Abstract
The genetic diversity and population composition of bradyrhizobial isolates collected from different parts of north-western and southern Ethiopia were studied. A total of 103 bacterial symbionts were trapped from the soils collected from Ethiopia. Genetic diversity and population composition of the bradyrhizobial isolates were assessed using restriction fragment length polymorphism (RFLP) of 16S–23S rRNA region. The results showed the presence of 30 distinct restriction pattern types in the population. The difference in bradyrhizobial communities between pairs of soil samples were estimated by alpha (H′α), beta (H′β) and gamma (H′γ) diversity. Southern Ethiopia showed the most diverse bradyrhizobial populations based on the many RFLP clusters occupied. The ratio of beta to gamma (H′β/ H′γ) diversity between the bradyrhizobial community compositions was greater in north-western than in the southern region of Ethiopia. The RFLP-based population composition suggested the frequent presence of individuals with admixture of ITS (16S–23S rDNA) region and showed the interlineage transfer of ITS genomic region. Phylogenetic analysis of 16S–23S rRNA sequences revealed the presence of a heterogenous group of Bradyrhizobium in Ethiopian soils. These results have provided new insight into the ecology of Bradyrhizobium nodulating soybean under different environmental conditions in Ethiopia.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soybean originated from north-eastern China and is currently cultivated worldwide under various climatic conditions (Appunu et al. 2008; Risal et al. 2010; Singh 2010). It may have been introduced to Africa in the nineteenth century by Chinese traders along the east coast of Africa (Shurtleff and Aoyagi 2009). In Ethiopia, it was introduced in the 1950s. During the following decades, a few trials were undertaken in some agricultural experimental stations in Ethiopia, but it was only in the 1970s that the actual production of soybean commenced with the introduction of high-yielding soybean varieties from Europe and the USA (Abate et al. 2012). The production of the crop at farm level could not take place until recently. Today, soybean cultivation has expanded in the country, occupying 11,261.12 ha of land and producing 15,824.41 tonnes in 2010 (Central Statistical Authority CSA 2009). The world average yield of soybean is estimated to be 2.35 tonnes ha−1 while the average yield in sub-Saharan Africa (SSA) is about 1 ton ha−1 (Abate et al. 2012). Though the actual yield of soybean is low in Ethiopia compared to the crop’s potential, the area under production is increasing considerably and is expected to continue increasing due to rising demand for domestic processing and use as animal feed. The low soybean production is often associated with the limited use of improved plant varieties, low input agricultural systems and poor soil fertility, particularly N limitation; thus, improving the crop’s performance is a major challenge for the country. The effectiveness of symbiotic N2 fixation between soybean cultivars and native soil rhizobia is an aspect to explore for increased yields.
Soybean can establish effective N2-fixing symbiosis with diverse groups of bacteria belonging to the genera Bradyrhizobium, Sinorhizobium (Ensifer) and Mesorhizobium (Vinuesa et al. 2008; Tan et al. 1997). They are categorized as fast or slow growers, with the slow growers being distributed across different species including Bradyrhizobium japonicum (Jordan 1982), Bradyrhizobium elkanii (Kuykendall et al. 1992), Bradyrhizobium liaoningense (Xu et al. 1995) and Bradyrhizobium yuanmingense (Yao et al. 2002). The fast growers include Sinorhizobium fredii and Sinorhizobium xinjiangense (Chen et al. 2000; Hungria et al. 2001; Keyser et al. 1982; Peng et al. 2002) while Mesorhizobium tianshanese (Chen et al. 1995), with varying generation time, has also been reported as soybean rhizobial species. However, other rhizobial isolates have also been reported that are genetically distinct from the already identified species (Appunu et al. 2009; Aserse et al. 2012; Vinuesa et al. 2008; Yang et al. 2006; Zhang et al. 2011).
Rhizobia are taxonomically very diverse; thus, efficient classification methods are required to identify different genotypes (Wolde-meskel et al. 2004). Molecular techniques have been developed that are easy and quick approaches for characterizing microorganisms including differentiation at the genera, species and even strain level (Giongo et al. 2008). Restriction fragment length polymorphism (RFLP) analysis of amplified 16S rRNA by polymerase chain reaction (PCR) provides a simplified method for characterization of rhizobial isolates at the molecular level (Laguerre et al. 1996; Neves and Rumjanek 1997). However, the use of 16S rRNA gene alone as a phylogenetic marker in differentiating closely related species, and strains within species, had the following drawbacks: (i) presence in multiple copies in the genome of some bacteria, (ii) susceptibility to genetic recombination and horizontal gene transfer and (iii) low divergence among closely related species (Aserse et al. 2012; Germano et al. 2006; Martens et al. 2008; Thies et al. 2001). Thus, the ITS and housekeeping genes are also currently used as markers for molecular systematics and for estimations of phylogenetic relationships among rhizobia. The sequences of 16S–23S rRNA provide more coherent results resembling DNA-DNA hybridization than 16S rRNA sequence analysis (Willems et al. 2003).
Soybean-nodulating bradyrhizobia are found over wide regions of the world, and their genetic diversity may reflect differences in geographical, climatic and soil conditions, as well as host diversity (Adhikari et al. 2012; Appunu et al. 2008; Paffetti et al. 1998; Saeki 2011). Inoculation of soybean with an effective rhizobial inoculum can increase soybean yield, but the symbiotic efficiency of introduced strain may be low due to poor competition with indigenous soybean-nodulating rhizobia in the soil which makes it unable to establish efficient symbiosis with the host plants. In Ethiopia, soybean cultivation was started in 1970 with cultivars from Europe and the USA, but still, the yield is very low, 0.712 tonnes ha−1 (Aserse et al. 2012; Central Statistical Authority CSA 2009). The reason behind low yield may be poor soil fertility and N limitation in soil. The efficient N2 fixation by symbiosis between soybean cultivars and native soil rhizobia is an aspect to explore for increased yields. This requires sufficient knowledge of the rhizobial population in terms of their genetic diversity, geographical distribution and compatibility with the host plant. In addition, knowing the environmental factors associated with the location and the dominance of the rhizobial strains in the Ethiopian soils is important for developing strategies to improve soybean yields at low cost. A recent study by Aserse et al. (2012) indicated the presence of diverse and effective soybean population in Ethiopian soils. However, that study was only based on 12 isolates. In order to obtain vast information of soybean-nodulating rhizobial isolates, this study was conducted to evaluate the diversity and population composition of soybean-nodulating bradyrhizobia from different agro-ecological zones of Ethiopia using molecular and mathematical approaches.
Materials and methods
Soil collection for trapping rhizobia
Soil samples were collected from five districts covering many sites in South and North-western Ethiopia (Table 1). To ensure the trapping of indigenous strains, forest and undisturbed fields with no history of cultivation were included. Soil characteristics like pH, organic C and total N contents and cation exchange capacity (CEC) were measured for all soils using standard procedures (Table 1). To make sure that many indigenous rhizobia were trapped in Ethiopian soils, 11 soybean varieties were used following the procedure by Bala et al. (2003). A randomized block design was used with three replicates per treatment. Soybean seeds were surface-sterilized with 70 % ethanol for 10 s, followed by treatment with sodium hypochlorite for 3 min, and washed thoroughly six or more times with sterilized distilled water (Somasegaran and Hoben 1994). The seeds were sown in sterilized pots containing different soils under glasshouse conditions. Nodules were harvested after 45 days. The collected nodules were preserved in vials containing silica gel.
Isolation of trapped root-nodule bacteria
Root-nodule bacteria from soybean were isolated, as described by Vincent (1970) and Somasegaran and Hoben (1994). Nodules preserved in silica gel were rehydrated in water and surface-sterilized with 95 % ethanol for 10 s, followed by treatment with sodium hypochlorite for 3 min, and rinsed several times with sterile distilled water. The nodules were squashed, and the nodule suspension was streaked on yeast-mannitol agar (YMA) plates. Bacterial colonies appeared after incubation at 28 °C for 5–10 days.
Nodulation test
Bacterial isolates were tested for their ability to nodulate soybean (TGx-1937-1F) using sterilized sand in pots (ca. 1-kg capacity) under glasshouse conditions with 14-h light and 10-h darkness (Somasegaran and Hoben 1994). The seeds of soybean were surface-sterilized as described above, and allowed to germinate on wetted filter paper in sterilized Petri dishes for 2–3 days at 28 °C. After germination, the seedlings were transferred onto potted sand and inoculated with 1 ml of rhizobial culture at exponential growth phase, which provided 108 cells per millilitre for each pot. Un-inoculated pots and those supplied with 5 mM NO− 3 solution were included as controls. Plants were watered with Broughton and Dilworth (1971) N-free solution twice a week and sterile distilled water when necessary. Plants were harvested after 5 weeks and their level of nodulation recorded. Deep green leaf colour and pink nodules were considered as an indication of effective N2 fixation.
PCR amplification
Bacterial genomic DNA was extracted using GenElute bacterial DNA isolation kit (Sigma-Aldrich, USA) according to the manufacturer’s instructions. The inter-transcribed spacer (ITS), that is the 16S–23S rRNA region was amplified using the primers FGPS1490-72 (5′ TGCGGCTGGATCACCTCCT3′) and FGPL132-38 (5′CCGGGTTTCCCCATTCGG3′) (Integrated DNA Technology, USA) with a thermal cycler (T100, Bio-Rad). Polymerase chain reaction (PCR) was carried out with 25–40 ng DNA in 25-μl reaction volume containing 5 μl (5×) My Taq PCR buffer, 0.1 μl (5 U/μl) Taq polymerase (Bioline, USA), 1 μl (10 pM) of each of the primers and double distilled water. The amplified products were separated by horizontal gel electrophoresis on 1.5 % agarose gel stained with ethidium bromide with standard DNA marker (GeneDirex, 1-kbp ladder) and photographed using a gel documentation system (Geldoc™ XR+, Bio-RAD, USA).
RFLP of ITS region
The PCR-amplified ITS region was digested with fast digest restriction endonucleases (HaeII, MspI, HindIII and TaqI) following the manufacturer’s instructions (Thermo Scientific). The digested fragments were separated by horizontal gel electrophoresis on 3 % agarose gel containing 1 μg ml−1 ethidium bromide. Electrophoresis was performed in 1× Tris acetic acid EDTA (TAE) buffer at 85 V for 2.5 h and photographed under UV light under Bio-Rad Gel documentation system.
ITS-RFLP cluster analysis
The cluster separation between isolates was determined by the equation
where, N AB represents the number of RFLP bands shared by strains A and B, and N A and N B represent the numbers of RFLP bands only found in strains A and B, respectively (Nei and Li 1979; Shiro et al. 2013).
The cluster analysis was carried out using the unweighted pair–group method considering arithmetic average (UPGMA) algorithm, using NTSYS-pc version 2.2 software (Rohlf 2009). Principal component analysis (PCA) was calculated from binary data generated from restriction endonucleases digestion of ITS region of the rhizobial isolates’ genome and used to evaluate the relationship between the isolates using NTSYS-pc version 2.2.
Diversity index analysis of bradyrhizobial communities
The RFLP pattern of each isolate digested by endonucleases and RFLP cluster results were used to estimate the difference between and among the soybean bradyrhizobial communities isolated from the different field sites in Ethiopia. The Shannon Wiener (Shiro et al. 2013) and Simpson (1-D) diversity indices (Palmer and Young 2000) were calculated as
where P i is the dominance of the isolates in the dendrogram cluster i, defined as n i /N, where N is the total number of isolates and n i is the number of isolates belonging to a particular cluster of constructed ITS-RFLP dendrogram i at each field site. The differences in the composition of bradyrhizobial communities between a pair of soil samples were estimated by alpha (H′α), beta (H′β) and gamma (H′γ) diversity (Shiro et al. 2013; Whittaker 1972).
Where
-
H′α is the average of the diversity indices of two rhizobial communities.
-
H′β is the differences between the rhizobial communities from two soil samples.
-
H′γ is the diversity of the total isolate communities from two soil samples.
The relationship among the above indices was expressed as
The composition of the bradyrhizobial communities was estimated by comparing the ratio of beta to gamma diversity (i.e. H′β/H′γ).
Analysis of bradyrhizobial population structure
The Markov Chain Monte Carlo (MCMC) algorithm of program STRUCTURE 2.3.2 was used to cluster bradyrhizobial individuals to populations on the basis of RFLP data (Hubisz et al. 2009; Pritchard et al. 2000) to investigate possible gene flow between the populations. STRUCTURE uses a Bayesian clustering framework and assumes that the observed data are derived from K populations (lineages). Each population was characterized by a set of allele frequencies P at each locus. Admixture ancestry model was used to detect individuals with mixed ancestry. Three replicate MCMC were performed for each value of K ranging from 3 to 5 using 50,000 iterations after a burn-in 25,000 iterations.
ITS sequencing and phylogenetic analysis
The ITS-PCR-amplified product was purified by Favour/Prep PCR purification kit (FAVORGEN, Sigma, USA). The purified samples were sequenced (Macrogen, Netherlands). The quality of all sequences was checked using BioEdit 7.0.0 software (Hall 2004). NCBI GenBank databases were used to identify closely related species with the tested strains by BLASTn program. The sequences were deposited in the NCBI GenBank database to get accession numbers. Bradyrhizobium type sequences were selected to align with sample sequences and construction of phylogenetic tree by MEGA 6.0 programme (Tamura et al. 2013). The phylogenetic tree was generated by Kimura-2 parameter model (Kimura 1980) using a neighbour-joining method (Saitou and Nei 1987) algorithm with 1000 bootstraps (Felsenstein 1985). The rate variation among sites was modelled with the gamma distribution (shape parameter = 1).
Results
Bacterial isolates
Not all 11 varieties were able to trap microsymbionts from the five tested soils. Soybean variety Crowford was only able to trap microsymbionts from three locations (Amaro, Dorebafano and Mambuk), while soybean varieties AGS-71 and Wegayen were only able to nodulate in Pawe and Borcha soils. Soybean varieties Crowford, William, Coker-240 and Clark-64 were able to nodulate in Mambuk soil. A total of 103 bacterial isolates were obtained from nodules of soybean planted in soils collected from the different locations in Ethiopia (Table 1). The majority of the strains were able to induce pink nodules on soybean with healthy, deep green leaves while some showed a yellowish leaf colour with stunted growth. The results revealed that 91 of the 103 (˜88 %) isolates were able to nodulate the host plant. Eighty two isolates were selected for analysis of PCR-amplified ITS (16S-23S rRNA) region. Nine isolates were excluded in ITS analysis because we did not find PCR amplification of this ITS genome region. All isolates produced a single band with a range of 900 to 1098 bp, while six isolates produced an extra band.
ITS-PCR-RFLP analysis
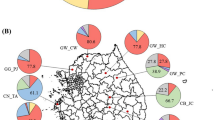
The ITS-amplified products were digested with two 4-bp cutters (MspI and TaqI) and two 6-bp cutters (HaeII and HindIII) restriction endonucleases. Overall, 30 distinct restriction pattern types among all strains were obtained, and one to four restriction fragments were observed with all used restriction enzymes. TaqI was the most discriminative restriction enzyme as it resolved the strains into 15 restriction pattern types (Table 2). To estimate the relationship between 30 restriction pattern types, a similarity matrix was calculated and a cluster generated by sharing RFLP bands. Nine different clusters were produced (Fig. 1). Three, 2, 21, 48 and 4 isolates grouped in clusters I, II, V, VI and VII, respectively, whereas isolates TUTSAGI-12, TUTSBAGS-33, TUTSFWI-63 and TUTSFCK-70 stood independently in a dendrogram. Figure 2 shows the distribution of all soybean-nodulating isolates that belonged to each cluster. The isolates from Amaro and Mambuk showed the highest number of clusters, with most of the isolates being grouped in cluster VI.
Distribution of clusters (I–IX) obtained from IGS-RFLP and population ratio of bradyrhizobia from Ethiopia. Isolates from Amaro occupied clusters I, II, V, VI and IX; isolates from Borcha occupied clusters V, VI, VII and VIII; isolates from Mambuk occupied clusters II, III, IV, VI and VII; isolates from Pawe occupied clusters VI and VII; and isolates from Dorebafano occupied clusters V and VI
The most diverse rhizobial isolates were trapped by William followed by Crowford and Coker-240 soybean varieties. Trapped isolates of William were present in six out of nine clusters while those of Crowford and Coker-240 were present in three clusters (Fig. 1).
The principal component analysis of data generated from restriction digestion of IGS (16S–23S rDNA) region gave two-dimensional PCs which collectively produced nearly 54 % of the variation among samples (Fig. 3). The results showed that TUTSACF-1, TUTSACF-2, TUTSACF-4, TUTSAGI-11, TUTSAGI-12, TUTSBAGS-33, TUTSFWI-63, TUTSFWI-64 and TUTSFCK-70 strains had zero values for PC1 and PC2 (Fig. 3; Table 2). The isolate grouping in PCs by UPGMA clustering analysis confirmed the same cluster distribution derived from the dendrogram (Fig. 1).
Principal component analysis (PCA) of restriction endonucleases digestion data of bradyrhizobial isolates. For clarity, the PC-1 and PC-2 values for each isolate are indicated in Table 2
Diversity index analysis
The differences in the indigenous bradyrhizobial communities sampled from the five sites in Ethiopia were estimated based on the H′β/H′γ ratio. The Shannon Wiener (H′) and Shimpson (1-D) diversity indices are shown in Table 3, with H′ varying from 0.18 to 1.49 and from 0.14 to 0.86, respectively. The indigenous bradyrhizobial communities isolated from Amaro region had the highest H′ (1.49) and 1-D (0.86), while Pawe exhibited the lowest H′ (0.18) and 1-D (0.14) diversity indices (Table 3). The gamma (γ) diversity index, which represents the total bradyrhizobial diversity, differed between the pair-wise comparison of the five locations and varied from 0.6 to 3.59 (Table 4). By comparing the bradyrhizobial communities of north-western and southern Ethiopia, the beta diversity index (H′β), which represents the differences between and among bacterial communities, varied from 0.1 to 2.98 (Table 4). The ratio of beta (H′β) to gamma diversity (H′β/H′γ) was the highest (>0.8) for the Borcha-Pawe (BO-PW) pair of regions (Fig. 4).
Population composition and ancestry analysis
Admixture model was used to characterize the bradyrhizobial population in clusters under F model with the assumption of correlating allele frequency of a population. The data for multiple STRUCTURE runs using the admixture model with independent allele frequencies indicated that the overall proportion of membership fraction of the isolates was 0.184, 0.388, 0.186 and 0.242 for lineages 1, 2, 3 and 4, respectively. The average distances (expected heterozygosity) between individuals in the same lineage were 0.0770, 0.0013, 0.1995 and 0.0008 for lineages 1, 2, 3 and 4, respectively. The bar plot for ancestry and admixture level of the 82 isolates are shown in Fig. 5, where each bradyrhizobial individual is represented by a single vertical line broken into K(4)-colored segments with length proportional to each of the K-inferred lineages. Any isolate with >90 % genetic material from one of the four ancestral lineages is considered to be representative of that lineage. Isolates assigned to lineage II were the most dominant (34 isolates), whereas lineage IV had the lowest number (13) of isolates. Most of the isolates in all lineages showed >95 % of their genetic material derived from the respective ancestral lineage. Out of 82, only 18 isolates of lineage III did not exhibit mixed ancestries, suggesting high interlineage flow of ITS (16S–23S rDNA) region of their (bradyrhizobial) genome. Isolates 15, 62, 48 and 29 of lineages I and III were highly admixed, possessing genetic material of IGS region inherited from all four ancestral lineages.
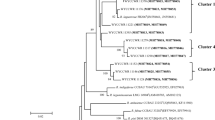
To identify the isolates, 32 representative strains from the different ITS types and ITS-RFLP clusters were selected for direct sequencing of the ITS region. All 32 isolates were identified as Bradyrhizobium from the NCBI BLASTn. NCBI GenBank database was used to construct the phylogenetic tree (see Fig. 6). The reconstructed phylogenetic tree based on ITS genomic region resulted in four (I to IV) clusters. Isolates grouped in cluster I with B. elkanii USDA76 had high 99 bootstrap support. Isolates in this cluster belonged to all studied regions. Six isolates from Amaro and Borcha regions grouped closely with B. cytisi and B. rifense with 56 bootstrap support. TUTSDCF-44 and TUTSAAW95-6 isolates were an outskirt of cluster II. TUTSBCK-28 isolate grouped together with B. ottawaense OO99 in cluster III. Monophyletic cluster IV included three (TUTSACF-2, TUTSACF-1 and TUTSACF-4) isolates without any reference or type strains. TUTSBCK-26 isolate was also found in the Bradyrhizobium group.
Discussion
Environmental factors can influence all aspects of nodulation and symbiotic N2 fixation, leading to reduced rhizobial survival and diversity in soil. The composition of soil bacterial populations depends on soil type, organic matter content and host plant. In other words, physico-chemical environment plays a major role in affecting microbial community composition and function (Girvan et al. 2003; Delmont et al. 2014). Our soils used for bradyrhizobial isolation were slight to moderately acidic in reaction and showed a low genetic diversity probably because they had a low plant-available P and high NO3 − concentration (Table 1). Analysis of ITS diversity showed a positive relationship between rhizobial diversity and available P content of soil, which was in contrast to what was reported by Palmer and Young (2000). Thus, Mambuk and Pawe soil had lower P content and lower H′ and 1-D values than Amaro and Borcha soils. The Pawe and Mambuk soils had high cation exchange capacity (CEC) which implied greater availability of nutrients and this could have affected isolate biodiversity as high levels of nutrients from fertilization is known to decrease symbiont diversity (Caballero-Mellado and Martinez-Romero 1999; Palmer and Young 2000). Furthermore, the diversity index (H′ and 1-D) was inversely related to CEC and positively related to P content, except for Dorebafano site which had very high P content but low diversity. Adhikari et al. (2012) also found that the genetic diversity of soybean-nodulating rhizobia was influenced by the soil and climatic conditions.
The ITS-PCR-RFLP fingerprint analysis showed five different major clusters, thus indicating that the isolates were genetically distinct from each other with some levels of intra-and inter-specific diversity (Palmer and Young 2000). The ITS is one of the strongest tools for the discriminatory distinction between bradyrhizobial populations (Willems et al. 2003). The ITS-PCR-RFLP cluster analysis showed that the Amaro and Mambuk isolates were more diverse and grouped in five different clusters whereas isolates from the other sites were less diverse and grouped in two to four clusters (Table 3). The diverse nature of these isolates was also proven by the high values of Shannon Wiener and Simpson diversity indices (Table 3). Due to the use of 11 soybean varieties, we were able to trap diverse types of Bradyrhizobium isolates. In other words, we can say the host plant influenced nodulation (Handley et al. 1998), as all 11 soybean varieties were not able to trap microsymbiont in the five soils tested. The reason behind this could be the release of different secondary metabolites by plants that positively or negatively affect the microbial colonization of the root (Liu et al. 2015).
The results of principal component analysis revealed zero values for PC1 and PC2, and zero value for Jaccard similarity coefficient (Fig. 3; Table 2), indicating that TUTSACF-1, TUTSACF-2, TUTSACF-4, TUTSAGI-11, TUTSAGI-12, TUTSBAGS-33, TUTSFWI-63, TUTSFWI-64 and TUTSFCK-70 strains played no role in the variability of isolates and stood alone in the dendrogram (Figs. 1 and 3). PCoA showed a large diversity of bradyrhizobial isolate population. Some of the isolates had same PC1 and PC2 values thus clustering together from the IGS-RFLP cluster analysis.
The estimated diversity indices showed that isolates from southern areas of Ethiopia occupied more clusters when compared to those from the north-western areas. The diversity of total isolate communities (H′γ) from two soils was higher in the southern compared to the north-western sites. By comparing the ratio of beta to gamma (H′β/H′γ), differences in bradyrhizobial diversity were smaller within each separate region than between different regions (Fig. 4).
STRUCTURE analysis suggests that most of the isolates exhibited mixed IGS (16S–23S rDNA) sequences or admixture. It is expected that gene flow occurs more often between members of the same bacterial species or closely related species than between different species (Didelot and Maiden 2010). Only 18 isolates of lineage III did not show mixed ancestries, suggesting that interlineage transfer of IGS region of the bradyrrhizobial genome occurs frequently in the rest of the isolates. The frequent IGS region flow between lineages showed the presence of substantial divergence in bradyrhizobial population. All isolates from Pawe, Mambuk and Amaro regions of Ethiopia were admixtures, and probably the intense gene flow depends on the good nutrient conditions as shown by high CEC of these soils; and the abundant bacterial population could provide suitable conditions for genetic recombination (Didelot and Maiden 2010; Tang et al. 2012).
PCR amplification of the ITS region of selected representative strains produced two bands on agarose gel which might be the result of DNA heteroduplex (Laguerre et al. 1996). Based on ITS gene sequence analysis, the strains isolated from soybean nodules in Ethiopia belonged to a heterogenous group of Bradyrhizobium with high genetic diversity (Doignon-Bourcier et al. 2000; Germano et al. 2006; Menna et al. 2009; Stępkowski et al. 2005). Our phylogenetic results have, therefore, indicated the presence of diverse groups of indigenous bradyrhizobia in Ethiopian soils, a finding consistent with reports by Abaidoo et al. (2000) and Aserse et al. (2012). We selected representative isolates from each cluster for sequence analysis along with more than four isolates which showed 100 % similarity in ITS-RFLP. TUTSAW04-120 and TUTSBWI-25 isolates were in same ITS-RFLP cluster but they were phylogenetically different. So, we cannot rely on the 100 % IGS-RFLP similarity, which implies the same species because these isolates could have the same restriction sites but different nucleotide sequences of that genomic region. Some isolates (TUTSDCF-44, TUTSAAW95-6, TUTSBWI-23, TUTSBAGS-35, TUTSBAW95-113, TUTSBAW04-120, TUTSBAGI-12, TUTSBAW95-111, TUTSBCK-26 and TUTSBCK-28) could not find any direct close relatives from Bradyrhizobium type strains in the database. The monophyletic groups of three (TUTSACF-2, TUTSACF-1 and TUTSACF-4) isolates stood alone in the phylogram. These unrelated strains require further study to know their exact species boundary. In the ITS phylogenetic tree, the bradyrhizobial isolates from Mambuk mainly grouped with B. elkanii, whereas those from Borcha and Amaro clustered with B. elkanii, or in a separate cluster without any relation with type strains. Clearly the Bradyrhizobium species nodulating soybean in Ethiopian soils are highly diverse, and this contradicts the misconception of absence of indigenous soybean-nodulating bradyrhizobia in Ethiopian soils and confirms what was reported by Aserse et al. (2012).
Soybean is not indigenous to Ethiopia. It was introduced in 1950. In a field trial, it was inoculated with the exotic B. japonicum strains USDA 136 and/or strain CC709 (TAL 378) from a NifTAL culture collection, Hawaii, USA (Aserse et al. 2012). But this strategy did not improve the yield of soybean in Ethiopia (Aserse et al. 2012) due to the poor competitiveness of the inoculants with indigenous rhizobia of Ethiopia soils. However, soybean production was renewed in Ethiopia in late 1970 with the introduction of European and American high-yielding soybean varieties (Aserse et al. 2012). Here, we were able to trap diverse Bradyrhizobium isolates by using 11 host soybean varieties, and the ITS-RFLP analysis revealed the presence of a high diversity of soybean bradyrhizobia in Ethiopia and most of these bradyrhizobia were admixtures. Our results confirm the presence of a wide range of rhizobial diversity in Ethiopian soils. All strains in each ITS-RFLP cluster of this study had the ability to form root nodules with soybean in Ethiopian soils thus indicating that they are well adopted to the local soil conditions and, therefore, there is the potential for the inoculant development in Ethiopia.
References
Abaidoo RC, Keyser HH, Singleton PW, Borthakur D (2000) Bradyrhizobium spp. (TGx) isolates nodulating the new soybean cultivars in Africa are diverse and distinct from bradyrhizobia that nodulate North American soybeans. Int J Syst Evol Microbiol 50:225–234
Abate T, Alene AD, Bergvinson D, Shiferaw B, Silim S, Orr A, Asfaw S (2012) Tropical grain legumes in Africa and south Asia: knowledge and opportunities. International Crops Research Institute for the Semi-Arid Tropics, ICRISAT
Adhikari D, Kaneto M, Itoh K, Suyama K, Pokharel BB, Gaihre YK (2012) Genetic diversity of soybean-nodulating rhizobia in Nepal in relation to climate and soil properties. Plant Soil 357:131–145
Appunu C, Angele N, Laguerre G (2008) Genetic diversity of native bradyrhizobia isolated from soybeans (Glycine max L.) in different agricultural-ecological-climatic regions of India. Appl Environ Microbiol 74:5991–5996
Appunu C, Sasirekha N, Prabavathy VR, Nair S (2009) A significant proportion of indigenous rhizobia from India associated with soybean (Glycine max L.) distinctly belong to Bradyrhizobium and Ensifer genera. Biol Fertil Soils 46:57–63
Aserse AA, Räsänen LA, Aseffa F, Hailemariam A, Lindström K (2012) Phylogenetically diverse groups of Bradyrhizobium isolated from nodules of Crotalaria spp., Indigofera spp., Erythrina brucei and Glycine max growing in Ethiopia. Mol Phylogen Evol 65:595–609
Bala A, Murphy P, Giller KE (2003) Distribution and diversity of rhizobia nodulating agroforestry legumes in soils from three continents in the tropics. Mol Ecol 12:917–929
Broughton W, Dilworth M (1971) Control of leghaemoglobin synthesis in snake beans. Biochem J 125:1075–1080
Caballero-Mellado J, Martinez-Romero E (1999) Soil fertilization limits the genetic diversity of Rhizobium in bean nodules. Symbiosis 26:111–121
Central Statistical Authority (CSA) (2009) Agricultural Sample Survey. 2008/9 Report on Area and Production for Major Crops (Private Peasant Holdings, Main Season). Addis Ababa, Ethiopia, p 45–47
Chen LS, Figueredo A, Pedrosa FO, Hungria M (2000) Genetic characterization of soybean rhizobia in Paraguay. Applied Environ Microbiol 66:5099–5103
Chen W, Wang E, Wang S, Li Y, Chen X, Li Y (1995) Characteristics of Rhizobium tianshanense sp. nov., a moderately and slowly growing root nodule bacterium isolated from an arid saline environment in Xinjiang, People’s Republic of China. Int J Syst Bacteriol 45:153–159
Delmont TO, Francioli D, Jacquesson S, Laoudi S, Mathieu A, Nesme J, Ceccherini MT, Nannipieri P, Simonet P, Vogel TM (2014) Microbial community development and unseen diversity recovery in inoculated sterile soil. Biol Fertil Soils 50:1069–1076
Didelot X, Maiden MC (2010) Impact of recombination on bacterial evolution. Trends Microbiol 18:315–322
Doignon-Bourcier F, Willems A, Coopman R, Laguerre G, Gillis M, de Lajudie P (2000) Genotypic characterization of Bradyrhizobium strains nodulating small Senegalese legumes by 16S-23S rRNA intergenic gene spacers and amplified fragment length polymorphism fingerprint analyses. Appl Environ Microbiol 66:3987–3997
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap Evol: 783–791
Germano MG, Menna P, Mostasso FL, Hungria M (2006) RFLP analysis of the rRNA operon of a Brazilian collection of bradyrhizobial strains from 33 legume species. Int J Syst Evol Microbiol 56:217–229
Giongo A, Ambrosini A, Vargas L, Freire J, Bodanese-Zanettini M, Passaglia L (2008) Evaluation of genetic diversity of bradyrhizobia strains nodulating soybean [Glycine max (L.) Merrill] isolated from South Brazilian fields. Appl Soil Ecol 38:261–269
Girvan MS, Bullimore J, Pretty JN, Osborn AM, Ball AS (2003) Soil type is the primary determinant of the composition of the total and active bacterial communities in arable soils. Appl Environ Microbiol 69:1800–1809
Hall T (2004) BioEdit version 7.0. 0 Distributed by the author, website: www mbio ncsu edu/BioEdit/bioedit html
Handley BA, Hedges AJ, Beringer JE (1998) Importance of host plants for detecting the population diversity of Rhizobium leguminosarum biovar viciae in soil. Soil Biol Biochem 30:241–9
Hubisz MJ, Falush D, Stephens M, Pritchard JK (2009) Inferring weak population structure with the assistance of sample group information. Mol Ecol Resour 9:1322–1332
Hungria M, de O Chueire LgM, Coca RG, Megias M (2001) Preliminary characterization of fast growing rhizobial strains isolated from soyabean nodules in Brazil Soil Biol Biochem 33:1349–1361
Jordan D (1982) Notes: Transfer of Rhizobium japonicum Buchanan 1980 to Bradyrhizobium gen. nov., a genus of slow-growing, root nodule bacteria from leguminous plants. Int J Syst Bacteriol 32:136–139
Keyser HH, Bohlool BB, Hu T, Weber DF (1982) Fast-growing rhizobia isolated from root nodules of soybean. Science 215:1631–1632
Kimura M (1980) A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16:111–120
Kuykendall L, Saxena B, Devine T, Udell S (1992) Genetic diversity in Bradyrhizobium japonicum Jordan 1982 and a proposal for Bradyrhizobium elkanii sp. nov Canad. J Microbiol 38:501–505
Laguerre G, Mavingui P, Allard MR, Charnay MP, Louvrier P, Mazurier SI, Rigottier-Gois L, Amarger N (1996) Typing of rhizobia by PCR DNA fingerprinting and PCR-restriction fragment length polymorphism analysis of chromosomal and symbiotic gene regions: application to Rhizobium leguminosarum and its different biovars. Appl Environ Microbiol 62:2029–2036
Liu Y, Guan D, Jiang X, Ma M, Li L, Cao F, Chen H, Shen D, Li J (2015) Proteins involved in nodulation competitiveness of two Bradyrhizobium diazoefficiens strains induced by soybean root exudates. Biol Fertil Soils 51:251–260
Martens M, Dawyndt P, Coopman R, Gillis M, De Vos P, Willems A (2008) Advantages of multilocus sequence analysis for taxonomic studies: a case study using 10 housekeeping genes in the genus Ensifer (including former Sinorhizobium). Int J Syst Evol Microbiol 58:200–214
Menna P, Barcellos FG, Hungria M (2009) Phylogeny and taxonomy of a diverse collection of Bradyrhizobium strains based on multilocus sequence analysis of the 16S rRNA gene ITS region and glnII, recA, atpD and dnaK genes. Int J Syst Evol Microbiol 59:2934–2950
Nei M, Li W-H (1979) Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc Nat Acad Sci 76:5269–5273
Neves MCP, Rumjanek NG (1997) Diversity and adaptability of soybean and cowpea rhizobia in tropical soils. Soil Biol Biochem 29:889–895
Paffetti D, Daguin F, Fancelli S, Gnocchi S, Lippi F, Scotti C, Bazzicalupo M (1998) Influence of plant genotype on the selection of nodulating Sinorhizobium meliloti strains by Madicago sativa. Antonie van Leeuwenhoek 73:3–8
Palmer K, Young J (2000) Higher diversity of Rhizobium leguminosarum biovar viciae populations in arable soils than in grass soils. Appl Environ Microbiol 66:2445–2450
Peng GX, Tan ZY, Wang ET, Reinhold-Hurek B, Chen WF, Chen WX (2002) Identification of isolates from soybean nodules in Xinjiang Region as Sinorhizobium xinjiangense and genetic differentiation of S. xinjiangense from Sinorhizobium fredii. Int J Syst Evol Microbiol 52:457–462
Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155:945–959
Risal CP, Yokoyama T, Ohkama-Ohtsu N, Djedidi S, Sekimoto H (2010) Genetic diversity of native soybean bradyrhizobia from different topographical regions along the southern slopes of the Himalayan Mountains in Nepal. Syst Appl Microbiol 33:416–425
Rohlf FJ (2009) NTSYS-pc Numerical Taxonomy System Version 2
Saeki Y (2011) Characterization of soybean-nodulating rhizobial communities and diversity. INTECH Open Access Publisher
Saitou N, Nei M (1987) The neighbour-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Shiro S et al (2013) Genetic diversity and geographical distribution of indigenous soybean-nodulating bradyrhizobia in the United States. Appl Environ Microbiol 79:3610–3618
Shurtleff W, Aoyagi A (2009) History of soybeans and soyfoods in Africa (1857–2009): extensively annotated bibliography and sourcebook. Soyinfo Center
Singh G (2010) The soybean: botany, production and uses. CABI
Somasegaran P, Hoben HJ (1994) Counting rhizobia by a plant infection method. Eds Handbook for Rhizobia. Springer-Verlag New York, Inc, pp 58–64
Stępkowski T, Moulin L, Krzyżańska A, McInnes A, Law IJ, Howieson J (2005) European origin of Bradyrhizobium populations infecting lupins and serradella in soils of Western Australia and South Africa. Appl Environ Microbiol 71:7041–7052
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729
Tang J, Bromfield E, Rodrigue N, Cloutier S, Tambong J (2012) Microevolution of symbiotic Bradyrhizobium populations associated with soybeans in east North America. Ecol Evol 2:2943–2961
Tan ZY, Xu XD, Wang ET, Gao JL, Martinez-Romero E, Chen WX (1997) Phylogenetic and genetic relationships of Mesorhizobium tianshanense and related rhizobia. Int J Syst Bacteriol 47:874–879
Thies J, Holmes E, Vachot A (2001) Application of molecular techniques to studies in Rhizobium ecology: a review. Animal Prod Sci 41:299–319
Vincent JM (1970) A manual for the practical study of the root-nodule bacteria . IBP Handbook No. 15, Burgess and Son (Abingdon) Ltd. Britain.
Vinuesa P, Rojas-Jiménez K, Contreras-Moreira B, Mahna SK, Prasad BN, Moe H, Selvaraju SB, Thierfelder H, Werner D (2008) Multilocus sequence analysis for assessment of the biogeography and evolutionary genetics of four Bradyrhizobium species that nodulate soybeans on the Asiatic continent. Appl Environ Microbiol 74:6987–6996
Whittaker RH (1972) Evolution and measurement of species diversity Taxon:213–251
Willems A, Munive A, de Lajudie P, Gillis M (2003) In most Bradyrhizobium groups sequence comparison of 16S-23S rDNA internal transcribed spacer regions corroborates DNA-DNA hybridizations. Syst Appl Microbiol 26:203–210
Wolde-meskel E, Terefework Z, Lindström K, Frostegård Å (2004) Rhizobia nodulating African Acacia spp. and Sesbania sesban trees in southern Ethiopian soils are metabolically and genomically diverse. Soil Biol Biochem 36:2013–2025
Xu L, Ge C, Cui Z, Li J, Fan H (1995) Bradyrhizobium liaoningense sp. nov., isolated from the root nodules of soybeans. Int J Syst Bacteriol 45:706–711
Yang JK, Zhang WT, Yuan TY, Zhou JC (2006) Genotypic characteristics of the rrn operon and genome of indigenous soybean bradyrhizobia in cropping zones of China. Canad J Microbiol 52:968–976
Yao ZY, Kan FL, Wang ET, Wei GH, Chen WX (2002) Characterization of rhizobia that nodulate legume species of the genus Lespedeza and description of Bradyrhizobium yuanmingense sp. nov. Int J Syst Evol Microbiol 52:2219–2230
Zhang YM, Li Y, Chen WF, Wang ET, Tian CF, Li QQ, Zhang YZ, Sui XH, Chen WX (2011) Biodiversity and biogeography of rhizobia associated with soybean plants grown in the North China Plain. Appl Environ Microbiol 77:6331–6342
Acknowledgments
This work was supported with grants from the Bill and Melinda Gates Foundation Project on Capacity Building in Legume Sciences in Africa, the South African Department of Science and Technology, the Tshwane University of Technology, the National Research Foundation in Pretoria, and the South African Research Chair in Agrochemurgy and Plant Symbioses. SMB is grateful for a competitive fellowship from the Bill and Melinda Gates Foundation Project on Capacity Building in Legume Sciences in Africa.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jaiswal, S.K., Beyan, S.M. & Dakora, F.D. Distribution, diversity and population composition of soybean-nodulating bradyrhizobia from different agro-climatic regions in Ethiopia. Biol Fertil Soils 52, 725–738 (2016). https://doi.org/10.1007/s00374-016-1108-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-016-1108-6