Abstract
Background and aims
Crop residues and soil types play an important role in soil C and N storage. The objectives of this study were to quantify the effects of crop residue quality and interactions with soil type on soil C and N, in the short- and medium-term, and to determine the responses related to the priming effect (PE).
Methods
Residues of vetch (Vicia sativa L.), pea (Pisum sativum L.) and wheat (Triticum aestivum L.) crops with different chemical compositions and labelled with 13C and 15N were left to decompose on the surface of either a sandy-loam soil or a clay soil incubated under laboratory conditions at 25 °C for 360 days. We measured the total CO2-C and CO2-13C emitted during decomposition, the soil mineral N content and the amounts of 13C and 15N remaining in both the surface residue particles and the bulk soil.
Results
Over the short-term, the vetch residues decomposed faster than those of wheat and pea on the soil surface due to their more favourable chemical composition for biodegradation; after one year, however, this difference disappeared. We observed extra soil C mineralization in all cases, i.e., the PE was positive for all treatments and was directly related to the water-soluble (vetch > pea > wheat) and soil C contents (clay soil > sandy-loam soil). Conversely, the fate of the added 15N and net N mineralization differed considerably between the three residues and was strongly related to the initial N content of the residue.
Conclusions
Crop residue quality and soil type affected the soil PE and soil C balance but not the fate of crop residue-C after one year. Net soil N mineralization was observed in all crop residues, with large early differences (vetch > pea > wheat), which were maintained on a medium-term basis. Our results emphasize the need to jointly consider C and N dynamics as well as short- and medium-term effects to manage agricultural and environmental services provided by the recycling of crop residues to agricultural soils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In agricultural systems, the main source of soil carbon (C) is crop residues. Their chemical composition and the amount of nutrients present in crop residues affect the dynamics of C and N in soil and strongly control the rate of decomposition and formation of microbial biomass and by-products (de Bruijn and Butterbach-Bahl 2010). However, the processes of crop residue decomposition and soil organic matter (SOM) stabilization are often considered separately (Sollins et al. 2007). While there is considerable evidence that crop residue quality parameters such as the nitrogen (N) concentration and the lignin and soluble fraction content control short-term C and N dynamics (Trinsoutrot et al. 2000; Abiven et al. 2005), less is known about the medium- to long-term fate of C and N from crop residues differing in quality (Gentile et al. 2010).
Crop residues with high concentrations of lignin and polyphenols, which provide characteristics linked to chemical recalcitrance (Gentile et al. 2011; Cotrufo et al. 2013), could incorporate large amounts of C in the soil through the modification and condensation of lignin, which was shown to be the primary pathway of SOM stabilization (Stevenson 1994). However, research characterizing the chemical structure of SOM showed a low stability of lignin in aerated soils (Rasse et al. 2005; Gentile et al. 2011). Others studies with plant residues enriched with 13C and 15N indicated that at an early stage of decomposition, leaves contributed more effectively to the accumulation of soil C and N than stems and roots (Bird et al. 2008; Rubino et al. 2010), mainly as a result of microbial by-products produced during crop residue degradation (Cotrufo et al. 2015). By promoting soil aggregation and the strong chemical bond to the soil mineral matrix, the microbial by-products would be the main contributing agents to SOM stabilization in the medium- and long-term (Kögel-Knabner et al. 2008). However, Castellano et al. (2015), after reviewing 15 studies that evaluated the effects of the quality of crop residues on C accumulation in soils, found that crop residue quality affected SOM stabilization in only 46% of the cases. Likewise, Gentile et al. (2010) reported that the quality of Tithonia, Calliandra and maize residues did not interfere with the stabilization of C and N in the soil, the most important factors being the inputs of C and N and the stabilizing capacity of the soil.
The addition of substrates with a high content of soluble C to soil can also affect the priming effect (PE) by changing soil microbial characteristics, resulting in acceleration in the decomposition of SOM (Wang et al. 2015). The PE has been a research focus of many authors in the past decade (e.g., Fontaine et al. 2003; Blagodatsky et al. 2010; Kuzyakov 2010). These studies most frequently examined simple compounds (i.e., glucose), several crop residues in a single soil, and substrates that were well mixed into soils. The PE can vary between soils (Kuzyakov 2002), can be modified by soil properties such as microbial biomass, nutritional stoichiometry and the total C content (Fontaine et al. 2011) and can also be affected by the contact between the soil and the C source added. Moreover, the occurrence and magnitude of the PE can influence the net changes in organic C stored in the soil, as a result of the balance between new C inputs, mainly from crop residues, and C losses promoted by the PE (Fontaine et al. 2003). Such a net C balance is assumed to be controlled by the interaction between crop residue quality and soil characteristics and to be affected by crop residue location.
The objectives of this study were to (i) quantify the contribution of crop residues of varied composition to total soil C and N in the short- and medium-term; (ii) determine how this contribution depends on soil type; and (iii) quantify the PE and how it responds to crop residue quality and soil type. Crop residues (vetch, pea and wheat) labelled with 13C and 15N were left on the surface of two different soils and incubated for one year under laboratory conditions. We hypothesized that a higher residue soluble fraction would increase both the decomposition rate and the amount of C stabilized in the soil, and this effect would be greater in the clay than in the sandy-loam soil. We also hypothesized that the PE would be more intense in the presence of residues with a higher initial soluble C fraction, i.e., vetch residues in the present study.
Materials and methods
Soils
Soils were collected from the 0–10-cm layer of two experimental areas that were managed for more than 10 years under a no-till system in Rio Grande do Sul State, southern Brazil. The soils were a sandy-loam, classified as a Typic Paluedalf, and a clay, classified as a Rhodic Hapludox (Soil Survey Staff 2010). The soils were initially gently crumbled, and visible organic residues were removed by hand. Then, the soils were sieved (4 mm), homogenized and stored at room temperature for 15 days until incubation. Selected soil properties are shown in Table 1. Three days prior to incubation, the soils were moistened to 80% of field capacity, which ensured aerobic conditions of decomposition in these soils. A potassium nitrate (KNO3) solution was added to the sandy-loam soil to reach the same initial soil mineral N content found in the clay soil.
Labelled plant residues
The plant residues (leaves + stems) used in this study were obtained from wheat (Triticum aestivum L.), pea (Pisum sativum L.) and vetch (Vicia sativa L.), which were labelled with 13C and 15N in situ under field conditions. The pulse labelling with 13CO2 started 20 days after the germination of the plants and was performed weekly thereafter until the plants had flowered, using portable labelling chambers as described by Tahir et al. (2016). In each pulse labelling event, the plants were enclosed inside labelling chambers that were sealed with air-tight water seals. Pulses of 13CO2 were generated from the reaction of 2 M HCL with a solution of NaH13CO3 (33 atom % 13C). 15N labelling was accomplished by adding (15NH4)2SO4 (66 atom % 15N) to the soil surface, split in equal doses for the first six 13CO2 labelling events. The aboveground plant residues of each species were collected at the same time, when wheat was at the early dough stage, pea was at maturity and vetch was at the pod formation stage. The plant shoots were separated into leaves and stems to determine their proportion and dried at 40 °C for 48 h in a forced-air oven to a constant weight. Then, all plant residues were cut manually into pieces of approximately 1.0 cm in length and stored in airtight jars until the start of incubation. One plant residue subsample was ground into 1-mm particles for proximate analysis using the Van Soest method described by Redin et al. (2014). Another subsample was oven-dried at 65 °C for 48 h for dry matter (DM) correction, and a third subsample was ground in a ball mill for the determination of the C and N contents and 13C and 15N isotope abundance analyses. The water-soluble organic C (Csw) and water-soluble total N (Nsw) was extracted using 0.5 g of chopped plant residue (1.0 cm) pre-dried at 40 °C. The residues were placed in a 60-mL snap cap with distilled water (20 °C) and mechanically stirred for 30 min. After mixing, the material was filtered (Whatman n°5), and the concentrations of Csw and Nsw were analysed using a total organic carbon (TOC) analyser equipped with a kit for N analysis (Shimadzu TOC-LCPH, Shimadzu, Kyoto, Japan). The characteristics of the plant residues are shown in Table 2.
Experimental design and incubation conditions
The experimental design consisted of two sets of jars prepared and monitored in parallel, one to evaluate the C mineralization dynamics and another to evaluate the C and N dynamics in the plant residues and soils. We conducted the incubations for 360 days in the dark at 25 ± 1 °C. The treatments consisted of the combination of two soils and three types of crops residues plus the non-amended soils, resulting in eight treatments: non-amended controls with sandy-loam soil (SS) and clay soil (CS); sandy-loam soil amended with wheat (SS-W), pea (SS-P) or vetch (SS-V) residues, and clay soil amended with wheat (CS-W), pea (CS-P) or vetch (CS-V) residues. The treatments were arranged as a completely randomized design, and each treatment was replicated three times. For each replicate, a sample of 122.7 g (sandy-loam soil) or 136.4 g (clay soil) of moist soil, at 0.137 g or 0.263 g H2O g−1 dry soil (80% of field capacity), respectively, equivalent to 108 g of dry soil, was added to each replicate pot. In each experimental unit, 50% of the soil mass was placed in a 110-ml cylindrical acrylic pot (5.0 cm in diameter and 5.0 cm in height) and compressed to a height of 2.5 cm. Then, the remaining 50% of the soil mass was placed in the same acrylic pot and compressed to a total height of 5.0 cm. Thus, the soil in each pot reached a final bulk density of 1.1 g cm−3. The plant residues (leaves + stems) of the wheat, pea and vetch were applied on the soil surface at a rate of 0.6 g DM pot−1, which was equivalent to the addition of 3 Mg DM ha−1. The plant residues were prepared as a mixture of the leaves and stems of each species, according to their actual mean proportion, which was determined at harvest time. The leaf:stem proportion used for each species was 0.15:0.85 (wheat), 0.65:0.35 (pea) and 0.40:0.60 (vetch).
In the series set up to evaluate C mineralization, each acrylic pot was incubated separately in a 1000-mL glass jar (1 pot per jar). In the series set up to assess the C and N dynamics in the plant residues and soil, the acrylic pots were incubated in 2000-mL glass jars, with three replicates of each treatment per jar. All the jars were opened periodically, aerated for a few minutes to renew the atmosphere in the jars, and the soil water content was checked by weighing and adjusted with a micropipette when necessary.
Sampling and analytical measurements
The C mineralization of the plant residues was assessed by quantifying the CO2 emitted, which was trapped in 10 mL of 1 M NaOH in a beaker placed inside each glass jar. The NaOH beakers were changed at each sampling time, i.e., at 3, 7, 14, 20, 40, 60, 80, 100, 120, 150, 180, 210, 240, 270, 300, 330 and 360 days. The carbonate trapped in the NaOH was precipitated with a 2 M BaCl2 solution, and the remaining NaOH was back titrated with 1 M HCl. In the treatments with added residues, the BaCO3 precipitates formed were separated by vacuum filtration (glass fibre filter, porosity 1.2 μm,) and dried at 65 °C for 24 h. The precipitates were then weighed in a tin cap with a catalyst (PbO2) for the analysis of 13C isotopic abundance.
The soil mineral N (NH4 + and NO2 −+NO3 −) was measured in all treatments at time 0 and at 3, 7, 20, 60, 180, and 360 days of incubation. At each sampling time, the visible plant residue particles were removed from the soil surface. The mineral N was extracted from fresh soil samples with 1 M KCl (30 min shaking) with a soil:solution ratio of 1:4. The soil KCl suspension was settled for 30 min until the supernatant liquid was clear. The mineral N in a soil extract aliquot was measured by steam distillation (Keeney and Nelson 1982). The soil mineral 15N was determined according to the micro-diffusion method (Brooks et al. 1989). Briefly, the aliquots of the supernatant of the soil KCl extract used for micro-diffusion were calculated to contain at least 70 μg N. After 7 days of diffusion, the glass fibre filter discs were retrieved, dried at 50 °C for 2 h, and enclosed in a tin capsule for the analysis of 15N isotopic abundance.
The amounts of C, 13C, N and 15N in the remaining residues particles and in the soils were estimated at 3, 7, 20, 60, 180 and 360 days. At each time, plant residues were removed from the soil surface, dried in an oven at 65 °C and weighed to determine the amount of DM remaining. The soil was then removed from the acrylic pots, separated into two layers (0–2.5 cm and 2.5–5.0 cm), homogenized and air dried. The dry plant residues and the soil samples were finely ground in a steel ball mill for C and N content determination and 13C and 15N isotope abundance analyses.
The total C and N contents of the plant residues and soil were analysed using an elemental analyser (Flash EA 1112, Thermo Electron Corporation, Milan, Italy). The isotope abundance of 13C and 15N in the plant residues and soil, 13C in the BaCO3 precipitates and 15N in the mineral N were analysed using an isotope ratio mass spectrometer (IRMS) (Delta V Advantage, Thermo Fisher Scientific, Bremen, Germany) interfaced with an elemental analyser (Flash EA 2000, Thermo Fisher Scientific, Bremen, Germany).
Calculations and statistical analysis
The apparent C mineralization of the plant residue-C was calculated by subtracting the amounts of CO2-C emitted from the non-amended control soil from those emitted from the residue-amended soil, for each soil and crop residue type (Trinsoutrot et al. 2000; Fontaine et al. 2004). The net N mineralization was calculated by subtracting the amounts of mineral N that accumulated in the non-amended control soil from those that accumulated in the residue-amended soil at each date.
The recovery of 13C in the soil, plant residues and CO2 pools was calculated using the following equation:
where C pool is the amount of C in the soil, plant residues or CO2; Atom pr is the atom % of 13C of the plant residues, soil or CO2 in the residue-amended soil; and Atom c is the atom % of 13C of the soil or CO2 in the non-amended control soil or the atom % of 13C of the international standard Vienna-Pee Dee Belemnite (1.1179 atom %) when the equation was applied to calculate the recovery of 13C in plant residues.
Similar calculations were performed for 15N. The atom % 15N of air (0.36765 atom %) was used to calculate the recovery of 15N in the plant residue pool. To calculate the recovery of 15N in the soil mineral N pool, the atom % of 15N of mineral N in the non-amended control soil was subtracted from the atom % of 15N of mineral N in the residue-amended control soil. The soil organic 15N was calculated by subtracting the amounts of soil mineral 15N from the amounts of soil total 15N.
The total 13C and 15N recovery was calculated on four sampling dates (7, 60, 180 and 360 days) based on the sum of 13C or 15N recovered in CO2, soil and plant pools and expressed as percentage of initial 13C and 15N added.
The PE was calculated as the difference between apparent CO2-C mineralization (calculated as described previously) and the actual mineralization of residue-C estimated from CO2-13C emitted; this corresponded to the difference in soil C mineralization between non-amended soils and treatments amended with crop residues.
The dynamics of 13C remaining in the residues (Cr) and 13C mineralization (Cm) were modelled using a 2-pool decomposition model according to Plante and Parton (2007) and Wang et al. (2004), respectively:
where Cr and Cm are the residue C and cumulative residue-derived CO2-C at time (t), respectively; C 1 and C 2 are the fast and slow C pools, respectively, present in the plant residue; and k 1 and k 2 (day−1) are the decomposition rates of C 1 and C 2 , respectively.
All data were subjected to analysis of variance (ANOVA), and for all parameters evaluated at each date, the interaction between the factors crop residues and soil type was tested. Differences between means were calculated using the Tukey test (P < 0.05). All data were analysed using SISVAR® statistical software (version 5.6).
Results
C dynamics
C and 13C mineralization
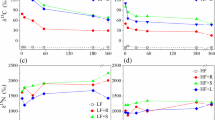
The cumulative CO2-C differed between treatments, with a significant interaction between residue quality and soil type, on all sampling dates (Fig. 1a, b and Table 3). In the sandy-loam soil, higher cumulative CO2-C was consistently obtained with vetch, e.g., at days 3 to 210 while pea and wheat were almost identical. However, at the end of the incubation, there was no significant difference between vetch, pea and wheat (on average, 2491 ± 71 mg CO2-C kg−1 soil). In the clay soil, on average, higher cumulative CO2-C was obtained compared to the sandy-loam soil, and the differences between vetch, pea and wheat were much larger. At the end of the incubation, the cumulative CO2-C was 3308 ± 138 mg kg−1 soil for vetch, 3042 ± 167 mg kg−1 soil for pea and 2573 ± 68 mg kg−1 soil for wheat.
Regarding the actual cumulative mineralization of residue-C (expressed as % 13C added) (Table 3), there was no interaction between residue quality and soil type during the experiment. The 13C mineralization pattern (Fig. 2a, b) differed greatly between the three species, with vetch residues decomposing much faster (20.3 ± 0.8% added 13C on day 7) than wheat and pea, which did not differ (12 ± 1.4% added 13C on day 7). After 180 days, we observed that the ranking between residues was reversed, with wheat > pea > vetch. This was confirmed at 360 days for both soil types, where 58 ± 2.7% and 55 ± 4.3% mineralization of added 13C was measured for the wheat and pea residues, respectively, while for the vetch residue, 49 ± 1.0% of added 13C had been mineralized (presented values are the average for the two soil types). The mean 13C mineralization was always significantly higher for the three treatments in the sandy-loam compared to the clay soil until 180 days; on day 360, however, differences were no longer observed between soils: 13C mineralization for the 3 residues reached 51 ± 3.3 and 47 ± 2.4% of added 13C in the sandy-loam and clay soil, respectively (Table 3).
The kinetics of 13C mineralization were well described using a two-compartment model that split the residue-C into fast and slow C pools, with R2 = 1.0 ((Supplementary material, Table S1). There was no interaction between residue quality and soil type for any of the parameters. The C 1 and C 2 pool sizes showed a significant effect of residue quality, with C 1 being significantly higher and C 2 significantly lower for the vetch treatments compared to pea and wheat. The mineralization rate of the fast C pool (k 1 ) was also significantly higher for the vetch residues, ranging from 0.066 for wheat to 0.118 for vetch. The soil type did not affect the size of the two C pools or the mineralization rate k 1 . However, the slow C pool showed significant differences with respect to soil type, with k 2 being higher in the sandy-loam than in the clay soil (0.011 vs. 0.007).
Priming effect
As observed in Fig. 3, positive differences (i.e., positive PE) were observed between the apparent mineralization (calculated as the difference between total CO2-C emitted from the amended and control soils) and the actual mineralization of crop residues-C, measured from CO2-13C emitted, in both soils for the three residues. On day 60, the PE differed significantly between the three residues, with vetch > pea > wheat (Table 3). When comparing the soils, a greater mean PE was observed in the clay soil treatments (+244 mg of CO2-C kg−1 soil) than in the sandy-loam soil treatments (+176 mg of CO2-C kg−1 soil). From day 80 until day 360, there was an interaction between residue quality and soil type. During this period, the PE remained almost constant in the sandy-loam soil (mean value over the 80–360 day period of +95 for wheat, +182 for pea and +438 mg CO2-C kg−1 soil for vetch), but the PE continued to increase for vetch and pea incubated in the clay soil. Conversely, the PE for wheat did not increase in the clay soil and was equal to the PE in the sandy-loam soil. Therefore, after 360 days, the cumulative PE varied greatly between the residues incubated in the clay soil, with +981, +665 and +82 mg CO2-C kg−1 soil for vetch, pea and wheat, respectively. Averaging the three residue treatments, the cumulative PE was much higher in the clay soil (+576 mg CO2-C kg−1 soil) than in the sandy-loam soil (+223 mg CO2-C kg−1 soil). When expressing this average PE as a function of the C content of each soil, the mean PE represented a similar proportion of the soil total C, i.e., 2.32% (sandy-loam soil) and 2.38% (clay soil).
Apparent (CO2-C) and actual (CO2-13C) C mineralization of wheat (a, b), pea (c, d) and vetch (e, f) residues in the sandy-loam (a, c, e) and clay (b, d, f) soils during the 360-day experiment. In each figure, the difference between apparent and actual mineralization represents the priming effect. The vertical bars indicate the standard error of the mean (n = 3)
The balance between new residue-C input into the soil, calculated using the 13C soil data (see next section), and extra C loss due to the PE was negative for vetch in the sandy-loam (−202 mg kg−1 soil) and clay soil (−637 mg kg−1 soil) and for pea in the clay soil (−329 mg kg soil). Conversely, the balance was positive for wheat in the sandy-loam (+358 mg kg−1 soil) and clay soil (+334 mg kg−1 soil) and for pea in the sandy-loam soil (+255 mg kg−1 soil).
13C of the remaining residue and soil 13C
Overall, there was a small but significant effect of residue quality or soil type on the 13C of the remaining residue, but no interaction was found between the two factors for any of the sampling dates (Table 3). The first 60 days of incubation corresponded to the period in which the largest decrease in residue-13C was observed, with the fastest decrease for vetch. From day 60 until the end of the experiment, the decrease in residue-13C was slower and progressively stabilized (Fig. 2c, d), with no significant differences between treatments. On day 360, an average of 24.8 ± 1.6% of added 13C was recovered as residue particles that remained on the soil surface.
The residue 13C kinetics of the remaining residue were also well described by a two-compartment model, with R2 = 0.99 (Supplementary material, Table S1). There was no interaction effect between residue quality and soil type on the parameters of the model. Similarly, there was no effect of residue quality or soil type on C 1 , C 2 and k2. We observed that vetch had a significantly higher k 1 value compared to the two other residues.
There was no interaction between residue quality and soil type for the amount of total 13C recovered in the 0–5-cm soil layer (calculated as the sum of the 0–2.5-cm plus 2.5–5.0-cm soil layers) during the experiment (Table 3). Differences between residues were observed only on day 7 when the 13C recovered was 6.4 ± 1.5% of added 13C for vetch, 5.0 ± 0.8% of added 13C for pea and 4.1 ± 0.6% of added 13C for wheat (Fig. 2e, f and Table 4). At the end of the incubation, the 13C recovered in the soil represented, on average, 16.6 ± 2.0% of the 13C added, with 80.1% of this amount being accumulated in the soils in the first 60 days and 87.5% being accumulated in the 0–2.5-cm soil layer (Table 4). The residue-13C recovered in the soil was not affected by soil type.
N dynamics
Net N mineralization
The N mineralization dynamics in soil showed a strongly contrasting pattern among the residues in both soil types, with net N mineralization for vetch and pea, while for wheat, N mineralization was negative (indicating net N immobilization) or close to 0 (Fig. 1c, d). Significant effects of residue quality and soil type were observed at 7, 60 and 180 days, with slight differences for pea and vetch, while for wheat, maximum N immobilization was reached on day 60 followed by net remineralization until day 360. At the end of the experiment, net N mineralization was +59.4 ± 4.7 mg N kg−1 soil (vetch), +20.2 ± 2.8 mg N kg−1 soil (pea), and +4.1 ± 5.4 mg N kg−1 soil (wheat). In the non-amended soils (control), there was little difference between the two soil types, and the net N mineralization over 360 days was +61 ± 1.6 mg N kg−1 soil and +69 ± 4.0 mg N kg−1 soil for the sandy-loam and clay soil, respectively (data not shown).
N and 15N of the remaining residues
The N concentration of the remaining residue particles increased for all crop residues until day 60 (vetch) or day 180 (pea and wheat) and then decreased until day 360. The initial % N in the residues occurred in the order: vetch > pea > wheat; as a result, the changes in C:N varied strongly between the residues (Supplementary material, Fig. S1a, b). The 15N atom excess % of the recovered residue particles decreased considerably for the wheat residues (Supplementary material, Fig. S1c). Conversely, this effect was much reduced or not observed for pea and vetch.
Only a significant effect of residue quality was found for the 15N of the remaining residues. The 15N of the remaining vetch decreased very rapidly over time in both soil types, with an average of 40 ± 2.8% of added 15N on day 60, i.e., significantly different from pea and wheat residues, which did not differ statistically (54.4 ± 8.6 and 65 ± 10.6% of added 15N, respectively) (Fig. 4a, b). On day 360, no differences were observed between treatments, and the remaining 15N recovered in the residue particles was 32 ± 6.1% of the 15N of the initial residue.
Mineral and organic soil 15N
Residue-derived mineral 15N increased rapidly in both soils for all residues and represented an important proportion of the initial 15N added (Fig. 4c, d). On day 180, significant differences were observed only between residues. Specifically, a greater proportion of the added 15N in soil, in the mineral 15N form, was observed for vetch (33.2 ± 6.0% of added 15N) compared to pea (20.9 ± 1.1% of added 15N) and wheat (17.6 ± 2.1% of added 15N). At the end of the experiment, the ranking was maintained with vetch > pea = wheat without any significant differences.
Overall, soil organic 15N increased rapidly until approximately 60 days, but with higher accumulation in wheat in the sandy-loam soil compared to pea and vetch (Fig. 4e). From day 60, there was little variation in the accumulation of organic 15N. After 360 days, the average soil organic 15N was 51.1 ± 12.7% of added 15N for wheat, which was significantly higher compared with vetch (31.4 ± 5.5% of added 15N) and pea (30.4 ± 7.1% of added 15N).
13C and 15N balances
The 13C balance, i.e., the sum of the 13C recovered in the various pools, was significantly higher in the sandy-loam soil than in the clay soil during the first 180 days of the incubation. The average recovery in the sandy-loam soil was 95 ± 2.6% compared with 87 ± 1.5% in the clay soil during this period (Table 4). However, on day 360, only a significant effect of residue type was observed: the 13C balance of vetch (88 ± 1.4% of added 13C; the average value for the two soils) was significantly lower than the wheat and pea treatments, which showed similar 13C recovery (100 ± 0.3% and 98 ± 2.2%, respectively) (Table 4).
Initially, the 15N balance did not differ significantly between the treatments. However, from day 60, 15N recovery for wheat treatment in the sandy-loam soil was higher than 100% of the added 15N. For the other two treatments, the 15N recovery ranged between 85% and 100% of added 15N. The higher recovery for wheat 15N in the sandy-loam soil resulted from a high 15N recovery in the 0–2.5-cm soil layer compared to the clay soil and the other residue types (Table 5).
Discussion
Effect of crop residue characteristics on C and N dynamics
We observed that the initial quality (as the initial N and soluble fraction contents) of the crop residues affected the short-term dynamics of the residue-C, which was expected and which agreed with previous studies (e.g., Martens 2000; Rasmussen et al. 2008). The mineralization of the residue-C, the C remaining in the residue particles and the residue-C recovered in soil varied with crop residue type in our study. The fastest decomposition was observed for vetch residues, which translated into higher C mineralization and greater C input into the soil over the short-term. According to the literature (e.g., Cobo et al. 2002; Abiven et al. 2005), this faster degradation in the early stages of decomposition is due to the presence of a large soluble fraction (SOL and Csw) combined with a high N content (N and Nsw) of crop residues. Compared to pea and wheat, the vetch tissues presented this favourable combination of factors that is known to support rapid microbial growth, which results in higher rates of decomposition as evidenced in several studies (Abiven et al. 2005; Thippayarugs et al. 2008) and higher diffusion of C and N into the underlying soil (Coppens et al. 2007). The C dynamics of wheat and pea did not differ despite significant differences in their initial tissues characteristics. While the C:N ratio of the pea residues (25.6) was lower than those of wheat C:N (51.0), both can be considered as limiting with respect to the growth of decomposing microorganisms. Other studies showed that decomposition is not limited provided the crop residue C:N ratio is less than 20 (Wang et al. 2015). Therefore, we assume that N availability limited wheat and pea decomposition at the soil surface, explaining the lack of response of degradation with respect to the initial tissue composition. This effect of residue quality was well modelled by the two-compartment model for C mineralization. Specifically, vetch had a larger C pool with rapid turnover, i.e., the labile pool (Wang et al. 2004), and a k1 mineralization rate two times higher than pea and wheat. However, the model suggests that while less C was allocated to the recalcitrant pool (C 2 ) for vetch, the rate of mineralization of this pool (k 2 ) did not differ between the three residues.
The effect of the residue quality was small and transient and after one year, the degree of decomposition of the three crop residues did not differ. Our results are in agreement with several studies showing that decay rates are rapid for residues with a high soluble fraction and are typically not sustained over the medium-term due to exhaustion of this most labile fraction (Cotrufo et al. 2013). Although the chemical quality of the residues is considered a key factor in stabilizing the SOM, results from the literature point to inconsistency regarding the effect of this factor on the accumulation of soil C (Gentile et al. 2011; Dungait et al. 2012; Castellano et al. 2015). However, the inconsistency could partly result from the fact that the crop residues were decomposing either on the soil surface or into the soil. Our results showed that at an annual scale, the initial quality of residues decomposing on the soil surface did not strongly impact the fate of added C.
However, the results were considerably different for the dynamics of N, which is a key issue in the recycling of crop residues in soils. Considerable differences in the net accumulation of mineral N were observed for the vetch and pea residues compared to the wheat residue. The N content of the plant tissues plays an important role in the process of decomposition when N limits microbial growth (Recous et al. 1995) but also determines the net balance between the simultaneous processes of gross N mineralization and immobilization (Jensen et al. 2005). In the vetch treatments, the higher initial residue N provided a higher availability of N to the soil microbial decomposers, and more inorganic N accumulated in the underlying soil. Conversely, for the wheat residue with a lower N content, N was taken up from the soil, probably by diffusion to the residue layer from colonizing fungal hyphae (Frey et al. 2000).
The difference in the N dynamics for the three residues is confirmed in this study by the 15N atom excess % dynamics of the recovered residue particles. Over time, we observed that the 15N enrichment decreased considerably for the wheat residue, indicating that part of the N measured in the remaining residue had a unlabelled N source, i.e., soil N, which was assimilated by the decomposers growing on the residue particles. Moreover, this effect was considerably reduced or not observed for pea and vetch where the residue-labelled N was available in sufficient amounts for microbial requirements throughout the incubation. When comparing the fate of residue-added N and C, we observed that a greater proportion of the residue-added N was retained as organic N in the soil or in remaining particles compared to the proportion of the residue-added C in these pools. This was expected, as a greater proportion of mineralized N was re-assimilated by the decomposers growing in the soil and on the residue particles, and this was probably enhanced by the fact that the residues were decomposing on the soil surface. Thus, we observed that the effects of the residue characteristics were considerably different when comparing the dynamics of C and N. Joint consideration of the N and C impacts of crop residue management is an important issue for agriculture because both affect the environment, particularly the greenhouse gas (GHG) balance.
Effect of soil type on C and N dynamics
There was no effect of soil type on N dynamics. However, the soil type affected the C mineralization rates until 180 days, but there was no effect after one year. On the short-term, higher C mineralization was observed in the sandy-loam than in the clay soil. This result is in line with that reported by Frøseth and Bleken (2015), who observed higher C mineralization of clover leaves incorporated into a sandy-loam soil compared to a clay soil. On the contrary, Finn et al. (2015) reported greater degradation of buffel grass, wheat and lucerne residues incorporated in a clay compared to a sandy-loam soil. The slower residue-C mineralization in clay soils is attributed to the protection of the soluble compounds in the residue and the reduction in the diffusion of enzymes to the decomposition sites promoted by clay (Frøseth and Bleken 2015).
The opposite effect of soil type was observed in the early stages of decomposition with respect to the 13C of the residue remaining pool, with faster depletion of the particle 13C pool in the clay soil compared to the sandy-loam soil, although the effect was transient. The clay soil, a fine textured soil, may have influenced the formation of a microenvironment more favourable for the microbes, thus enabling faster colonization of fresh litter by decomposer microorganisms. This effect could have been increased by the fact that the clay soil supported much higher initial microbial biomass than the sandy-loam soil (Table 1). Some results were also found in the literature confirming this process: higher decomposition of wheat, pea and sawdust residues was observed in clay soils in the short-term by Roychand and Marschner (2013). On the other hand, no significant effect of soil texture on the decomposition of crop residues was found by Scott et al. (1996) during a 91-day incubation period. The fact that we did not observe differences between the two soils in the medium-term and only a slight difference was observed in the short-term reinforces the idea that there is greater dependence of the decomposition rate of crop residues on their chemical composition than on the soil physicochemical characteristics (Manzoni et al. 2010; Finn et al. 2015), particularly the decomposition of crop residues on the surface.
It was expected that a larger amount of new C accumulated in the clay than in the sandy-loam soil because of the higher SOM stabilizing ability of fine-textured soils rich in oxides and phyllosilicates (Cotrufo et al. 2015), but this hypothesis was not confirmed by our results. In addition, Finn et al. (2015) did not observe differences in the stabilization of C derived from residues in different soils during a one-year incubation, whereas over a longer-term (3 to 17 years), the soil texture influences the stabilization of C in the field (Baer et al. 2010; de Oliveira et al. 2013). We believe that the time scale (one year) and the single pulse of crop residues in our study did not allow differences to be detected between the two soils in their ability to store new C, which is expected when comparing sandy-loam and clay soils. However, our results showed strong interactions between added C and “native” soil C, which should influence the medium-term C storage.
Priming effect: interaction between soil and added C
Despite little or no effect of soil type and litter quality on the fate of the added C at the scale of one year as discussed above, our study with 13C showed a remarkably strong interaction between the short-term (added residue-C) and long-term (soil C dynamics) fate of C, and this interaction was affected by residue quality and soil type. The mechanisms responsible for the PE are much more complex than is commonly believed, and the use of enriched crop residues is necessary to quantify and understand this process (Fontaine et al. 2003). It has been recently suggested that extra CO2 mineralization due to the PE is the result of different mechanisms, sometimes antagonists, interacting together (Blagodatskaya and Kuzyakov 2008; Guenet et al. 2010). In this study, the PE was greater with a higher initial soluble C pool of the residue (vetch) - confirming our initial hypothesis - and this priming was even stronger than when the C content and soil microbial biomass were high (i.e., in the clay soil).
Residue addition can stimulate soil priming by providing a labile C source for the soil microbial biomass (Kuzyakov 2010). The higher PE observed with vetch and pea compared to wheat suggests that the intensity of the PE resulted from the ability of the added C to interact with the soil C, which is favoured with a substrate having a higher soluble C. This allowed greater interactions with soil C, which is important in no-till systems where crop residues are maintained on the soil surface. In the clay soil, both the higher C content and the higher initial microbial biomass pool might allow a greater PE, as observed, but only when soluble C was available (wheat < pea < vetch). A series of long-term studies summarized by Kuzyakov (2002) showed that the PE was higher in clay than in sandy-loam soils and higher in C rich than in C poor soils. Likewise, Frøseth and Bleken (2015) concluded that soil type influences the PE; a greater PE was observed for clay soils compared with sandy-loam soils, both of which were incubated with clover residues for 142 days.
The balance between new C input and C losses due to the PE indicates that the net C balance can be negative. This is the case in this study for vetch in both soils and pea in the clay soil. This possibility of a negative C balance was earlier discussed by Fontaine et al. (2004, 2011) and Paterson and Sim (2013), and other authors such as Hoosbeek and Scarascia-Mugnozza (2009) observed a net decrease in SOC. Many authors have, however, reported a positive C balance (Hoosbeek et al. 2004; Qiao et al. 2014), which is in agreement with our results for the wheat residues. Our results therefore suggest that the C balance is strongly controlled by the nature of the added residues, with a risk of high extra C mineralization with crop residues containing labile C, e.g., non-mature crop residues such as cover crops. Considering both the C and N impacts of recycling residues, cover crops, which are often used to efficiently capture and recycle soil mineral N, could be less efficient in storing C in the soil depending on their chemical characteristics (Tribouillois et al. 2015).
Conclusions
This study confirms that the quality of crop residues, related to their initial chemical composition, affects the short-term dynamics of crop residue decomposition, the fate of residue-C and the dynamics of residue-N and soil N. However, the effects of the residue characteristics differed considerably when comparing the C and N dynamics. This occurred because there was a transient effect on the C dynamics when there were small differences between the crop residues, while the decomposition of the three crop residues rapidly led to large differences in soil mineral N accumulation, due to N mineralization-immobilization processes during decomposition, which were maintained over the medium-term. Extra mineralization of soil C due to residue decomposition, quantified using 13C residues, showed that the PE responded significantly to the quality of the crop residue, the soil type and their interaction. The greater the initial soluble C fraction, the greater the PE, which ultimately caused a negative balance in the soil organic C at the scale of the duration of the experiment, i.e., one year at 25 °C. This work emphasizes the need for setting up cropping systems with crop rotations (based on different species and botanical families) that allow optimization of the trade-off between several ecosystem services.
References
Abiven S, Recous S, Reyes V, Oliver R (2005) Mineralisation of C and N from root, stem and leaf residues in soil and role of their biochemical quality. Biol Fertil Soils 42:119–128. doi:10.1007/s00374-005-0006-0
Baer SG, Meyer CK, Bach EM, Klopf RP, Six J (2010) Contrasting ecosystem recovery on two soil textures: implications for carbon mitigation and grassland conservation. Ecosphere 1:1–22. doi:10.1890/ES10-00004.1
Bird JA, Kleber M, Torn MS (2008) 13C-and 15N stabilization dynamics in soil organic matter fractions during needle and fine root decomposition. Org Geochem 39:465–477. doi:10.1016/j.orggeochem.2007.12.003
Blagodatskaya Е, Kuzyakov Y (2008) Mechanisms of real and apparent priming effects and their dependence on soil microbial biomass and community structure: critical review. Biol Fertil Soils 45:115–131. doi:10.1007/s00374-008-0334-y
Blagodatsky S, Blagodatskaya E, Yuyukina T, Kuzyakov Y (2010) Model of apparent and real priming effects: linking microbial activity with soil organic matter decomposition. Soil Biol Biochem 42:1275–1283. doi:10.1016/j.soilbio.2010.04.005
Brooks PD, Stark JM, McInteer BB, Preston T (1989) Diffusion method to prepare soil extracts for automated nitrogen-15 analysis. Soil Sci Soc Am J 53:1707–1711. doi:10.2136/sssaj1989.03615995005300060016x
Castellano MJ, Mueller KE, Olk DC, Sawyer JE, Six J (2015) Integrating plant litter quality, soil organic matter stabilization, and the carbon saturation concept. Glob Change Biol 21:3200–3209. doi:10.1111/gcb.12982
Cobo JG, Barrios E, Kass DCL, Thomas RJ (2002) Decomposition and nutrient release by green manures in tropical hillside agroecosystem. Plant Soil 240:331–342. doi:10.1023/A:1015720324392
Coppens F, Garnier P, Findeling A, Merckx R, Recous S (2007) Decomposition of mulched versus incorporated crop residues: modelling with PASTIS clarifies interactions between residue quality and location. Soil Biol Biochem 39:2339–2350. doi:10.1016/j.soilbio.2007.04.005
Cotrufo MF, Wallenstein MD, Boot CM, Denef K, Paul E (2013) The microbial efficiency-matrix stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: do labile plant inputs form stable soil organic matter? Glob Change Biol 19:988–995. doi:10.1111/gcb.12113
Cotrufo MF, Soong JL, Horton AJ, Campbell EE, Haddix M, Wall DH, Parton WJ (2015) Formation of soil organic matter via biochemical and physical pathways of litter mass loss. Nat Geosci 8:776–779. doi:10.1038/ngeo2520
de Bruijn AMG, Butterbach-Bahl K (2010) Linking carbon and nitrogen mineralization with microbial responses to substrate availability—the DECONIT model. Plant Soil 328:271–290. doi:10.1007/s11104-009-0108-9
de Oliveira FA, Amado TJC, da Silveira NR, de Moraes Sá JC, Fiorin JE, Hansel DS, Menefee D (2013) Soil carbon stratification affected by long-term tillage and cropping systems in southern Brazil. Soil Tillage Res 133:65–74. doi:10.1016/j.still.2013.05.011
Dungait JAJ, Hopkins DW, Gregory AS, Whitmore AP (2012) Soil organic matter turnover is governed by accessibility not recalcitrance. Glob Change Biol 18:1781–1796. doi:10.1111/j.1365-2486.2012.02665.x
Finn D, Page K, Catton K, Strounina E, Kienzle M, Robertson F, Armstrong R, Dalal R (2015) Effect of added nitrogen on plant litter decomposition depends on initial soil carbon and nitrogen stoichiometry. Soil Biol Biochem 91:160–168. doi:10.1016/j.soilbio.2015.09.001
Fontaine S, Mariotti A, Abbadie L (2003) The priming effect of organic matter: a question of microbial competition? Soil Biol Biochem 35:837–843. doi:10.1016/S0038-0717(03)00123-8
Fontaine S, Bardoux G, Abbadie L, Mariotti A (2004) Carbon input to soil may decrease soil carbon content. Ecol Lett 7:314–320. doi:10.1111/j.1461-0248.2004.00579.x
Fontaine S, Henault C, Aamor A, Bdioui N, Bloor JMG, Maire V, Mary B, Revaillot S, Maron PA (2011) Fungi mediate long term sequestration of carbon and nitrogen in soil through their priming effect. Soil Biol Biochem 43:86–96. doi:10.1016/j.soilbio.2010.09.017
Frey SD, Elliott ET, Paustian K, Peterson GA (2000) Fungal translocation as a mechanism for soil nitrogen inputs to surface residue decomposition in a no-tillage agroecosystem. Soil Biol Biochem 32:689–698. doi:10.1016/S0038-0717(99)00205-9
Frøseth RB, Bleken MA (2015) Effect of low temperature and soil type on the decomposition rate of soil organic carbon and clover leaves, and related priming effect. Soil Biol Biochem 80:156–166. doi:10.1016/j.soilbio.2014.10.004
Gentile R, Vanlauwe B, Kavoo A, Chivenge P, Six J (2010) Residue quality and N fertilizer do not influence aggregate stabilization of C and N in two tropical soils with contrasting texture. Nutr Cycl Agroecosystems 88:121–131. doi:10.1007/s10705-008-9216-9
Gentile R, Vanlauwe B, Six J (2011) Litter quality impacts short- but not long-term soil carbon dynamics in soil aggregate fractions. Ecol Appl 21:695–703. doi:10.1890/09-2325.1
Guenet B, Neill C, Bardoux G, Abbadie L (2010) Is there a linear relationship between priming effect intensity and the amount of organic matter input? Appl Soil Ecol 46:436–442. doi:10.1016/j.apsoil.2010.09.006
Hoosbeek MR, Scarascia-Mugnozza GE (2009) Increased litter build up and soil organic matter stabilization in a poplar plantation after 6 years of atmospheric CO2 enrichment (FACE): final results of POP-EuroFACE compared to other forest FACE experiments. Ecosystems 12:220–239. doi:10.1007/s10021-008-9219-z
Hoosbeek MR, Lukac M, van Dam D, Godbold DL, Velthorst EJ, Biondi FA, Peressotti A, Cotrufo MF, de Angelis P, Scarascia-Mugnozza G (2004) More new carbon in the mineral soil of a poplar plantation under free air carbon enrichment (POPFACE): cause of increased priming effect? Glob Biogeochem Cycles 18:1–7
Jensen LS, Salo T, Palmason F, Breland TA, Henriksen TM, Stenberg B, Pedersen A, Lundström C, Esala M (2005) Influence of biochemical quality on C and N mineralisation from a broad variety of plant materials in soil. Plant Soil 273:307–326. doi:10.1007/s11104-004-8128-y
Keeney DR, Nelson DW (1982) Nitrogen in organic forms. In: Page AL (ed) Methods of soil analysis: part 2. Chemical and microbiological, 2nd edn. American Society of Agronomy, Madison, WI, pp 643–698
Kögel-Knabner I, Guggenberger G, Kleber M, Kandeler E, Kalbitz K, Scheu S, Eusterhues K, Leinweber P (2008) Organo-mineral associations in temperate soils: integrating biology, mineralogy, and organic matter chemistry. J Plant Nutr Soil Sci 171:61–82. doi:10.1002/jpln.200700048
Kuzyakov Y (2002) Review: factors affecting rhizosphere priming effects. J Plant Nutr Soil Sci 165:382–396. doi:10.1002/1522-2624(200208)165:4<382::AID-JPLN382>3.0.CO;2-#
Kuzyakov Y (2010) Priming effects: interactions between living and dead organic matter. Soil Biol Biochem 42:1363–1371. doi:10.1016/j.soilbio.2010.04.003
Manzoni S, Trofymow JA, Jackson RB, Porporato A (2010) Stoichiometric controls on carbon, nitrogen, and phosphorus dynamics in decomposing litter. Ecol Monogr 80:89–106. doi:10.1890/09-0179.1
Martens D (2000) Plant residue biochemistry regulates soil carbon cycling and carbon sequestration. Soil Biol Biochem 32:361–369. doi:10.1016/S0038-0717(99)00162-5
Paterson E, Sim A (2013) Soil-specific response functions of organic matter mineralization to the availability of labile carbon. Glob Change Biol 19:1562–1571. doi:10.1111/gcb.12140
Plante AF, Parton WJ (2007) The dynamics of soil organic matter and nutrient cycling. In: Paul EA (ed) Soil microbiology, ecology, and biochemistry, 3rd edn. Academic Press, Amsterdam, pp. 433–464
Qiao NA, Schaefer D, Blagodatskaya E, Zou X, Xu X, Kuzyakov Y (2014) Labile carbon retention compensates for CO2 released by priming in forest soils. Glob Change Biol 20:1943–1954. doi:10.1111/gcb.12458
Rasmussen C, Southard RJ, Horwath WR (2008) Litter type and soil minerals control temperate forest soil carbon response to climate change. Glob Change Biol 14:2064–2080. doi:10.1111/j.1365-2486.2008.01639.x
Rasse DP, Rumpel C, Dignac M (2005) Is soil carbon mostly root carbon? Mechanisms for a specific stabilisation. Plant Soil 269:341–356. doi:10.1007/s11104-004-0907-y
Recous S, Robin D, Darwis D, Mary B (1995) Soil inorganic N availability: effect on maize residue decomposition. Soil Biol Biochem 27:1529–1538. doi:10.1016/0038-0717(95)00096-W
Redin M, Guénon R, Recous S, Schmatz R, de Freitas LL, Aita C, Giacomini SJ (2014) Carbon mineralization in soil of roots from twenty crop species, as affected by their chemical composition and botanical family. Plant Soil 378:205–214. doi:10.1007/s11104-013-2021-5
Roychand P, Marschner P (2013) Respiration in a sand amended with clay – effect of residue type and rate. Eur J Soil Biol 58:19–23. doi:10.1016/j.ejsobi.2013.05.005
Rubino M, Dungait JAJ, Evershed RP, Bertolini T, De Angelis P, D’Onofrio A, Lagomarsino A, Lubritto C, Merola A, Terrasi F‘A (2010) Carbon input belowground is the major C flux contributing to leaf litter mass loss: evidences from a 13C labelled-leaf litter experiment. Soil Biol Biochem 42:1009–1016. doi:10.1016/j.soilbio.2010.02.018
Scott NA, Cole CV, Elliott ET, Huffman SA (1996) Soil textural control on decomposition and soil organic matter dynamics. Soil Sci Soc Am J 60:1102–1109. doi:10.2136/sssaj1996.03615995006000040020x
Soil Survey Staff (2010) Keys to soil taxonomy, 11th edn. USDA Natural Resources Conservation Service, Washington, DC
Sollins P, Swanston C, Kramer M (2007) Stabilization and destabilization of soil organic matter - a new focus. Biogeochemistry 85:1–7. doi:10.1007/s10533-007-9099-x
Stevenson FJ (1994) Humus chemistry: genesis, composition, reactions, 2nd edn. John Wiley and Sons, New York, 1994
Tahir MM, Recous S, Aita C, Pilecco GE, Schmatz R, Giacomini SJ (2016) In situ roots decompose faster than shoots left on the soil surface under subtropical no-till conditions. Biol Fert Soils 52:853–865. doi:10.1007/s00374-016-1125-5
Thippayarugs S, Toomsan B, Vityakon P, Limpinuntana V, Patanothai A, Cadisch G (2008) Interactions in decomposition and N mineralization between tropical legume residue components. Agroforest Syst 72:137–148. doi:10.1007/s10457-007-9062-9
Tribouillois H, Cruz P, Cohan J, Justes É (2015) Modelling agroecosystem nitrogen functions provided by cover crop species in bispecific mixtures using functional traits and environmental factors. Agric Ecosyst Environ 207:218–228. doi:10.1016/j.agee.2015.04.016
Trinsoutrot I, Recous S, Bentz B, Linères M, Chèneby D, Nicolardot B (2000) Biochemical quality of crop residues and carbon and nitrogen mineralization kinetics under non limiting nitrogen conditions. Soil Sci Soc Am J 64:918–926. doi:10.2136/sssaj2000.643918x
Wang WJ, Baldock JA, Dalal RC, Moody PW (2004) Decomposition dynamics of plant materials in relation to nitrogen availability and biochemistry determined by NMR and wet-chemical analysis. Soil Biol Biochem 36:2045–2058. doi:10.1016/j.soilbio.2004.05.023
Wang H, Boutton TW, Xu W, Hu G, Jiang P, Bai E (2015) Quality of fresh organic matter affects priming of soil organic matter and substrate utilization patterns of microbes. Sci Rep 5:10102. doi:10.1038/srep10102
Acknowledgements
This work was supported by the Brazilian government through the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). The bilateral Brazilian and French collaboration was funded under Program CNPq—Ciência sem Fronteiras, Process Number 207530/2015-7, and by INRA (the Environment & Agronomy Division) during Schmatz’s leave at UMR FARE in Reims, France.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Elizabeth M Baggs.
Rights and permissions
About this article
Cite this article
Schmatz, R., Recous, S., Aita, C. et al. Crop residue quality and soil type influence the priming effect but not the fate of crop residue C. Plant Soil 414, 229–245 (2017). https://doi.org/10.1007/s11104-016-3120-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-016-3120-x