Abstract
The fate and turnover of microbial carbon (C) in an arable soil following crop residue addition likely depends on the quality of both native soil organic matter (SOM) and residues. We labeled the microbial biomass with 13C-glucose and followed the microbial 13C turnover into different SOM pools under the influence of three plant amendments (mature wheat, immature wheat, and vetch) in a laboratory incubation experiment using a soil with two different contents of organic C (0.9 and 1.3 %) owing to different soil management. At the end of incubation, more labeled glucose C was assimilated into microbial biomass in amended samples compared to an unamended control. The addition of plant residues also caused a positive priming effect, enhancing mineralization of soil organic C, which led to overall less glucose-derived C being incorporated into soil C pools. This was more pronounced in the soil with lower soil organic C content. Recovery of microbial C as recalcitrant C ranged between 6.0 and 7.1 %, with no significant effect of initial SOM content and addition of plant materials. Although the short-term fate of C present in microbial biomass was clearly affected by residue additions and initial native SOM, the extent to which it was stabilized as recalcitrant C during this time was not affected and must therefore be controlled by other factors than soil amendments and land management.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The mineralization rate of organic material is a slow process carried out by soil microbiota (Jenkinson 1978). The soil microbial biomass (SMB) represents only a small fraction of the total soil C and N but turns over relatively rapidly in comparison to soil organic matter (SOM) (Amato and Ladd 1991). Although abiotic factors such as pH, temperature, and moisture can affect C turnover (Blagodatskaya and Kuzyakov 2008), the composition and activity of the soil microbial community are key factors controlling the amounts of C and N mineralized (Fontaine and Barot 2005).
Addition of 13C-labeled substrates, such as labeled litter material, to SOM (Rasmussen et al. 2008) is an established method for evaluating the turnover of the SMB and its contribution to soil organic C (Crossman et al. 2004; Evershed et al. 2006). Several studies have used 13C-glucose to determine its consumption, modification, and accumulation as soil organic C through time (Aoyama et al. 2000; Fontaine et al. 2004; Evershed et al. 2006; Dungait et al. 2011). Addition of C substrates to soil can change the turnover of SOM, accelerating or retarding mineralization of native organic C with the supply of organic inputs; this is called, respectively, positive or negative priming effect (PE). In addition, many field and controlled observations have demonstrated that despite the incorporation of organic inputs into the soil in large quantities, soil total organic C content does not necessarily increase, although microbial biomass and activity rapidly respond to substrate inputs (Fontaine et al. 2004). When extra CO2-C evolved following addition of organic substrates comes directly from an acceleration of microbial biomass turnover, it is called apparent PE, while if it comes from mineralization of SOM by the stimulated microbial growth (co-metabolism), it is termed real PE, both being governed by microbial activity (Blagodatskaya and Kuzyakov 2008).
Although generally acknowledged as an important source of C for SOM (Kögel-Knabner et al. 2008; Mambelli et al. 2011), the contribution of SMB turnover to recalcitrant SOM and PE remains poorly understood. Up to our knowledge, no studies have examined the influence of the content of native SOM and incorporation of external inputs on soil C turnover and formation of recalcitrant SOM directly mediated by SMB, despite the prospect that the majority of plant C passes through microbial biomass prior to its conversion to CO2 or stabilization as SOM (Mambelli et al. 2011). Balser and Wixon (2009) suggested that a better understanding of the link between SMB and SOM stabilization would improve predictions of soil C dynamics in response to climate change.
For the current study, the SMB was labeled with 13C-glucose to assess the influence of native SOM content and plant residue quality and their interaction on the microbially mediated processes of decomposition and contribution to stable soil C. We pre-incubated soils with two different contents of SOM owing to different management systems with 13C-glucose for 8 days and followed the label through the microbial biomass into various soil fractions. After pre-incubation, three different plant materials (immature wheat, mature wheat, and vetch) were applied to soil, which was incubated under laboratory conditions for 64 days to monitor soil organic C mineralization and stabilization derived from SMB. While soil C turnover has been extensively studied, our aim was to investigate several less understood aspects of the processes involved, by specifically determining the influence of different content of native SOM and addition of plant residues of varying composition on (1) the incorporation of microbial biomass C into recalcitrant SOM and (2) the PE on mineralization of microbial biomass-derived C resulting from the addition of plant material. We chose the labeling approach described above as particularly appropriate for these objectives, as this method would isotopically enrich the microbial biomass without stimulating growth, therefore not affecting its natural turnover. Plant residues with different chemical composition were included in order to describe how the composition of external organic inputs affects any potential PE and formation of recalcitrant SOM, providing a relevant connection to what occurs in field soils.
Materials and methods
Soil and plant materials used
Soils were sampled from the Russell Ranch Sustainable Agricultural Facility at the University of California, Davis, CA. The site has a Mediterranean climate with a mean annual temperature of 17 °C and a mean annual rainfall of 460 mm. The soil is a silty clay loam and classified as Mollic Haploxeralf (Soil Survey Staff 2014). Samples were collected from two contrasting management systems in the same soil: (1) a conventional system receiving only synthetic fertilizers, with a soil organic C content of 0.95 % (low SOC) and (2) an organic system with cover crops and addition of composted poultry manure, with a soil organic C content of 1.32 % (high SOC). The management systems have been in place for 15 years. Three random soil samples per replicate of each management system were taken from the upper 20 cm layer, which were homogenized to obtain a composite sample for each management system. Samples were sieved <4 mm, roots and visible plants residues removed, stored at 4 °C, and used within 10 days.
The added plant materials used in the incubations described below consisted of mature wheat (Triticum aestivum L.), immature wheat, and vetch (Vicia sativa L.). The residue was dried at 55 °C and ground to less than 0.5 mm before application (Table 1).
Soil labeling and plant amendments
The optimal amount of 13C-labeled glucose amendment was determined as the minimum concentration of glucose that produces the maximal respiration rate (Anderson and Domsch 1978; Horwath and Paul 1994). This ensures the most efficient incorporation of 13C into the standing microbial biomass while at the same time minimizing its growth. We tested a range of glucose concentrations: 84, 169, 253, 337, 422, 843, 1686, and 3372 μg C g−1 soil. The respiratory response (mg CO2 h−1) was determined by CO2 evolution using an Infrared Gas Analyzer (IRGA, S151, Qubit systems). The minimum concentration of glucose invoking maximal respiratory response was 169 μg C g−1 soil for both high- and low-SOC soils. A solution of 13C-labeled glucose solution (169 μg C g−1 dry soil, 99 atom% 13C) was added to soil samples (22 g of dry soil). Nitrogen was added with the glucose as ammonium sulfate (17 μg N g−1, C:N ratio = 10) to ensure no limitation of N. The amended soils were incubated for 8 days to ensure the incorporation of glucose C and N. The pre-incubation conditions were 21 °C and 55 % water holding capacity, which was maintained for the remainder of the incubations described below.
Three different plant materials were applied in both soils with different SOM content and stability: mature wheat (MW), immature wheat (IW), and vetch (V). For each soil management, a control (CT) receiving the initial 13C-glucose input without any plant material addition was included. In total, there were eight treatments with three replicates per treatment. The plant residues were thoroughly mixed at a rate of 1 % of the dry mass of soil (corresponding to 4.23, 4.09, and 4.29 g C kg−1 for MW, IW, and V treatments, respectively). Soil samples were weighed into 60-mL plastic cups and incubated for an additional 64 days. Moisture levels were gravimetrically maintained every 3 days adding deionized water when necessary.
Analytical methods
The added plant materials were analyzed for lignin by a two-stage sulphuric acid hydrolysis (Hatfield et al. 1994), cellulose content was measured following the procedure of Brendel et al. (2000), and soluble C was extracted in water (1:500 w/v) and determined by UV-persulfate digestion (Teledyne-Tekmar Phoenix 8000). Biochemical analysis of the plant materials are shown in Table 1.
Soils were air dried following incubation and total C and 13C content were determined by combustion GC (Elementar Vario EL Cube) interfaced to a PDZ Europa 20–20 isotope ratio mass spectrometer (IRMS) (Stable Isotope Facility, UC Davis). Recalcitrant C was defined as the C remaining following acid hydrolysis as described by Rovira and Vallejo (2007); its quantity and 13C content were determined as above in soil samples from day 22 and day 64. A recalcitrance index was calculated for both total C and glucose-derived C as the ratio of recalcitrant C to organic C.
Microbial biomass C (MBC) was measured at days −8, 0, 7, 14, 22, 29, 37, 50, and 64 using the chloroform fumigation-extraction method proposed by Vance et al. (1987). K2SO4-extractable C was measured with UV-persulfate digestion (Teledyne-Tekmar Phoenix 8000), while the 13C content of these extracts was determined using an O.I. Analytical Model 1030 TOC Analyzer interfaced to a PDZ Europa 20–20 IRMS. MBC was calculated by subtraction of the extractable C of non-fumigated soil from that of the fumigated soil and then multiplied by the factor 2.64. We calculated nonliving organic C as the difference between soil total organic C and MBC.
To estimate the rate of 13C-CO2 mineralization by the soil microbiota, the soil vials were placed in a 500-mL mason jar for several hours in ten separate measurement events. The 13C-CO2 was measured using a ThermoScientific PreCon-GasBench system interfaced to a ThermoScientific Delta V Plus IRMS (Stable Isotope Facility, UC Davis). Three jars without soil or plant residue were used blanks to correct for background CO2 concentration and isotopic enrichment. The total amount of 13C mineralized during the incubation (64 days) was calculated by integrating the rate of 13C-CO2 measured at each date, taking the rate measured at a given date to be the average rate for the interval represented by that date.
Data analysis and statistical procedures
Data were checked to ensure normal distribution using the Kolmogorov-Smirnov test and transformed when necessary to ensure normal distribution. The 13C-glucose labeling of the microbial biomass allowed for the separation of C respiration derived from the original glucose addition from respiration derived from soil or plant residue. The priming effect (PE, mg CO2-C g−1 soil) induced by the addition of plant materials was calculated as the difference between the amount of glucose-derived CO2-C released from soils amended with plant materials and that released from controls receiving no addition of plant materials but similarly pre-incubated with 13C-glucose. For each interval, the amount of glucose-derived C respired as CO2 (“X”, mg g−1 soil) was calculated as follows:
The total amount of glucose-derived CO2-C produced during the course of the incubation was calculated as the sum of the values of X for all intervals.
The PE was then calculated as the percent change in production of glucose-derived CO2 in a given treatment relative to that produced without addition of plant materials:
In order to describe the effect of plant material addition on the PE, the amount of glucose-derived CO2 at each sampling event was fitted versus time to different kinetics functions. The coefficients of regression (R 2) and assumption of normal distribution of residuals were used as criteria to decide the best fit. A one-way ANOVA was carried out to assess differences among plant materials and soil management systems separately for each sampling event. The comparison of means was made according to Tukey’s honestly significant difference test (P < 0.05). Relationships among properties were determined using Pearson correlation coefficients. The fits and kinetic parameters were carried out using the SigmaPlot 10.0 software. ANOVA, and correlations were performed with the software SPSS for Windows, Version 17.
Results
Recovery of glucose addition in soil organic C pools
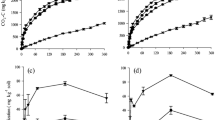
The soil with high SOC showed the highest significant (P < 0.05) content in nonliving organic 13C at the beginning of the incubation. As a general trend, nonliving organic 13C contents significantly decreased as the incubation progressed from day 0 to day 64 for all treatments (Fig. 1a). IW and V in the soil with low SOC showed the lowest values in total organic 13C. The recovery of the 13C added from glucose in nonliving organic C decreased during incubation, being slightly higher in CT than in the plant residue-amended soil within same the management. The lowest recovery was usually found in IW and V, especially for the low-SOC soil (Fig. 2a).
The amount of nonliving organic C (a), microbial biomass C (b), and CO2-C (c) derived from initial glucose addition. Data are mean ± standard deviation (n = 3). Solid lines and black symbols indicate high-SOC soil; open lines and white symbols indicate low-SOC soil. Treatments are as follows: control (crosses), mature wheat (squares), immature wheat (triangles), and vetch (circles). Significant differences among treatments at the same date are indicated as asterisk for P < 0.05; no symbol indicates a significant difference at P < 0.001; “ns” indicates no significant differences among treatments for a given date
Percentage of initial glucose C addition recovered in nonliving soil organic matter (a) and soil microbial biomass (b). Solid lines and black symbols indicate high-SOC soil; open lines and white symbols indicate low-SOC soil. Treatments are as follows: control (crosses), mature wheat (squares), immature wheat (triangles), and vetch (circles)
The 13C content of the microbial biomass was similar at day 0 for both soils, without significant differences between them. After plant residue addition, the amount of 13C incorporated in the microbial biomass reached a maximum at day 7 in the order IW ≈ V > MW, regardless of the initial content of SOC (Fig. 1b). The 13C content of the microbial biomass significantly decreased in all treatments from day 7 to day 14; from that date forward, it remained more or less stable until day 37, after which an increasing trend was observed. The presence of C derived from glucose (recovery) in MBC reached a maximum 7 days after incorporation of plants (Fig. 2b). At the end of the incubation, the recovery of glucose C was significantly higher for plant-amended treatments, with highest values in the high-SOC soil with MW, the low-SOC soil with MW, and the high-SOC soil with IW (5.5, 4.8, and 4.7 % recovery, respectively). Mineralization of glucose-derived C followed a similar pattern as microbial biomass 13C (Fig. 1c). However, the 13C released as CO2 during the first days of incubation was higher for the soil with low SOC, although these differences disappeared as the incubation progressed.
The results for recalcitrant 13C did not show significant differences among treatments at 22 or 64 days. At the end of the incubation, the average recovery of 13C derived from glucose was ∼6.4 % in all treatments (Fig. 3).
Priming effect
The addition of plant materials to soil provoked positive PEs (increases in mineralization of native SOM mineralization compared to unamended treatments; Fig. 1c; Table 2). This increased mineralization was reflected in a decrease in nonliving organic C, for which the plant-amended soils showed a larger decrease in total organic 13C compared to the control soils (Fig. 1a), with the lowest values observed in the low-SOC soil, especially with more labile plant materials (IW and V). Similarly, the observed PEs in the low-SOC soil were greater than those in the high-SOC soil. Production of 13C-CO2 after addition of plant materials over time suggested the presence of two distinct pools of mineralizable 13C, and data were fitted to a double first-order exponential model: Cmin = C1 (1 − e−k1t) + C2 (1 − e−k2), where C1 and C2 represent the sizes of the active and slow pools of mineralizable C (mg C g−1) in the plant residues, respectively, and k1 and k2 are the corresponding mineralization rate constants for each pool (day−1). These models explained more than 99 % of the variation in the results. Values of C1 indicated a much larger presence of easily biodegradable organic compounds in V, being higher in the high-SOC soil. Except for the V treatment, C2 values indicate that most of the organic 13C in the samples was slowly mineralized, particularly for MW. The highest values of the C1 × k1 product were observed for IW and V in both soils, while the highest values of the C2 × k2 product were found in IW and V in the low-SOC soil. The PE at the end of the incubation was higher in the low-SOC soil with IW > V > MW (Table 2).
Discussion
Fate of initial addition of glucose
The lowest recovery of 13C derived from the initial glucose addition in all measured soil organic pools occurred with addition of plant material, especially with more labile plant materials (IW and V) with the low-SOC soil. This is likely due to the higher mineralization of organic matter as a result of the PE. Following the pre-incubation period, at the time of plant material addition (day 0), the 13C enrichment of the MBC showed no significant differences between either soil, which indicates that initial efficiency of glucose utilization by the soil microbiota was similar in both systems. Glucose, a soluble low-molecular substrate, presumably diffuses relatively readily within the soil matrix to sites where decomposer organisms are located, where it is utilized rapidly and with high efficiencies (Amato and Ladd 1991). The amount of glucose-derived C in the microbial biomass followed a very similar trend to the overall size of the microbial biomass C throughout the incubation (r = 0.83; P < 0.001; data not shown). From the beginning of the incubation onward, the amount of this original glucose-derived C present in the microbial biomass was influenced by plant composition, supported by a negative correlation between the 13C content of the MBC and plant cellulose and lignin contents (r = −0.88; P < 0.001 for both), as well as a positive correlation with plant soluble C and N (r = 0.72 and 0.80; P < 0.001). As such, the IW and V treatments showed a similar and significantly higher microbial immobilization of 13C compared to the more recalcitrant MW residue, owing to increased microbial activity in response to easily degradable compounds in IW and V. The influence of organic matter quality of crop residues is known to affect the size of microbiota (De Nobili et al. 2001; Hoyle et al. 2008). However, as the incubation progressed, this trend reverted and 13C recovery in microbial biomass for the MW treatment became significantly higher. This may be likely due to lower losses of 13C as 13CO2 by mineralization (more intense in IW and V), leaving more 13C available in the soil for assimilation by the biomass.
The greater propensity for easily decomposable residues like IW and V to cause loss of initially immobilized C is reflected in the amount of the initial addition of glucose C present in soil (including MBC and nonliving C): lower recoveries were observed following addition of IW and V compared to MW. Furthermore, overall less initial glucose C was found in the low-SOC soil for all residue treatments, indicating a more active microbial community in regards to the processes observed in this experiment. No effect of plant materials or initial native SOM was observed with regard to the recovery of microbial C as recalcitrant C, with similar values in both soils under all treatments. Thus, although the short-term fate of C present in the soil microbiota was clearly affected by external inputs and initial native SOM, the extent to which it was stabilized as recalcitrant C during this time was not affected and must therefore be controlled by other factors other than soil amendments and land management. Similarly, Comeau et al. (2013), who cultivated labeled crops, reported that changes in different soil organic 13C pools (very light, light, heavy, and water-extractable organic fractions) were similar among the different studied crops, without significant effect of different growing plants.
Priming effect
The PE of plant residue amendments was evident in increased mineralization of an initially immobilized C addition (glucose) relative to unamended soils, as well as a steady decrease in the amount of C derived from this addition present in SOC relative to unamended soils. Increased production of 13C-CO2 in soils amended with plant material directly indicates greater mineralization of newly formed organic matter from soil microbiota, itself derived from the 13C-glucose addition. This could be explained by the plant materials causing an accelerated turnover of microbial biomass. Upon adding plant residues, an overproduction of enzymes degrading cellulose and lignin is the likely cause of the real PE because these enzymes are also involved in SOM decomposition (Fontaine and Barot 2005). Guenet et al. (2010) also found a positive PE when straw was added to soil. We observed that the nature of the plant material impacts the intensity of the PE, with higher mineralization of native organic C in soils receiving more labile residues (IW and V). Thus, applying fresh organic inputs that act as a source of energy-rich substrates for the soil microbiota increases its activity and growth (Fontaine et al. 2004; Blagodatskaya and Kuzyakov 2008). The double first-order model fitted to PEs appears to be consistent with some mechanistic models that divide SOM into an active pool, rapidly mineralized, and slow pool which breaks down slowly during a second phase (Fernández et al. 2007; Turrión et al. 2012).
The initial organic matter content of the soil amended, as a consequence of different management history, also affected the PE: the soil containing a lower SOM content showed an increased PE, and this coincided with lower recoveries of 13C, suggesting higher a microbial turnover rate. This confirms that PEs are related to the quality or ease of metabolism of inputs. Similar results were found by Toosi et al. (2012) who found lower positive PEs amending with ryegrass residue compared with its more labile water extract. Both the nature of a residue amendment as well as the amount of existing SOM can affect microbial community composition and activity, thereby affecting processes such as those observed here (Fontaine et al. 2004; Cayuela et al. 2009).
Conclusions
The retention of microbial biomass C in soil and its loss via mineralization were affected by the nature of plant residue amendments as well as the content of soil organic matter, a product of management history. A positive priming effect was observed in all instances, in which addition of residue stimulated greater mineralization of C initially contained in the microbial biomass, although this effect was greater with more labile plant residues and in soil with lower organic matter content. At the same time, the amount of initial microbial biomass C transformed into recalcitrant C was the same for all treatments. Clearly, there are factors other than initial content of native organic matter and lability of plant amendments which control the persistence of microbial biomass C in the form of recalcitrant organic C. Nevertheless, turnover of microbial C, including the relative amount of loss and retention as non-recalcitrant soil organic matter, is indeed affected by variables such as those examined in this study. This study has provided new insights about the incorporation of microbial biomass C into recalcitrant SOM, which had not been addressed so far. Future research is needed to identify the factors implied in the formation of recalcitrant SOM through soil microbiota and assess the environmental conditions or management practices which may influence this process.
References
Amato M, Ladd JN (1991) Decomposition of 14C-labelled glucose and legume material in soils: properties influencing the accumulation of organic residue and microbial biomass C. Soil Biol Biochem 24:455–464
Anderson JPE, Domsch KH (1978) A physiologocal method for the quantitative measurement of microbial biomass in soils. Soil Biol Biochem 10:215–221
Aoyama M, Angers DA, N’Dayegamiye A, Bissonnette N (2000) Metabolism of 13C-labeled glucose in aggregates from soils manure application. Soil Biol Biochem 31:295–300
Balser TC, Wixon DL (2009) Investigating biological control over soil carbon temperature sensitivity. Glob Chang Biol 15:2935–2949
Blagodatskaya EV, Kuzyakov Y (2008) Mechanisms of real and apparent priming effects and their dependence on soil microbial biomass and community structure: critical review. Biol Fertil Soils 45:115–131
Brendel O, Iannetta PPM, Stewart D (2000) A rapid and simple method to isolate pure alpha-cellulose. Phytochem Anal 11:7–10
Cayuela ML, Sinicco T, Mondini C (2009) Mineralization dynamics and biochemical properties during initial decomposition of plant and animal residues in soil. Appl Soil Ecol 41:118–127
Comeau LP, Lemke RL, Knight JD, Bedard-Haughn A (2013) Carbon input from 13C-labeled crops in four soil organic matter fractions. Biol Fertil Soils 49:1179–1188
Crossman ZM, Abraham F, Evershed RP (2004) Stable isotope pulse-chasing and compound specific stable carbon isotope analysis of phospholipid fatty acids to assess methane oxidizing bacterial populations in landfill cover soils. Environ Sci Technol 38:1359–1367
De Nobili M, Contin M, Mondini C, Brookes PC (2001) Soil microbial biomass is triggered into activity by trace amounts of substrate. Soil Biol Biochem 33:1163–1170
Dungait JAJ, Kemmit SJ, Michallon L, Guo S, Wen Q, Brookes PC, Evershed RP (2011) Variable responses of the soil microbial biomass to trace concentrations of 13C-labelled glucose, using 13C-PLFA analysis. Eur J Soil Sci 62:117–126
Evershed RP, Crossman ZM, Bull ID, Mottram H, Dungait JAJ, Maxfield PJ, Brennand EL (2006) 13C-labelling of lipids to investigate microbial communities in the environment. Curr Opin Biotechnol 17:72–82
Fernández JM, Plaza C, Hernandez D, Polo A (2007) Carbon mineralization in an arid soil amended with thermally dried and composted sewage sludge. Geoderma 137:497–503
Fontaine S, Barot S (2005) Size and functional diversity of microbe populations control plant persistence and long-term soil carbon accumulation. Ecol Lett 8:1075–1087
Fontaine S, Bardoux G, Abbadie L, Mariotti A (2004) Carbon input to soil may decrease soil organic carbon content. Ecol Lett 7:314–320
Guenet B, Neill C, Bardoux G, Abbadie L (2010) Is there a linear relationship between priming effect intensity and the amount of organic matter input? Appl Soil Ecol 46:436–442
Hatfield RD, Jung HJG, Ralph J, Buxton DR, Weimer PJ (1994) A comparison of the insoluble residues produced by the Klason lignin and acid detergent lignin procedures. J Sci Food Agric 65:51–58
Horwath WR, Paul EA (1994) Microbial biomass. Chapter 36. In: Weaver RW (ed) Methods of soil analysis. Part 2. Microbiological and biochemical properties. Soil Science Society of America. Inc, Madison, pp 760–761
Hoyle FC, Murphy DV, Brookes PC (2008) Microbial response to the addition of glucose in low-fertility soils. Biol Fertil Soils 44:571–579
Jenkinson DS (1978) The soil biomass. CSIRO Report
Kögel-Knabner I, Guggenberger G, Kleber M, Kandeler E, Kalbitz K, Scheu S, Eusterhues K, Leinweber P (2008) Organo-mineral associations in temperate soils: integrating biology, mineralogy and organic matter chemistry. J Plant Nutr Soil Sci 171:61–82
Mambelli S, Bird JA, Gleixer G, Dawson TE, Tom MS (2011) Relative contribution of foliar and fine root pine litter to the molecular composition of soil organic matter after in situ degradation. Org Geochem 42(9):1099–1108
Rasmussen C, Southard RJ, Horwath WR (2008) Litter type and soil minerals control temperate forest soil carbon response to climate change. Glob Chang Biol 14:2064–2080
Rovira P, Vallejo VR (2007) Labile, recalcitrant, and inert organic matter in Mediterranean forest soils. Soil Biol Biochem 39:202–215
Soil Survey Staff (2014) Keys to soil taxonomy, 12th edn. Natural Resources Conservation Service (NRCS), Washington DC
Toosi E, Doane TA, Horwath WR (2012) Abiotic solubilization of soil organic matter, a less-seen aspect of dissolved organic matter production. Soil Biol Biochem 50:12–21
Turrión MB, Lafuente D, Mulas R, Ruipérez C, Pando V (2012) Effects on soil organic matter mineralization and microbiological properties of applying compost to burned and unburned soils. J Environ Manag 95:245–259
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19:703–707
Acknowledgments
Dr. Moreno-Cornejo thanks the Universidad Politécnica de Cartagena for her FPU fellowship and providing a visiting fellowship during the course of this study and the J. G. Boswell Endowed Chair in Soil Science for funding.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moreno-Cornejo, J., Zornoza, R., Doane, T.A. et al. Influence of cropping system management and crop residue addition on soil carbon turnover through the microbial biomass. Biol Fertil Soils 51, 839–845 (2015). https://doi.org/10.1007/s00374-015-1030-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-015-1030-3