Abstract
Particle-size soils were fractionated for evaluating changes in the composition of bacterial community and enzyme activity in response to 13 years of fertilization. This study focused on Mollisol and its particle-size fractions of 200–2,000 μm (coarse sand sized), 63 to 200 μm (fine sand sized), 2 to 63 μm (silt sized), and 0.1 to 2 to μm (clay-sized). Long-term chemical fertilization lowered the pH of all particle fractions, whereas organic fertilizer application mitigated soil acidification. Nutrient concentrations depended on both fertilizer treatment and particle fractions and enzymes were unevenly active throughout the soil. Generally, the highest enzyme activities were observed in the silt and clay fractions of control soil and the soil treated with chemical fertilizer (N, P, and K (NPK)) and in the sand-sized fraction of soil treated with manure and chemical fertilizer (MNPK). Except for acid phosphomonoesterase, the other tested enzyme activities in coarse-sized fractions of MNPK soil were significantly higher than those of the control and NPK soils. Fertilization and soil fraction interactively (p < 0.05) affected the enzyme activity. Denaturing gradient gel electrophoresis analysis showed that the bacterial community structure significantly differed in different particle sizes with a higher bacterial diversity in small-sized than in coarse-sized fractions. Dominant bands were excised and sequenced. We have found the following bacterial groups: Actinobacteria, γ-proteobacteria, and Acidobacteria. In addition, enrichment of organic matter in coarser fractions was related to greater bacterial diversity than any other treatment. Principal component analysis showed a smaller variability among fractions of the organic amended treatment. Redundancy analysis showed that the tested properties significantly affected the composition of bacterial community with the exception of C/N and available P. No significant correlation between enzyme activity and bacterial community composition was detected, whereas positive correlations between other soil properties and enzyme activities were observed to various extents. Probably, enzyme activities might be affected by specific functional bacterial communities rather than by the overall bacterial community. We concluded that the long-term application of organic manures contributed to the increase of soil organic matter content of particles higher than 200 mm, with higher bacterial diversity and increases in most of the enzyme activities.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mollisol (Black soil) are the most fertile and productive soils in China (Wang et al. 2009) and are distributed mainly in the Heilongjiang and Jinlin Provinces in Northeast China Plain. These soils, often called “the king of soils” in China, are rich in organic matter (SOC) (Ding et al. 2012). In recent decades, a significant depletion of C stocks in the soil occurred due to intensive tillage and cultivation, with low return of organic matters. The serious losses in soil fertility are threatening sustainable production of grains in Northeast China Plain. To maintain or restore the fertility of Mollisols, several long-term experiments involving agronomic practices have been conducted in the Northeast China Plain with changes in crop yield and soil chemical properties by varying soil managements (Wang et al. 2009).
Under these conditions, organic amendment can be an important strategy, which has been widely reported to improve soil organic content and soil fertility by improving soil structure, microbial activity, and nutrient status (Ai et al. 2013; Li et al. 2010). Most organic residues added into soil contain polymeric compounds, and thus the decomposition of these residues depends on the microbial production of extracellular enzymes and their break down should occur before taking up of low molecular weight organic molecules by microbial cells (Allison and Jastrow 2006; Nannipieri et al. 2012). Soil organic matter (SOM) dynamics and turnover, along with other factors, are also affected by the types of the inputs and their position within the soil matrix (Kandeler et al. 1999). Therefore, the specific locations of enzyme activities within the soil matrix have attracted attention, especially as these enzyme activities are affected by SOM quality (Kandeler et al. 1999) and turnover (Stemmer et al. 1998a). Different soil particle-size fractions can show different role in SOM dynamics and turnover (Allison and Jastrow 2006; Kandeler et al. 1999). In addition, enzyme activities depend on the soil microbial diversity (Nannipieri et al 2012). However, it is not known how microbial diversity and enzyme activities of different soil fractions of Mollisol are affected by fertilization.
Fertilizer application can affect both diversity and activity of microbial communities (Yevdokimov et al. 2008; Zhong et al. 2010). Both changes in the bacterial community composition and enzyme synthesis has been often studied in bulk soil (Renella et al. 2006), whereas information on the specific locations of enzyme activities and their relationship to the diversity of bacterial populations within the soil structure is not known. Biochemical and microbiological characterization of soil size fractions after soil physical fractionation can provide an insight of changes in SOM quality and turnover under different managements (Stemmer et al. 1998a, b; Kandeler et al. 1999; Allison and Jastrow 2006; Lagomarsino et al. 2011, 2012). Information on the enzyme activity and bacterial communities and their nutrient allocation in the structure of Mollisols has not been studied so far.
Therefore, we have carried out a study on the composition of bacteria and enzyme activities of different particle-size fractions of a Mollisol after long-term fertilization with the application of either inorganic fertilizers or inorganic and organic mixed fertilizers. We employed a physical fractionation procedure based on the low-energy sonication to obtain soil particle-size fractions. Bacterial diversity was determined by denaturing gradient gel electrophoresis (DGGE) analysis. We determined the following enzyme activities: acid phosphomonoesterase, sulfatase, β-glucosidase, β-cellobiosidase, N-acetyl-glucosaminidase, β-xylosidase, and α-glucosidase because they are linked to C, N, P, and S transformation in soil. Dominant bands were excised from gel before being sequenced. The aim was to study how the relationship between the composition of bacterial communities and enzyme activities can be affected by adding inorganic fertilizers with or without organic manure to soil.
Materials and methods
Field design and sampling
A long-term field fertilization experiment was initiated in 1990 at Gongzhuling Agro-ecological Experimental Station (124° 48′ 33.9″ E, 43° 30′ 23″ N) located in the Northeast China Plain in a typical plain agricultural area of Jilin Province, China. This region has a temperate and semi-humid climate with an annual average temperature of 4–5 °C and 450–600 mm of precipitation. The cropping regime is dominated by one maize crop per year. The soil was classified as a Mollisol developed from Quaternary loess-like sediments. At the beginning of the experiment, the soil had a pH (H2O) of 7.6, 22.8 g/kg organic matter, 1.40 g/kg total N (TN), 27 mg/kg available P (AP), and 190 mg/kg available K (AK). The experiment included three treatments (with three replicates per each treatment) by using 400 m2 plots. The treatments were: soil with no fertilizer application (control (CK)), soil treated with chemical fertilizer application containing N, P, and K (NPK), and soil treated with organic manure and chemical fertilizer (MNPK). Chemical fertilizers were applied as annual rate of 165 kg N ha−1, 82.5 kg P2O5 ha−1, and 82.5 kg K2O ha−1. The N, P, and K were applied as urea, superphosphate, and potassium chloride, respectively. In the MNPK treatment, the same rates of chemical fertilizers as NPK treatment were used in addition to 60,000 kg/ha of organic manures (pig manure). Organic manure contained 10–15 % organic matter, 0.15–0.55 % TN, 0.1–0.5 % P2O5, 0.35–0.45 % K2O, and 35–40 % water content.
Soil samples from the three replicates of each treatment were collected before planting maize in May. Ten soil cores (5 cm diameter) were collected at a depth of 0–20 cm from each plot. All samples were carefully mixed to form a composite sample. Moist soils were gently broken apart along the natural break points and passed through a 2-mm sieve to remove visible organic debris. After thoroughly mixing, the field-moist soil was used for particle-size fractionation.
Fractionation procedure
The size fractionation procedure was carried out by the method of Stemmer et al. (1998b). Briefly, the soil–water suspension was dispersed by low-energy sonication (output energy of 0.2 kJ/g) and subsequently fractionated by a combination of wet sieving and repeated centrifugation to avoid disruption of micro-aggregates. Finally, four fractions were obtained for each sample: (1) coarse sand-sized fraction (200 to 2,000 μm), fine sand-sized fraction (63 to 200 μm), silt-sized fraction (2 to 63 μm), and clay-sized fraction (0.1 to 2 μm). Field-moist soils (140 g equivalent dry weight for each sample) were suspended in 400 ml of distilled water and then equally placed into four 150-ml glass beakers, and the resulting same size soil fractions from the glass beakers of the same sample were pooled together. The fractions were freeze-dried and then analyzed for soil chemical properties, soil enzyme activities, and bacterial diversity.
Soil analysis
Soil pH was measured in a 1:2.5 soil/water ratio solution with a compound electrode (PE-10, Sartorious, Germany). SOM was determined by the method of dichromate oxidation, and TN by a vario MACRO cube element analyzer (Elementar Analysensysteme, Germany). The AP was extracted by sodium bicarbonate and then determined by the molybdenum-blue method (Zhong et al 2010). The AK was analyzed using a flame atomic absorption spectrophotometer (Zhong et al 2010).
Enzyme activity
Seven enzyme (acid phosphomonoesterase, sulfatase, β-glucosidase, β-cellobiosidase, N-acetyl-glucosaminidase, β-xylosidase, and α-glucosidase) activities were measured using 4-methylumbelliferyl-esters as substrates, producing fluorescent 4-methylumbeliferone (MUF) after hydrolysis (Deng et al. 2013). All the substrates were purchased from Sigma Company. Briefly, each soil suspension was prepared by mixing 1 g of soil with 120 ml of deionized water. Aliquots (100 μl each) of the soil suspension were placed into microplate wells that each contained 50 μl of modified universal buffer at pH optimal for the assayed enzyme activities. Subsequently, 50 μl of 5-mM MUF-labeled substrate solutions were added to each microplate well. The well contents were mixed by pipetting and discharging several times before incubating the microplate at 37 °C for 1 h. A 96-well microplate was used for measurements following the detailed procedure described by Deng et al. (2011) and Ai et al. (2012). The fluorescence intensity was quantified using a microplate fluorometer (Scientific Fluoroskan Ascent FL, Thermo) with 365 nm excitation and 450 nm emission filters. Enzyme activities were expressed by nanomoles per hour per gram.
DNA extraction, PCR amplification, and DGGE analysis
The total DNA of each fraction was extracted using an Ultra Clean™ Soil DNA Isolation Kit (MOBIO Laboratories, Carlsbad, CA, USA) according to the manufacturer’s instructions. DNA was finally eluted with 100 ml of the DNA elution solution included in the kit. DNA size was checked by electrophoresis experiments on 1 % agarose gels.
The diversity of soil bacterial community was determined by the PCR-DGGE technique, after PCR amplification. Each sample was replicated for three times in three independent gels. The PCR primers nucleotide sequences were: U968-GC (5′-GC-Clamp-[CGC CCG GGG CGC GCC CCG GGC GGG GCG GGGGCA CGG GGG G]-AAC GCG AAG AAC CTT AC-3′) and L1401 (5′-GCG TGT GTA CAA GAC CC-3′). PCR mixtures consisted of 12.5 ml PCR Premix Taq™ (Takara Bio INC., Dalian, China) containing a 0.5-mM concentration of each primer and 1 μl of DNA template diluted to a final volume of 25 ml. The DGGE analysis was conducted according to Muyzer et al. (1993) using a DCode system (Bio-Rad Laboratories, Hercules, CA, USA).
Sequencing of the dominant DGGE band
The dominant bands were excised from the gel, and the DNA was extracted with 20 μl sterile water. The supernatant (1 μl) was used as the template in the re-amplification PCR using the U968 and L1401 primers without a GC clamp (Muyzer et al. 1993). The DNA was sequenced by Genescript Company (Nanjing, China) and aligned in the NCBI GenBank by the BLAST program. The nucleotide sequences of the bands excised from the DGGE gel have been deposited in the GenBank databases with accession numbers: KF789464–KF789493. A phylogenetic tree among the homologous sequences was constructed using the neighbor-joining method and the MEGA software package (version 3.1). Bootstrap support (>50 %) from 1,000 replications was displayed at the nodes of the trees (Yong et al. 2011).
Statistical analysis
Statistical analysis was performed using IMB SPSS statistics version 20 (IBM Corporation, New York, USA). One-way ANOVA using Fisher’s least significant difference (p < 0.05) was analyzed to compare the means for each variable. Two-way ANOVA was used to compare the soil fractions and fertilization treatments.
DGGE bands were analyzed with Quantity One software (p < 0.05; version 4.6.3, Bio-Rad). The relative intensity of a specific band was expressed as the ratio between the intensity of that band and the total intensity of all bands in that lane. The intensities were used for determining the Shannon–Wiener diversity index (H′) and were calculated by the formula H′ = −∑p ilnp i = −∑(n i/N)ln(n i/N), where p i is the ratio between the number in a specific group and the total number, n i is the intensity of a band, and N is the sum of all band intensities in the densitometry profile.
Principal component analysis (PCA) was carried out by Canoco for Windows (version 4.5). Redundancy analysis (RDA) was carried by Canoco for Windows version 4.5 with the Monte Carlo permutations test (499 permutations) to determine whether the bacterial community structures could be correlated to enzyme activity and properties of soil.
Results
Properties of soil
The soil pH and nutrient concentrations determined after fractionation of soils from different long-term fertilization are shown in Table 1. The pH values were significantly affected by long-term fertilizer application, and they were lower in the NPK-treated soil than in the control soil and MNPK-treated soil. The lowest pH value was found in clay samples treated with NPK (pH = 6.04). The total or available nutrient concentrations were the highest in the clay fractions regardless of the fertilization type, with the exception of AP. Long-term organic amendment (MNPK) significantly increased SOM, TN, AP, and AK contents of all soil fractions. No similar trend was observed for the C/N of soil fractions. Two-way ANOVA analysis (Table 2) revealed that both fertilization and soil fractionation significantly affected soil pH and nutrient concentrations with the exception of AK.
Enzyme activity
The activities of seven soil enzymes of all soil fractions exhibited different trends according to the fraction and fertilization type. The maximum acid phosphomonoesterase activity was found in the clay-sized fractions of CK and MNPK-treated soil and in the silt-sized fraction of the NPK-treated soil. The α-glucosidase, β-glucosidase, β-d-xylosidase, N-acetyl-glucosaminidase, and β-cellobiosidase activities were higher in silt-sized fractions than in other fractions of CK and NPK soils and were higher in coarse sand-sized fractions than in other fractions of the MNPK soil (Table 3). The sulfatase activity increased with the decrease of soil particle size in CK and NPK soils (other than in MNPK), but the maximum sulfatase activity was found in the clay-sized fractions for each treatment. In coarse sand-sized fraction of the MNPK soil, the activities of α-glucosidase, β-glucosidase, β-d-xylosidase, N-acetyl-glucosaminidase, sulfatase, and β-cellobiosidase were significantly higher than in those from CK and NPK treatments.
Both fertilization and soil fractions had significant effects on soil enzymes activities, and their interactions also had a remarkable effect on the enzyme activities, as assessed by two-way ANOVA (Table 4).
Community structure of bacteria in fractions of treated soil
DGGE analysis was performed to monitor changes in the composition of soil bacterial communities of particle-size fractions of the Mollisol after long-term fertilization. The analysis of replicates showed negligible variation among replicated gels (shown in Figs. S1 and S2), and thus we only showed one replicate (Fig. 1). Differences in the DGGE profiles were found in the different treated samples, and the microbial composition varied among the size fractions. As shown in the similarity dendrograms, the fine particle sizes (silt and clay) of different treatments had similar bacterial communities, whereas the impact of fertilization on bacterial community composition was mainly observed in the larger (especially in coarse sand) than smaller particle-size fractions.
DGGE profiles and similarity dendrogram (neighbor joining) from DGGE analysis of different fractions of treated soils. CK soil with no fertilizer application, NPK soil treated with chemical fertilizer application containing N, P, and K, MNPK soil treated with organic manure and chemical fertilizer. Numbers (1, 2, 3, and 4) following the treatment indicate the particle sizes of coarse sand, fine sand, silt, and clay particles, respectively. Bands with the number are those excised, re-amplified, and sequenced
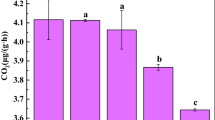
The bacterial diversity of coarse sand-size fraction, as indicated by the Shannon–Weaver index (H′), was the highest in the MNPK soil (Fig. 2). In all treatments, the smaller particle-size fractions generally showed the richest bacterial diversity. The bacterial diversity of the NPK soil was the lowest than the control soil (Fig. 2), whereas those of fractions of MNPK soil were the highest.
The Shannon–Weaver index (H′) of bacterial diversity of the soil bacterial community in different soil fractions from different fertilizer-treated soils. CK soil with no fertilizer application, NPK soil treated with chemical fertilizer application containing N, P, and K, MNPK soil treated with organic manure and chemical fertilizer. The bars indicate the standard errors of the mean
The numbered bands of the DGGE lanes (Fig. 1) were sequenced for bacterial phylogenetic analysis (Fig. 3). Thirty dominant clones were obtained and could be classified into three main groups (Phylum): Actinobacteria, γ-Proteobacteria, and Acidobacteria. However, most of the sequenced bands were not aligned to any of known bacterial species probably being of uncultured bacteria. Bands 10, 11, and 19 were commonly present in all lanes. The first two bands belong to Acidobacteria, and the last one to γ-proteobacteria. Interestingly, a high intensity unclassified band (band 17) observed in the slit- and clay-size fractions was only detected in the NPK soil.
The PC1 and PC2 accounted for 21.9 and 19.3 %, respectively, of the total variation when the PCA of all DGGE bands was carried out. The PC scores on axes were well decentralized on the basis of soil particle-size fractions or fertilization treatments (Fig. 4). There were, however, smaller changes in bacterial diversity among soil fractions of the organic fertilizer-amended MNPK soil than in the other two soils (Fig. 4).
Plot of the first two principle components (PC1 and PC2) grouped in the fractions of treated soils according to bacterial diversity. CK soil with no fertilizer application, NPK soil treated with chemical fertilizer containing N, P, and K, MNPK soil treated with organic manure and chemical fertilizer. Numbers (1, 2, 3, and 4) following the treatment indicate the particle sizes of coarse sand, fine sand, silt, and clay, respectively
Correlations of soil properties and bacterial community structures
Results of the RDA between soil properties with bacterial community structure are shown in Fig. 5. The first and second axes accounted for 21.1 and 16.8 %, respectively, of the total variation in bacterial community structure. The bacterial community was significantly correlated with pH (F = 2.207, P = 0.004) and OM (F = 1.901, P = 0.0320), TN (F = 1.855, P = 0.014), and AK (F = 1.937, P = .0120) contents. On the contrary, C/N (F = 1.225, P = 0.1860) and AP (F = 1.169, P = 0.2580) content had no significant correlation with bacterial diversity of different particle-size fractions of the three soils.
Correlations between soil properties and composition of bacterial community as determined by redundancy analysis (RDA). CK soil with no fertilizer application, NPK soil treated with chemical fertilizer containing N, P, and K, MNPK soil treated with organic manure and chemical fertilizer. Numbers (1, 2, 3, and 4) following the treatment indicate the particle sizes of coarse sand, fine sand, silt, and clay, respectively
Correlations
The RDA showed the relationships of soil enzyme activities with bacterial community structures (Fig. 6), and the first and second axes accounted for 25.4 and 14.5 % of the total variation. All soil enzyme activities and that is acid phosphomonoesterase (F = 1.317, P = 0.1640), sulfatase (F = 1.342, P = 0.1660), α-glucosidase (F = 1.108, P = 0.3320), β-glucosidase (F = 0.962, P = 0.4860), β-d-xylosidase (F = 1.121, P = 0.3160), N-acetyl-glucosaminidase (F = 0.986, P = 0.428), and β-cellobiosidase (F = 0.750, P = 0.7560) activities of different soil fractions, were not significantly correlated with bacterial diversity after long-term fertilization.
Correlations of soil enzyme activities and bacterial community structure as determined by redundancy analysis (RDA). CK soil with no fertilizer application, NPK soil treated with chemical fertilizer containing N, P, and K, MNPK soil treated with organic manure and chemical fertilizer. Numbers (1, 2, 3, and 4) following the treatment indicate coarse sand, fine sand, silt, and clay particles, respectively
The RDA was carried out for enzyme activities and soil properties of soil fractions of the three soils. Soil properties were used as environmental variables (Fig. 7). The first and second axes accounted for 42.8 and 16.1 % of the total variation between enzyme activity and soil properties. The AP contents were significantly and negatively correlated (P < 0.05) with acid phosphomonoesterase activity. The other soil properties showed positive correlation with all enzyme activities.
Discussion
This research revealed the effects of inorganic fertilizers with or without organic amendment on the relationship between composition of bacterial communities and enzyme activities and nutrient content of different soil fractions of a Mollisol. The results revealed that 13 years of chemical fertilizer application to the Mollisol soil decreased significantly (P < 0.05) the pH, which confirms that the overuse of N fertilizer contributes to soil acidification in China (Guo et al. 2010). The acidification occurred in all soil fractions after long-term application of chemical fertilizer with the lowest effects by the organic manure amendment. Moreover, organic matter, TN, AP, and AK contents of all particle-size fractions were significantly (P < 0.05) increased by long-term organic amendment. It is well established that the long-term organic fertilization can increase the organic matter, soil nutrient (N, P, and K) contents, and both soil microbial biomass and activity (Bastida et al. 2008; Elfstrand et al. 2007). Therefore, organic fertilizer plays a more crucial role than mineral fertilizers in improving soil fertility for a sustainable field (Mader et al. 2002). In particular, our results showed that these improvements were much more significant for large than smaller size soil fractions. There were little differences in the macronutrient contents between soil fractions of CK and NPK soils, probably due to the inherently high fertility of Mollisol soils. The organic matter content of all fractions of the NPK soil was lower than that of the corresponding fractions of the CK soil probably because the input of N fertilizer activated microbial activity with the consequent accelerated degradation of organic matter (Dijkstra et al. 2008).
Physical separation procedures offer the opportunity to study the small-scale distribution of soil microbes and enzyme activities and to analyze which environmental factors affect their distributions (Lagomarsino et al. 2012; Stemmer et al. 1998a). The general pattern of soil enzyme activities is often dominated by the amount and quality of organic substances as well as by various physical and chemical protection mechanisms (Allison and Jastrow 2006; Nannipieri et al. 2012; Lagomarsino et al. 2012). Therefore, the different distribution of nutrients and different soil pH values may affect enzyme activities of different soil particles. In the present study, the highest enzyme activities were observed in silt- and clay-sized fractions of CK and NPK soils and in the sand-sized fraction of the MNPK soil except for acid phosphomonoesterase. Both silt- and clay-sized fractions of CK and NPK soils contained higher SOM content than sand-size fraction. The long-term application of organic manures contributed to the increase in the SOM content of all fractions. In the MNPK soil probably, active microbes used the C for growth and synthesized enzymes and the increased enzyme activities were higher in the particles greater than 200 mm (Kandeler et al. 1999). Marx et al. (2005) also found positive relationships between hydrolase activity and C concentrations of grassland soil particles separated by size, particularly for fractions with abundant labile C, such as those greater than 200 mm. ANOVA displayed that soil fractions, fertilization conditions, and their interactions significantly affected the activities of all tested enzymes (Table 4). The results further showed that enzyme activity of soil fractions was altered by fertilization but not in the similar manner. Therefore, our results are in agreement with previous conclusions that the long-term compost amendment can increase the organic C content of soil by increasing organic C in all aggregates, thus changing the soil enzyme activities of different soil fractions (Yu et al. 2012). In addition, RAD was carried out to evaluate the correlation between soil enzyme activities and soil properties (Fig. 7). The cosine of the angle between the soil enzyme activity and the soil property shows the type of relationship. The negative correlation was only observed between AP content and acid phosphomonoesterase activities, whereas there were positive relationships between other soil properties and enzyme activities. These results are also in agreement with previous findings that soil enzyme activities are affected by soil properties mainly organic inputs and are significantly correlated with the SOM content of soil (Bastida et al. 2008; Nannipieri et al. 2012; Nicolás et al. 2012). Differences in enzyme activity can also depend on the type of humic compounds in soil (Benitez et al. 2005; Nannipieri et al. 2002).
Bacterial community structure was significantly influenced by particle sizes, and small soil fractions harbored higher bacterial diversity than larger particles thus confirming what was reported by Sessitsch et al. (2001). In addition, the microbial biomass is higher in smaller- than large-sized soil fractions (Jocteur Monrozier et al. 1991; Kandeler et al. 2000). Higher nutrient availability in smaller-sized particles of soil might lead to higher bacterial diversity. According to van Gestel et al. (1996), the vicinity between bacteria, organic matter, and clay is required for the survival of bacteria, as both organic matter and clay particles offer substrates and nutrients to bacteria. This can explain why our silt- and clay-sized fractions showed higher bacterial diversity than other soil fractions. Additionally, fine fractions (silt and clay size) offer physical protection to the microbial communities, thus allowing for high microbial activity in these fractions (Nicolás et al. 2012). This was also consistent with the report that bacterial diversity and abundance increased by decreasing the soil particle size as shown by 16S rRNA-based analysis (Gerzabek et al. 2002). The enrichment of organic matter in coarser fractions by organic fertilizer amendment seems to be the reason why MNPK maintained a relatively richer bacterial diversity than other treatments. This result was also reflected by the higher enzyme activities in coarser than finer fractions of MNPK soils.
Phylogenetic assignment of sequenced 16S rRNA genes showed a great abundance of Actinobacteria, γ-proteobacteria, and Acidobacteria belonging to the unculturable bacteria (Fig. 3). The function of these uncultured bacteria in soil needs to be investigated. In addition, inorganic fertilization and particle-size effects were two major factors affecting composition of soil bacterial communities, whereas the MNPK treatment resulted in the least variation in the composition of bacterial communities of soil fractions.
Except for C/N and AP contents, the other tested soil properties affected the composition of bacterial communities in soil fractions of different treatments as shown by RDA analysis. The organic matter of small-sized aggregates such as silt- and clay-sized fractions is probably more recalcitrant than that of larger fractions (Six et al. 1998). Particles with different sizes can have different contents of available C and N and probably for this reason the C/N was not an important factor affecting the composition of bacterial communities. The finer fractions (silt and clay size fractions) exhibited high C/N, which could be caused by the high potential of these fractions to accumulate organic C (Nicolás et al. 2012), and this result contradicts the findings that fine fractions can accumulate microbial compounds, which had low C/N ratios (Kiem and Kögel-Knabner 2003; Poll et al. 2003).
Among soil properties, soil pH may have the most impact on the composition of soil bacterial communities (Fierer and Jackson 2006), but other evidences indicates that the P content, altitude, and the presence of cations (Ca2+, Mg2+, and Al3+) in the soil are the most important (Faoro et al. 2010). We have shown that several soil properties can affect the composition of soil bacterial communities acting in a synergistic way (Chaparro et al. 2012). It is widely accepted that the response of microbes to their environment results in the production of enzymes (Nannipieri et al 2002, 2012). However, no significant relationship was observed between enzyme activities and composition of bacterial communities. Soil enzyme activity has been reported to provide a unique integrative biochemical assessment of soil function and condition and may be useful as an indicator of soil functional diversity (Epelde et al. 2008). Perhaps the key aspect determining this relationship is not taxonomic diversity, but rather functional diversity (Chaparro et al. 2012). Therefore, relationship may be observed between the enzyme activities and functional bacterial activities rather than the composition of bacterial communities (Nannipieri et al 2012).
In conclusion, soil particle size and fertilization regimes have different effects on soil enzyme activities and bacterial community structure in the Mollisol soils. The long-term application of organic manures contributed to the increase in the OM content of particles higher than 200 mm, and this enriched bacterial diversity of these fractions. Furthermore, the activities of α-glucosidase, β-glucosidase, β-d-xylosidase, N-acetyl-glucosaminidase, sulfatase, and β-cellobiosidase in coarse sand-sized soil were significantly increased by the organic amendment. We recommend soil fractionation as a promising approach that offers potential in analyzing the relationship between soil functional diversity and soil fertility. Nevertheless, further information is necessary about functions of the observed uncultured bacteria and the relationship between functional microbial activity and enzyme activities.
References
Ai C, Liang G, Sun J, Wang X, Zhou W (2012) Responses of extracellular enzyme activities and microbial community in both the rhizosphere and bulk soil to long-term fertilization practices in a fluvo-aquic soil. Geoderma 173–174:330–338
Ai C, Liang G, Sun J, Wang X, He P, Zhou W (2013) Different roles of rhizosphere effect and long-term fertilization in the activity and community structure of ammonia oxidizers in a calcareous fluvo-aquic soil. Soil Biol Biochem 57:30–42
Allison SD, Jastrow JD (2006) Activities of extracellular enzymes in physically isolated fractions of restored grassland soils. Soil Biol Biochem 38:3245–3256
Bastida F, Kandeler E, Hernandez T, Garcia C (2008) Long-term effect of municipal solid waste amendment on microbial abundance and humus-associated enzyme activities under semiarid conditions. Microb Ecol 55:651–661
Benitez E, Sainz H, Nogales R (2005) Hydrolytic enzyme activities of extracted humic substances during the vermicomposting of a lignocellulosic olive waste. Bioresour Technol 96:785–790
Chaparro JM, Sheflin AM, Manter DK, Vivanco JM (2012) Manipulating the soil microbiome to increase soil health and plant fertility. Biol Fertil Soils 48:489–499
Deng S, Kang H, Freeman C (2011) Microplate fluorimetric assay of soil enzymes. In: Dick RP (ed) Methods in soil enzymology. Soil Science Society of America, Madison, WI, pp 311–318
Deng S, Popova IE, Dick L, Dick R (2013) Bench scale and microplate format assay of soil enzyme activities using spectroscopic and fluorometric approaches. Appl Soil Ecol 64:84–90
Dijkstra P, LaViolette CM, Coyle JS, Doucett RR, Schwartz E, Hart SC, Hungate BA (2008) 15 N enrichment as an integrator of the effects of C and N on microbial metabolism and ecosystem function. Ecol Lett 11:389–397
Ding X, Han X, Liang Y, Qiao Y, Li L, Li N (2012) Changes in soil organic carbon pools after 10 years of continuous manuring combined with chemical fertilizer in a Mollisol in China. Soil Till Res 122:36–41
Elfstrand S, Hedlund K, Mårtensson A (2007) Soil enzyme activities, microbial community composition and function after 47 years of continuous green manuring. Appl Soil Ecol 35:610–621
Epelde L, Becerril JM, Hernández-Allica J, Barrutia O, Garbisu C (2008) Functional diversity as indicator of the recovery of soil health derived from Thlaspi caerulescens growth and metal phytoextraction. Appl Soil Ecol 39:299–310
Faoro H, Alves AC, Souza EM, Rigo LU, Cruz LM, Al-Janabi SM, Monteiro RA, Baura VA, Pedrosa FO (2010) Influence of soil characteristics on the diversity of bacteria in the Southern Brazilian Atlantic Forest. App Environ Microb 76:4744–4749
Fierer N, Jackson RB (2006) The diversity and biogeography of soil bacterial communities. Proc Natl Acad Sci U S A 103:626–631
Gerzabek MH, Haberhauer G, Kandeler E, Sessitsch A, Kirchmann H (2002) Response of organic matter pools and enzyme activities in particle size fractions to organic amendments in a long-term field experiment. In: Violante A, Huang PM, Bollag J-M, Gianfreda L (eds) Soil mineral-organic matter-microorganism interactions and ecosystem health, vol 28B, Development in Soil Science. Elsevier, London, pp 329–344
Guo JH, Liu XJ, Zhang Y, Shen JL, Han WX, Zhang WF, Christie P, Goulding KW, Vitousek PM, Zhang FS (2010) Significant acidification in major Chinese croplands. Science 327:1008–1010
Jocteur Monrozier L, Ladd JN, Fitzpatrick RW, Foster RC, Rapauch M (1991) Components and microbial biomass content of size fractions in soils of contrasting aggregation. Geoderma 50:37–62
Kandeler E, Stemmer M, Klimanek E-M (1999) Response of soil microbial biomass, urease and xylanase within particle size fractions to long-term soil management. Soil Biol Biochem 31:261–273
Kandeler E, Tscherko D, Bruce KD, Stemmer M, Hobbs PJ, Bardgett RD, Amelung W (2000) Structure and function of the soil microbial community in microhabitats of a heavy metal polluted soil. Biol Fertil Soils 32:390–400
Kiem R, Kögel-Knabner I (2003) Contribution of lignin and polysaccharides to the refractory carbon pool in C-depleted arable soils. Soil Biol Biochem 35:101–118
Lagomarsino A, Mench M, Marabottini R, Pignataro A, Grego G, Renella G, Stazi SR (2011) Copper distribution and hydrolase activities in a contaminated soil amended with dolomitic limestone and compost. Ecotox Environ Safe 74:2013–2019
Lagomarsino A, Grego S, Kandeler E (2012) Soil organic carbon distribution drives microbial activity and functional diversity in particle and aggregate-size fractions. Pedobiologia 55:101–110
Li Z, Liu M, Wu X, Han F, Zhang T (2010) Effects of long-term chemical fertilization and organic amendments on dynamics of soil organic C and total N in paddy soil derived from barren land in subtropical China. Soil Till Res 106:268–274
Mader P, Fliessbach A, Dubois D, Gunst L, Fried P, Niggli U (2002) Soil fertility and biodiversity in organic farming. Science 296:1694–1697
Marx MC, Kandeler E, Wood M, Wermbter N, Jarvis SC (2005) Exploring the enzymatic landscape: distribution and kinetics of hydrolytic enzymes in soil particle-size fractions. Soil Biol Biochem 37:35–48
Muyzer G, de Waal EC, Uitterlinden AG (1993) Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl Environ Microbiol 59:695–700
Nannipieri P, Kandeler E, Ruggiero P (2002) Enzyme activities and microbiological and biochemical processes in soil. In: Burns RG, Dick R (eds) Enzymes in the environment. Marcel Dekker, New York, pp 1–33
Nannipieri P, Giagnoni L, Lagomarsino G, Puglisi E, Ceccanti B, Masciandaro G, Fornasier F, Moscatelli MC, Marinari S (2012) Soil enzymology: classical and molecular approaches. Biol Fertil Soils 48:743–762
Nicolás C, Hernández T, García C (2012) Organic amendments as strategy to increase organic matter in particle-size fractions of a semi-arid soil. Appl Soil Ecol 57:50–58
Poll C, Thiede A, Wermbter N, Sessitsch A, Kandeler E (2003) Micro-scale distribution of microorganisms and microbial enzyme activities in a soil with long-term organic amendment. Eur J Soil Sci 54:715–724
Renella G, Landi L, Ascher MT, Ceccherini MT, Pietramellara G, Nannipieri P (2006) Phosphomonoesterase production and persistence and composition of bacterial communities during plant material decomposition in soils in soil with different pH values. Soil Biol Biochem 38:795–802
Sessitsch A, Weilharter A, Gerzabek MH, Kirchmann H, Kandeler A (2001) Microbial population structures in soil particle size fractions of a long-term fertilizer field experiment. Appl Environ Microbiol 67:4215–4224
Six J, Elliott ET, Paustian K, Doran JW (1998) Aggregation and soil organic matter accumulation in cultivated soils and native grassland soils. Soil Sci Soc Am J 62:1367–1377
Stemmer M, Gerzabek MH, Kandeler E (1998a) Invertase and xylanase activity of bulk soil and particle-size fractions during maize straw decomposition. Soil Biol Biochem 31:9–18
Stemmer M, Gerzabek MH, Kandeler E (1998b) Organic matter and enzyme activity in particle-size fractions of soils obtained after low-energy sonication. Soil Biol Biochem 30:9–17
Van Gestel M, Merckx R, Vlassak K (1996) Spatial distribution of microbial biomass in microaggregates of a silty-loam soil and the relation with the resistance of microorganisms to soil drying. Soil Biol Biochem 28:503–510
Wang G-H, Jin J, Liu J-J, Chen X-L, Liu J-D, Liu X-B (2009) Bacterial community structure in a Mollisol under long-term natural restoration, cropping, and bare fallow history estimated by PCR-DGGE. Pedosphere 19:156–165
Yevdokimov I, Gattinger A, Buegger F, Munch J, Schloter M (2008) Changes in microbial community structure in soil as a result of different amounts of nitrogen fertilization. Biol Fertil Soils 44:1103–1106
Yong X, Cui Y, Chen L, Ran W, Shen Q, Yang X (2011) Dynamics of bacterial communities during solid-state fermentation using agro-industrial wastes to produce poly-gamma-glutamic acid, revealed by real-time PCR and denaturing gradient gel electrophoresis (DGGE). Appl Microbiol Biotechnol 92:717–725
Yu HY, Ding WX, Luo JF, Donnison A, Zhang JB (2012) Long-term effect of compost and inorganic fertilizer on activities of carbon-cycle enzymes in aggregates of an intensively cultivated sandy loam. Soil Use Manage 28:347–360
Zhong W, Gu T, Wang W, Zhang B, Lin X, Huang Q, Shen W (2010) The effects of mineral fertilizer and organic manure on soil microbial community and diversity. Plant Soil 326:511–522
Acknowledgements
This work was supported by the National Basic Research Program of China (2013CB127403), Agricultural Ministry of China (201103004), and Priority Academic Program Development of Jiangsu Higher Education Institutions. The authors especially thank Dr. Joshua Kendall and Dr. Waseem Raza for the kind help in the language editing of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ling, N., Sun, Y., Ma, J. et al. Response of the bacterial diversity and soil enzyme activity in particle-size fractions of Mollisol after different fertilization in a long-term experiment. Biol Fertil Soils 50, 901–911 (2014). https://doi.org/10.1007/s00374-014-0911-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-014-0911-1