Technology Development of Rural Development Administration, Republic of Korea (Project No. PJ014253022019).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. LD collected data, conducted the laboratory and statistical analyses and wrote the manuscript while JL helped him with procuring the required materials and in some aspects of data collection and analysis. TKO and JS supervised the experiment and offered technical guidance throughout the entire research period.
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Luyima, D., Lee, JH., Sung, J. et al. Co-pyrolysed animal manure and bone meal-based urea hydrogen peroxide (UHP) fertilisers are an effective technique of combating ammonia emissions. J Mater Cycles Waste Manag 22, 1887–1898 (2020). https://doi.org/10.1007/s10163-020-01074-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10163-020-01074-7